Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
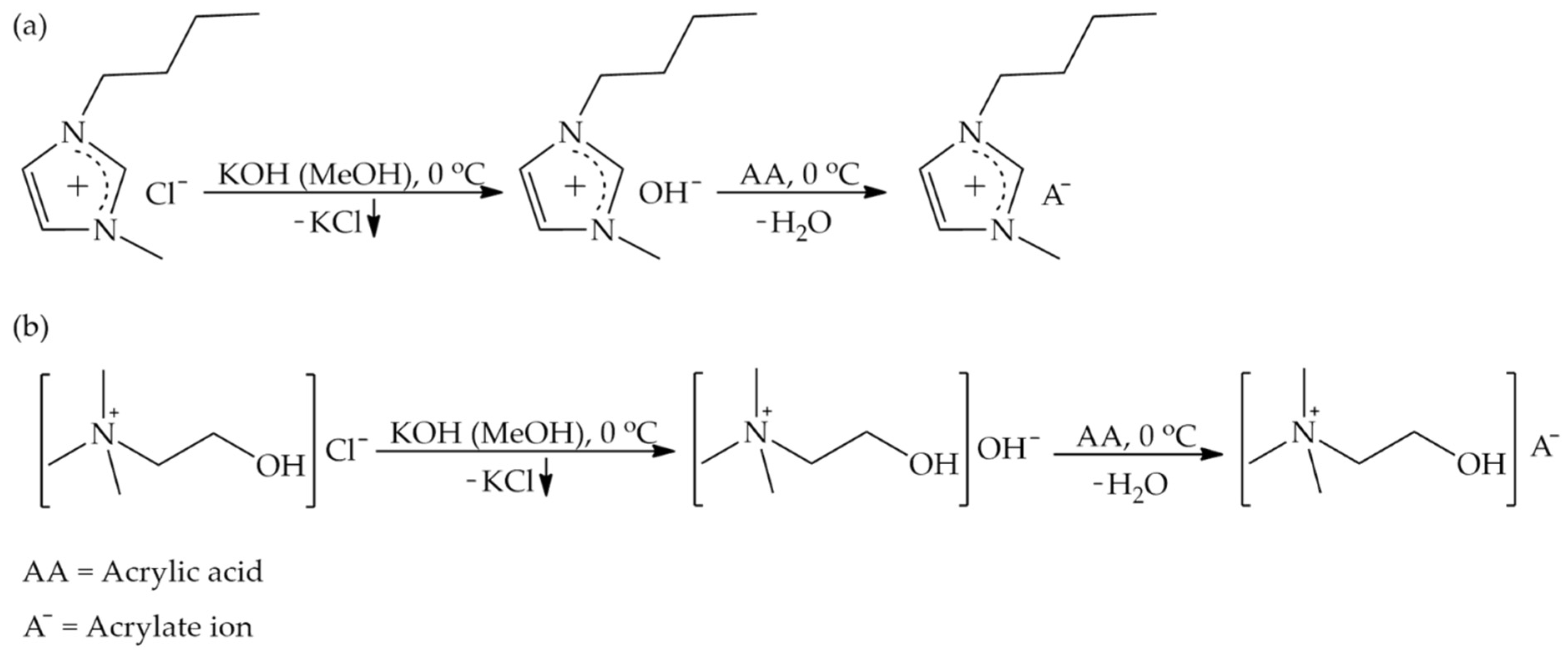
2.2.1. Synthesis of mILs
2.2.2. Dissolution and Regeneration of Cellulose
2.2.3. Structural Study of mILs
2.2.4. Wide-Angle X-ray Diffraction Study
2.2.5. Microscopic Investigation
2.2.6. Investigation of Thermal Properties of mILs
2.2.7. Rheological Studies
2.2.8. Application of ChA and [C4mim]A for 3D Printing with BC
3. Results and Discussion
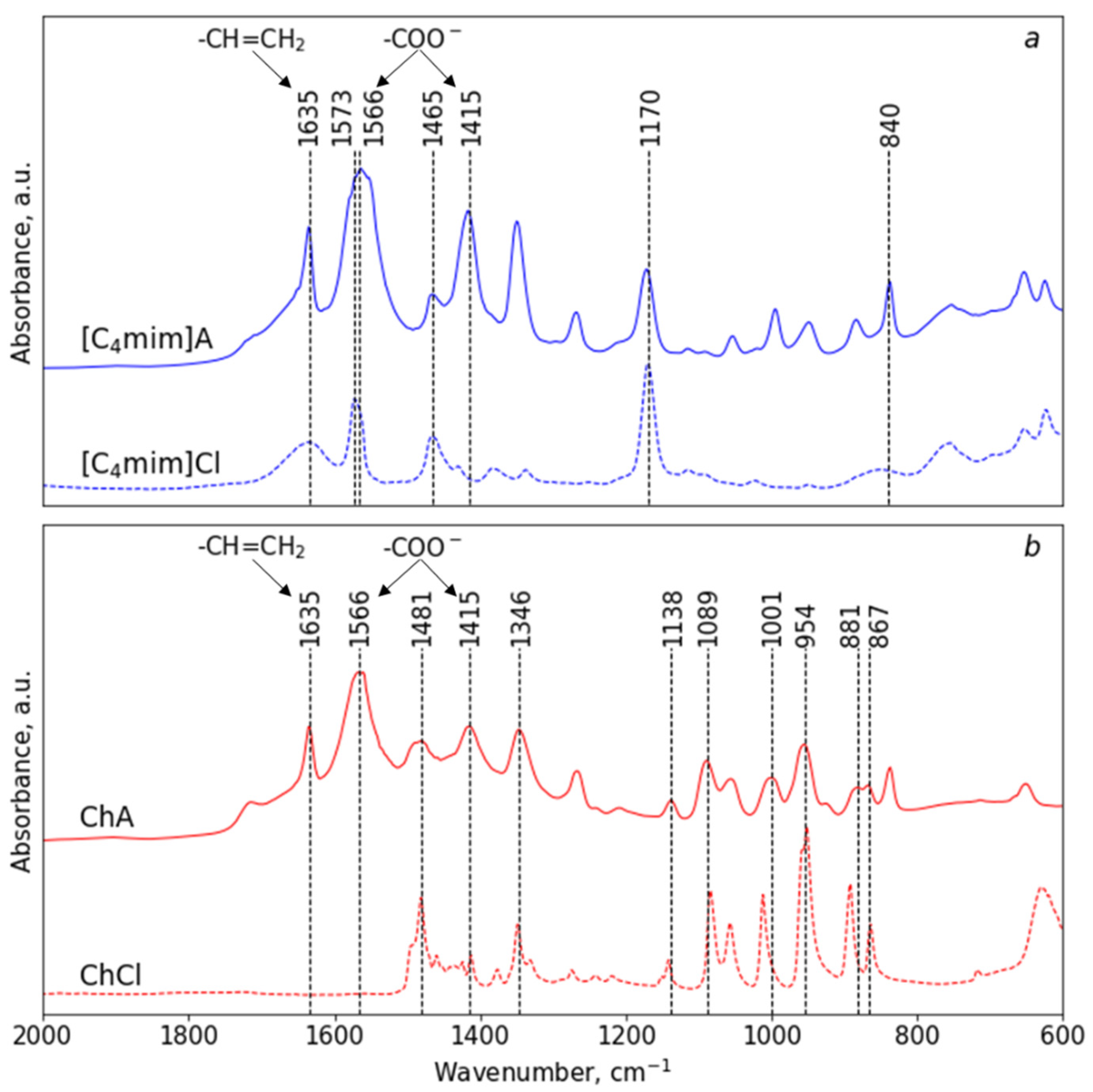
3.1. Synthesis and Spectroscopic Study of Acrylate-Based mILs
3.2. Thermal Properties of Acrylate Containing mILs
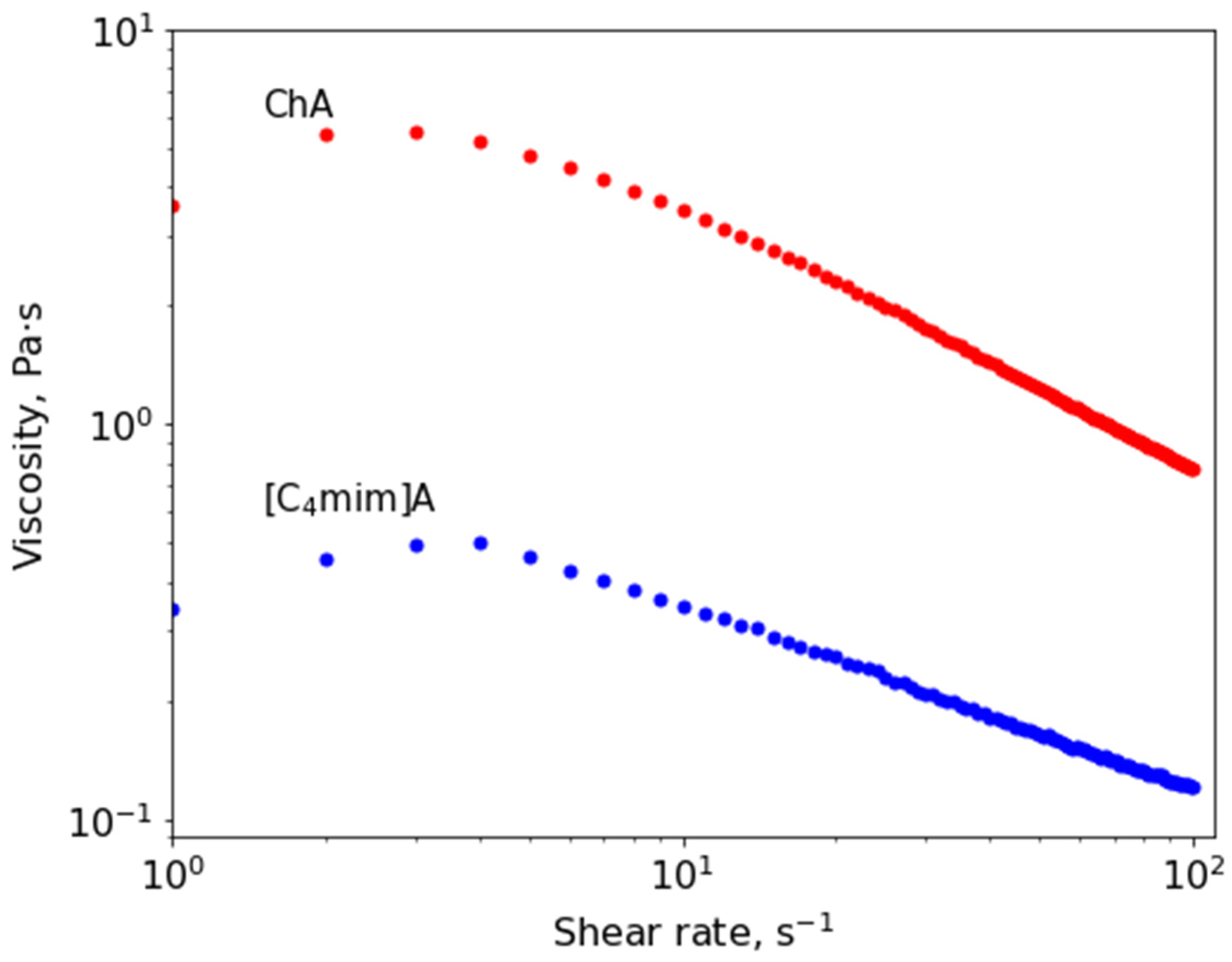
3.3. Rheological Properties of Acrylate-Based mILs
3.4. Application of ChA and [C4mim]A for Cellulose Treatment
3.5. Structure and Morphology of BC after Regeneration from mILs
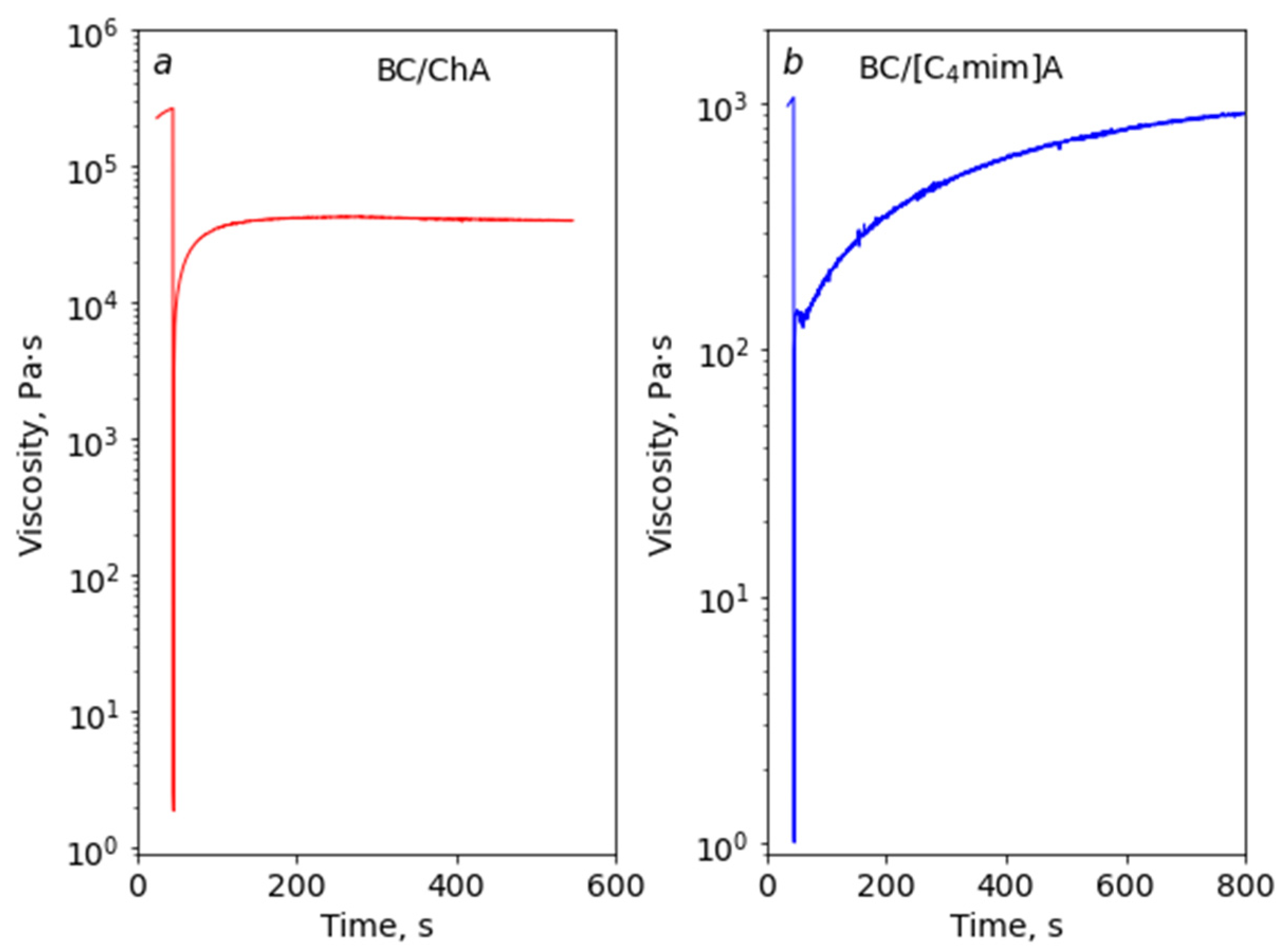
3.6. Rheological Properties of Acrylate-Based mILs with BC
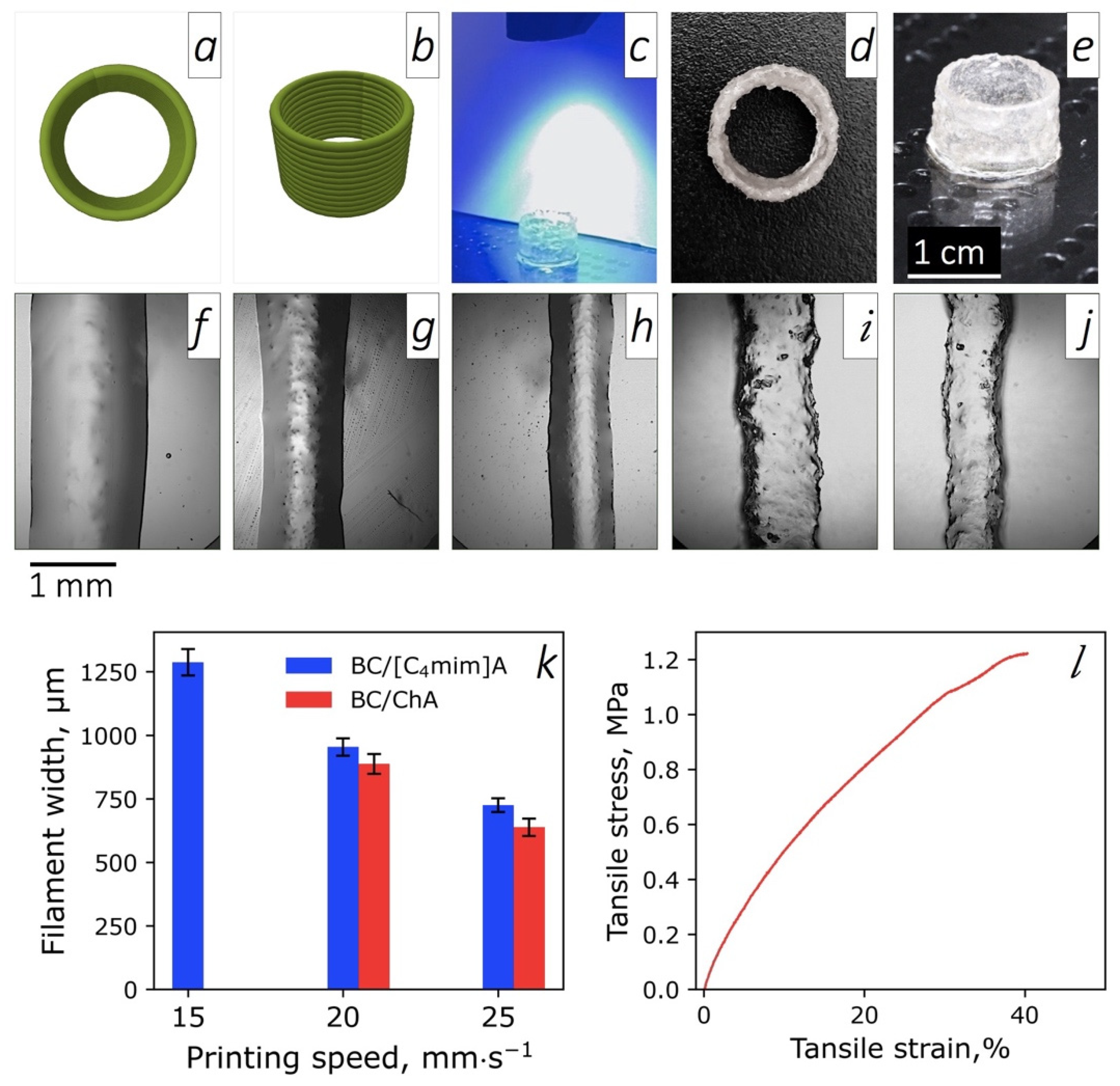
3.7. 3D Printing and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Namboodiri, V.V.; Varma, R.S. An improved preparation of 1,3-dialkylimidazolium tetrafluoroborate ionic liquids using microwaves. Tetrahedron Lett. 2002, 43, 5381–5383. [Google Scholar] [CrossRef]
- Stepnowski, P.; Mrozik, W.; Nichthauser, J. Adsorption of Alkylimidazolium and Alkylpyridinium Ionic Liquids onto Natural Soils. Environ. Sci. Technol. 2007, 41, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, E.A.; Ivanov, A.S.; Maslakov, K.I.; Savilov, S.V.; Lunin, V.V. Effect of cation structure of tetraalkylammonium- and imidazolium-based ionic liquids on their conductivity. Electrochim. Acta 2019, 297, 842–849. [Google Scholar] [CrossRef]
- Yuan, J.; Schlaad, H.; Giordano, C.; Antonietti, M. Double hydrophilic diblock copolymers containing a poly(ionic liquid) segment: Controlled synthesis, solution property, and application as carbon precursor. Eur. Polym. J. 2011, 47, 772–781. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Tokuda, H.; Hayamizu, K.; Watanabe, M. Magnitude and Directionality of Interaction in Ion Pairs of Ionic Liquids: Relationship with Ionic Conductivity. J. Phys. Chem. B 2005, 109, 16474–16481. [Google Scholar] [CrossRef]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Marszałł, M.P.; Baczek, T.; Kaliszan, R. Evaluation of the silanol-suppressing potency of ionic liquids. J. Sep. Sci. 2006, 29, 1138–1145. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Wei, G.-T.; Yang, Z.; Chen, C.-J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Strehmel, V.; Laschewsky, A.; Krudelt, H.; Wetzel, H.; Görnitz, E. Free Radical Polymerization of Methacrylates in Ionic Liquids. In Ionic Liquids in Polymer Systems; Brazel, C.S., Rogers, R.D., Eds.; ACS Publications: Washington, DC, USA, 2005; pp. 17–36. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef] [Green Version]
- Nulwala, H.; Mirjafari, A.; Zhou, X. Ionic liquids and poly(ionic liquid)s for 3D printing—A focused mini-review. Eur. Polym. J. 2018, 108, 390–398. [Google Scholar] [CrossRef]
- Abdullah, M.M.; AlQuraishi, A.A.; Allohedan, H.A.; AlMansour, A.O.; Atta, A.M. Synthesis of novel water soluble poly(ionic liquids) based on quaternary ammonium acrylamidomethyl propane sulfonate for enhanced oil recovery. J. Mol. Liq. 2017, 233, 508–516. [Google Scholar] [CrossRef]
- Pillai, P.; Mandal, A. A comprehensive micro scale study of poly-ionic liquid for application in enhanced oil recovery: Synthesis, characterization and evaluation of physicochemical properties. J. Mol. Liq. 2020, 302, 112553. [Google Scholar] [CrossRef]
- Jeon, E.H.; Nguyen, M.D.; Chung, C.I.; Kim, Y.J.; Kim, H.S.; Cheong, M.; Lee, J.S. Polymer-supported methylselenite for the oxidative carbonylation of aniline. Appl. Catal. A Gen. 2007, 332, 65–69. [Google Scholar] [CrossRef]
- Yan, F.; Texter, J. Surfactant ionic liquid-based microemulsions for polymerization. Chem. Commun. 2006, 25, 2696–2698. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Membr. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- López, M.S.-P.; Mecerreyes, D.; López-Cabarcos, E.; López-Ruiz, B. Amperometric glucose biosensor based on polymerized ionic liquid microparticles. Biosens. Bioelectron. 2006, 21, 2320–2328. [Google Scholar] [CrossRef]
- Ohno, H. Design of Ion Conductive Polymers Based on Ionic Liquids. Macromol. Symp. 2007, 249–250, 551–556. [Google Scholar] [CrossRef]
- Ito, K.; Nishina, N.; Ohno, H. Enhanced ion conduction in imidazolium-type molten salts. Electrochim. Acta 2000, 45, 1295–1298. [Google Scholar] [CrossRef]
- Zaoui, A.; Cherifi, Z.; Belbachir, M. Ultrasound-induced synthesis of an imidazolium based poly(ionic liquid) in an aqueous media: A structural, thermal and morphological study. Ultrason. Sonochem. 2019, 55, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.I.C.; Lozinskaya, E.I.; Meyer, F.; Shaplov, A.S.; Goujon, L.; Malyshkina, I.A.; Chevrot, C.; Teyssie, D. Ionic IPNs as Novel Candidates for Highly Conductive Solid Polymer Electrolytes. J. Polym. Sci. 2009, 47, 4245–4266. [Google Scholar]
- Mel’Nik, O.A.; Shaplov, A.S.; Lozinskaya, E.I.; Popova, N.A.; Makarov, M.V.; Odinets, I.L.; Lysenko, K.A.; Timofeeva, G.I.; Malyshkina, I.A.; Vygodskii, Y.S. Polymers based on ionic monomers with side phosphonate groups. Polym. Sci. Ser. B 2010, 52, 316–326. [Google Scholar] [CrossRef]
- Ye, Y.; Elabd, Y.A. Anion exchanged polymerized ionic liquids: High free volume single ion conductors. Polymer 2011, 52, 1309–1317. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, F.; Wang, C.; Liu, W. A novel gel polymer electrolyte based on poly ionic liquid 1-ethyl 3-(2-methacryloyloxy ethyl) imidazolium iodide. Eur. Polym. J. 2007, 43, 2699–2707. [Google Scholar] [CrossRef]
- Fan, F.; Wang, Y.; Hong, T.; Heres, M.F.; Saito, T.; Sokolov, A.P. Ion Conduction in Polymerized Ionic Liquids with Different Pendant Groups. Macromolecules 2015, 48, 4461–4470. [Google Scholar] [CrossRef]
- He, H.; Chung, H.; Roth, E.; Luebke, D.; Hopkinson, D.; Nulwala, H.; Matyjaszewski, K. Low glass transition temperature poly(ionic liquid) prepared from a new quaternary ammonium cationic monomer. Polym. Adv. Technol. 2015, 26, 823–828. [Google Scholar] [CrossRef]
- Fukaya, Y.; Iizuka, Y.; Sekikawa, K.; Ohno, H. Bio ionic liquids: Room temperature ionic liquids composed wholly of biomaterials. Green Chem. 2007, 9, 1155–1157. [Google Scholar] [CrossRef]
- Isik, M.; Gracia, R.; Kollnus, L.C.; Tomé, L.C.; Marrucho, I.; Mecerreyes, D. Cholinium-Based Poly(ionic liquid)s: Synthesis, Characterization, and Application as Biocompatible Ion Gels and Cellulose Coatings. ACS Macro Lett. 2013, 2, 975–979. [Google Scholar] [CrossRef]
- Kohno, Y.; Saita, S.; Men, Y.; Yuan, J.; Ohno, H. Thermoresponsive polyelectrolytes derived from ionic liquids. Polym. Chem. 2015, 6, 2163–2178. [Google Scholar] [CrossRef]
- Kohno, Y.; Deguchi, Y.; Ohno, H. Ionic liquid-derived charged polymers to show highly thermoresponsive LCST-type transition with water at desired temperatures. Chem. Commun. 2012, 48, 11883–11885. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa, M.; Ogihara, W. Development of new class of ion conductive polymers based on ionic liquids. Electrochim. Acta 2004, 50, 255–261. [Google Scholar] [CrossRef]
- Sharma, M.; Mondal, D.; Mukesh, C.; Prasad, K. Preparation of tamarind gum based soft ion gels having thixotropic properties. Carbohydr. Polym. 2014, 102, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room Temperature Ionic Liquids from 20 Natural Amino Acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef] [PubMed]
- Korchak, P.; Alopina, E.; Pukinsky, I.; Safonova, E. Liquid-liquid equilibria of aqueous biphasic systems containing 1-alkyl-3-methylimidazolium amino acid ionic liquids with different anions (L-Leucine, L-Valine, L-Lysine) and inorganic salt (tripotassium phosphate, potassium carbonate). Fluid Phase Equilib. 2020, 525, 112789. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Liimatainen, H. Cellulose Nanofibrils from Nonderivatizing Urea-Based Deep Eutectic Solvent Pretreatments. ACS Appl. Mater. Interfaces 2017, 9, 2846–2855. [Google Scholar] [CrossRef] [Green Version]
- Vorobiov, V.K.; Sokolova, M.P.; Bobrova, N.V.; Elokhovsky, V.Y.; Smirnov, M.A. Rheological properties and 3D-printability of cellulose nanocrystals/deep eutectic solvent electroactive ion gels. Carbohydr. Polym. 2022, 290, 119475. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Zuo, M.; Zeng, X.; Tang, X.; Sun, Y.; Lin, L. Stretchable, freezing-tolerant conductive hydrogel for wearable electronics reinforced by cellulose nanocrystals toward multiple hydrogen bonding. Carbohydr. Polym. 2022, 280, 119018. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. A self-healing water-dissolvable and stretchable cellulose-hydrogel for strain sensor. Cellulose 2022, 29, 341–354. [Google Scholar] [CrossRef]
- Lai, C.-W.; Yu, S.-S. 3D Printable Strain Sensors from Deep Eutectic Solvents and Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2020, 12, 34235–34244. [Google Scholar] [CrossRef]
- Firmanda, A.; Syamsu, K.; Sari, Y.W.; Cabral, J.; Pletzer, D.; Mahadik, B.; Fisher, J.; Fahma, F. 3D printed cellulose based product applications. Mater. Chem. Front. 2022, 6, 254–279. [Google Scholar] [CrossRef]
- Yuan, R.; Wu, K.; Fu, Q. 3D printing of all-regenerated cellulose material with truly 3D configuration: The critical role of cellulose microfiber. Carbohydr. Polym. 2022, 294, 119784. [Google Scholar] [CrossRef]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2019, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Thomann, C.A.; Münzner, P.; Moch, K.; Jacquemin, J.; Goodrich, P.; Sokolov, A.P.; Böhmer, R.; Gainaru, C. Tuning the dynamics of imidazolium-based ionic liquids via hydrogen bonding. I. The viscous regime. J. Chem. Phys. 2020, 153, 194501. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Fedotova, V.S.; Sokolova, M.P.; Nikolaeva, A.L.; Elokhovsky, V.Y.; Karttunen, M. Polymerizable Choline- and Imidazolium-Based Ionic Liquids Reinforced with Bacterial Cellulose for 3D-Printing. Polymers 2021, 13, 3044. [Google Scholar] [CrossRef]
- Valtasaari, L. The configuration of cellulose dissolved in iron/sodium tartrate. Die Makronwlekulare Chem. 1971, 150, 117–126. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rao, G.R. Synthesis and characterization of hybrid molecular material prepared by ionic liquid and silicotungstic acid. Mater. Chem. Phys. 2008, 112, 853–857. [Google Scholar] [CrossRef]
- Rao, G.R.; Rajkumar, T.; Varghese, B. Synthesis and characterization of 1-butyl 3-methyl imidazolium phosphomolybdate molecular salt. Solid State Sci. 2009, 11, 36–42. [Google Scholar] [CrossRef]
- Feng, W.-Q.; Lu, Y.-H.; Chen, Y.; Lu, Y.-W.; Yang, T. Thermal stability of imidazolium-based ionic liquids investigated by TG and FTIR techniques. J. Therm. Anal. Calorim. 2016, 125, 143–154. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Samarov, A.A.; Bobrova, N.V.; Vorobiov, V.K.; Popova, E.N.; Filippova, E.; Geydt, P.; Lahderanta, E.; Toikka, A.M. Plasticizing of chitosan films with deep eutectic mixture of malonic acid and choline chloride. Carbohydr. Polym. 2018, 197, 548–557. [Google Scholar] [CrossRef]
- Paschoal, V.H.; Faria, L.F.O.; Ribeiro, M.C.C. Vibrational Spectroscopy of Ionic Liquids. Chem. Rev. 2017, 117, 7053–7112. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR spectral band shifts explained by OM–cation interactions. J. Plant Nutr. Soil Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Ueno, K.; Imaizumi, S.; Hata, K.; Watanabe, M. Colloidal Interaction in Ionic Liquids: Effects of Ionic Structures and Surface Chemistry on Rheology of Silica Colloidal Dispersions. Langmuir 2008, 25, 825–831. [Google Scholar] [CrossRef]
- Comtet, J.; Chatté, G.; Niguès, A.; Bocquet, L.; Siria, A.; Colin, A. Pairwise frictional profile between particles determines discontinuous shear thickening transition in non-colloidal suspensions. Nat. Commun. 2017, 8, 15633. [Google Scholar] [CrossRef] [Green Version]
- de Souza, F.; Ribeiro, M.C. A Raman spectroscopy and rheology study of the phase transitions of the ionic liquid choline acetate. J. Mol. Liq. 2021, 322, 114530. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of Pure Ionic Liquids and Their Complex Fluids: A Review. ACS Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef] [Green Version]
- Haron, G.A.S.; Mahmood, H.; Noh, M.H.; Alam, Z.; Moniruzzaman, M. Ionic Liquids as a Sustainable Platform for Nanocellulose Processing from Bioresources: Overview and Current Status. ACS Sustain. Chem. Eng. 2021, 9, 1008–1034. [Google Scholar] [CrossRef]
- Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Swelling and dissolution kinetics of natural and man-made cellulose fibers in solvent power tuned ionic liquid. Cellulose 2020, 27, 7399–7415. [Google Scholar] [CrossRef]
- Kirchner, B. Ionic Liquids. In Topics in Current Chemistry; Kirchner, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bentivoglio, G.; Röder, T.; Fasching, M.; Buchberger, M.; Schottenberger, H.; Sixta, H. Cellulose Processing with Chloride-Based Ionic Liquids. Lenzing. Ber. 2006, 86, 154–161. [Google Scholar]
- De Silva, R.; Byrne, N. Utilization of cotton waste for regenerated cellulose fibres: Influence of degree of polymerization on mechanical properties. Carbohydr. Polym. 2017, 174, 89–94. [Google Scholar] [CrossRef]
- Michud, A.; Hummel, M.; Haward, S.; Sixta, H. Monitoring of cellulose depolymerization in 1-ethyl-3-methylimidazolium acetate by shear and elongational rheology. Carbohydr. Polym. 2015, 117, 355–363. [Google Scholar] [CrossRef]
- Zhou, L.; Pan, F.; Liu, Y.; Kang, Z.; Zeng, S.; Nie, Y. Study on the regularity of cellulose degradation in ionic liquids. J. Mol. Liq. 2020, 308, 113153. [Google Scholar] [CrossRef]
- Ky, I.; Le Floch, A.; Zeng, L.; Pechamat, L.; Jourdes, M.; Teissedre, P.L. Tannins. In Encyclopedia of Food and Health; Elsevier: New York, NY, USA, 2015; pp. 247–255. ISBN 9780123849533. [Google Scholar]
- Keshk, S.M. Bacterial Cellulose Production and its Industrial Applications. J. Bioprocess. Biotech. 2014, 4, 1000150. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.-L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2010, 84, 96–102. [Google Scholar] [CrossRef]
- Sugiyama, J.; Persson, J.; Chanzy, H. Combined infrared and electron diffraction study of the polymorphism of native celluloses. Macromolecules 1991, 24, 2461–2466. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Schade, A.; Behme, N.; Spange, S. Dipolarity versus Polarizability and Acidity versus Basicity of Ionic Liquids as a Function of Their Molecular Structures. Chem. Eur. J. 2014, 20, 2232–2243. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing Ionic Liquids on the Basis of Multiple Solvation Interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [Green Version]
- Pinkert, A.; Marsh, K.N.; Pang, S.; Staiger, M.P. Ionic Liquids and Their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Rinaldi, R. Instantaneous dissolution of cellulose in organic electrolyte solutions. Chem. Commun. 2010, 47, 511–513. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef]
- Barnes, H.A. The yield stress—A review—Everything flows? J. Nonnewton. Fluid Mech. 1999, 81, 133–178. [Google Scholar] [CrossRef]
- Huang, L.; Du, X.; Fan, S.; Yang, G.; Shao, H.; Li, D.; Cao, C.; Zhu, Y.; Zhu, M.; Zhang, Y. Bacterial cellulose nanofibers promote stress and fidelity of 3D-printed silk based hydrogel scaffold with hierarchical pores. Carbohydr. Polym. 2019, 221, 146–156. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Kechagias, J.D.; Mountakis, N.; Argyros, A.; Boura, O.; Grammatikos, S. High-performance medical-grade resin radically reinforced with cellulose nanofibers for 3D printing. J. Mech. Behav. Biomed. Mater. 2022, 134, 105408. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Sundberg, J.; Gatenholm, P. 3D Bioprinting of Cellulose Structures from an Ionic Liquid. 3D Print. Addit. Manuf. 2014, 1, 115–121. [Google Scholar] [CrossRef]
- Gunasekera, D.H.A.T.; Kuek, S.; Hasanaj, D.; He, Y.; Tuck, C.; Croft, A.K.; Wildman, R.D. Three dimensional ink-jet printing of biomaterials using ionic liquids and co-solvents. Faraday Discuss. 2016, 190, 509–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaszyńska, J.; Rachocki, A.; Bielejewski, M.; Tritt-Goc, J. Influence of cellulose gel matrix on BMIMCl ionic liquid dynamics and conductivity. Cellulose 2017, 24, 1641–1655. [Google Scholar] [CrossRef]
- Rana, H.H.; Park, J.H.; Gund, G.S.; Park, H.S. Highly conducting, extremely durable, phosphorylated cellulose-based ionogels for renewable flexible supercapacitors. Energy Storage Mater. 2020, 25, 70–75. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Xu, G.; Wang, Q.; Liu, S.; Li, J.; Chen, C.; Yu, H.; Hu, L. A Dynamic Gel with Reversible and Tunable Topological Networks and Performances. Matter 2020, 2, 390–403. [Google Scholar] [CrossRef]
- Zhao, D.; Pang, B.; Zhu, Y.; Cheng, W.; Cao, K.; Ye, D.; Si, C.; Xu, G.; Chen, C.; Yu, H. A Stiffness-Switchable, Biomimetic Smart Material Enabled by Supramolecular Reconfiguration. Adv. Mater. 2022, 34, 2107857. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Feng, X.; Dang, C.; Dai, F.; Yin, X.; He, M.; Liu, D.; Qi, H. Cellulose Nanofiber-Reinforced Ionic Conductors for Multifunctional Sensors and Devices. ACS Appl. Mater. Interfaces 2020, 12, 27545–27554. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Bu, X.; Wang, L.; Wu, L.; Ma, X.; Diao, W.; Lu, D. Ultratough and recoverable ionogels based on multiple interpolymer hydrogen bonding as durable electrolytes for flexible solid-state supercapacitor. J. Appl. Polym. Sci. 2021, 138, 50259. [Google Scholar] [CrossRef]
- Chen, L.; Fu, J.; Lu, Q.; Shi, L.; Li, M.; Dong, L.; Xu, Y.; Jia, R. Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization. Chem. Eng. J. 2019, 378, 122245. [Google Scholar] [CrossRef]




| Sample | DP |
|---|---|
| Initial BC | 730 |
| BC after ChA | 715 |
| BC after [C4mim]A | 370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotova, V.S.; Sokolova, M.P.; Vorobiov, V.K.; Sivtsov, E.V.; Ribeiro, M.C.C.; Smirnov, M.A. Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers 2022, 14, 5148. https://doi.org/10.3390/polym14235148
Fedotova VS, Sokolova MP, Vorobiov VK, Sivtsov EV, Ribeiro MCC, Smirnov MA. Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers. 2022; 14(23):5148. https://doi.org/10.3390/polym14235148
Chicago/Turabian StyleFedotova, Veronika S., Maria P. Sokolova, Vitaliy K. Vorobiov, Eugene V. Sivtsov, Mauro C. C. Ribeiro, and Michael A. Smirnov. 2022. "Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids" Polymers 14, no. 23: 5148. https://doi.org/10.3390/polym14235148
APA StyleFedotova, V. S., Sokolova, M. P., Vorobiov, V. K., Sivtsov, E. V., Ribeiro, M. C. C., & Smirnov, M. A. (2022). Synthesis and Physicochemical Properties of Acrylate Anion Based Ionic Liquids. Polymers, 14(23), 5148. https://doi.org/10.3390/polym14235148










