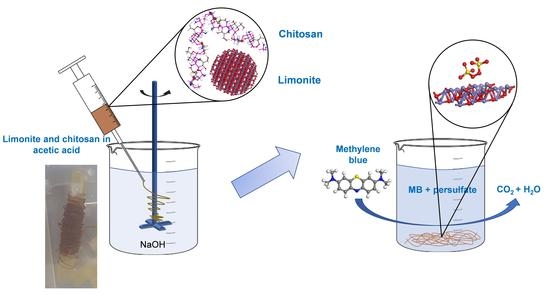
Chitosan Fibers Loaded with Limonite as a Catalyst for the Decolorization of Methylene Blue via a Persulfate-Based Advanced Oxidation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan Fibers Loaded with Limonite
2.3. Adsorption and Decolorization of MB Using Chitosan Fibers Loaded with Limonite
2.4. Computational Analysis
3. Results and Discussion
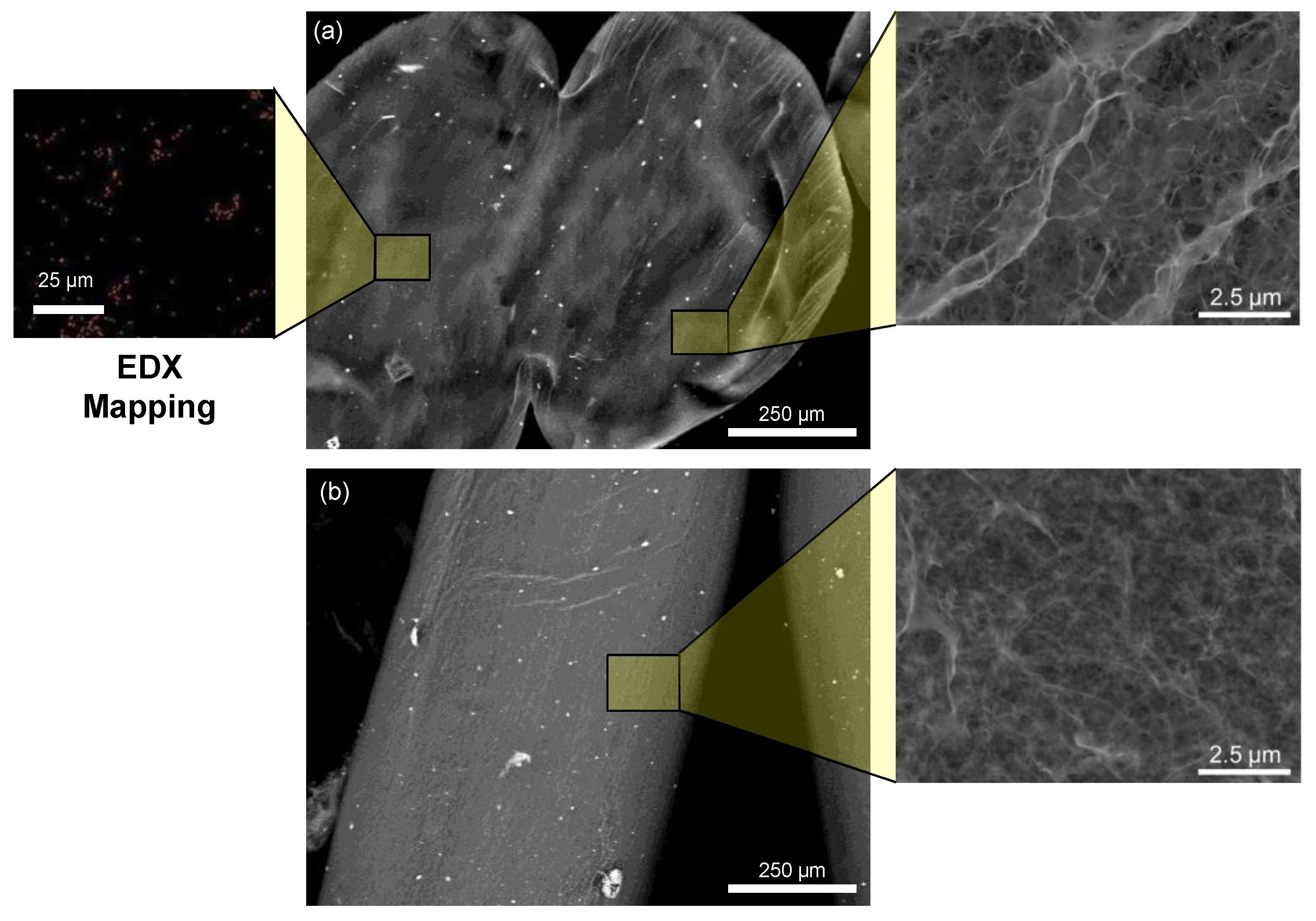
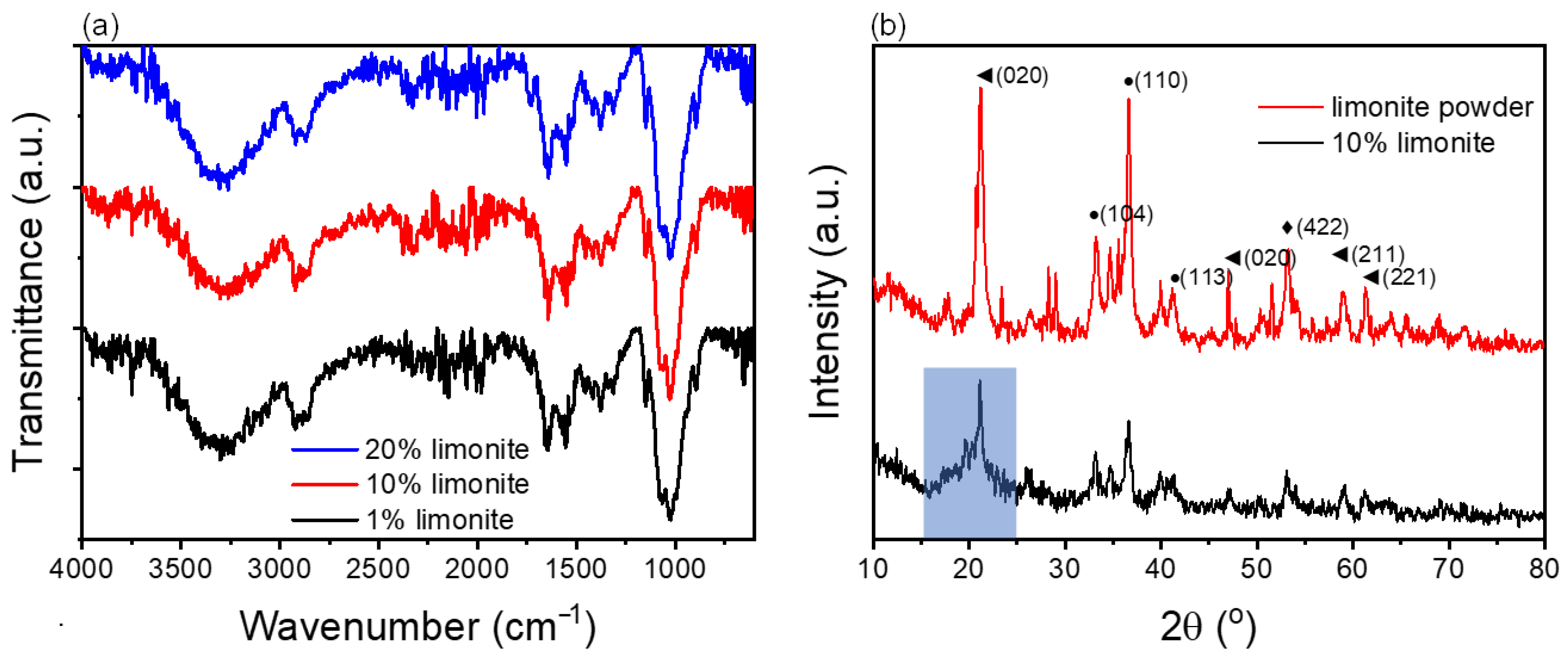
3.1. Characterization of the Chitosan-Limonite Fibers
3.2. Adsorption and SO4−·-Based AOPs Decolorization of MB Using Chitosan-Limonite Fibers
3.3. Computational Adsorption Energy Calculation of the Adsorption of PS onto Limonite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nayl, A.A.; Abd-Elhamid, A.I.; Awwad, N.S.; Abdelgawad, M.A.; Wu, J.; Mo, X.; Gomha, S.M.; Aly, A.A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Lan, D.; Zhu, H.; Zhang, J.; Li, S.; Chen, Q.; Wang, C.; Wu, T.; Xu, M. Adsorptive Removal of Organic Dyes via Porous Materials for Wastewater Treatment in Recent Decades: A Review on Species, Mechanisms and Perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of Pharmaceuticals from Water by Homo/Heterogeneous Fenton-Type Processes—A Review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Zoroufchi Benis, K.; Behnami, A.; Aghayani, E.; Farabi, S.; Pourakbar, M. Water Recovery and On-Site Reuse of Laundry Wastewater by a Facile and Cost-Effective System: Combined Biological and Advanced Oxidation Process. Sci. Total Environ. 2021, 789, 148068. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jamal, R.; Abdiryim, T.; Liu, X. Polydopamine-Based Polysaccharide Materials for Water Treatment. Cellulose 2022, 29, 8025–8064. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Ye, X.; Shang, S.; Zhao, Y.; Cui, S.; Zhong, Y.; Huang, L. Ultra-Efficient Adsorption of Copper Ions in Chitosan–Montmorillonite Composite Aerogel at Wastewater Treatment. Cellulose 2021, 28, 7201–7212. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Ahmed, I.M.; El-Shanshory, A.A.; Abu-Saied, M.A.; Soliman, H.M.A.; Abdelgawad, M.A.; Ali, H.M.; Bräse, S. Chitosan-Functionalized-Graphene Oxide (GO@CS) Beads as an Effective Adsorbent to Remove Cationic Dye from Wastewater. Polymers 2022, 14, 4236. [Google Scholar] [CrossRef]
- Cárdenas Bates, I.I.; Loranger, É.; Mathew, A.P.; Chabot, B. Cellulose Reinforced Electrospun Chitosan Nanofibers Bio-Based Composite Sorbent for Water Treatment Applications. Cellulose 2021, 28, 4865–4885. [Google Scholar] [CrossRef]
- Căprărescu, S.; Zgârian, R.G.; Tihan, G.T.; Purcar, V.; Eftimie Totu, E.; Modrogan, C.; Chiriac, A.-L.; Nicolae, C.A. Biopolymeric Membrane Enriched with Chitosan and Silver for Metallic Ions Removal. Polymers 2020, 12, 1792. [Google Scholar] [CrossRef]
- Thamer, B.M.; Aldalbahi, A.; Moydeen, A.M.; El-Newehy, M.H. In Situ Preparation of Novel Porous Nanocomposite Hydrogel as Effective Adsorbent for the Removal of Cationic Dyes from Polluted Water. Polymers 2020, 12, 3002. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, V.; Mohammadian, M. Photo-Assisted Advanced Oxidation Processes for Efficient Removal of Anionic and Cationic Dyes Using Bentonite/TiO2 Nano-Photocatalyst Immobilized with Silver Nanoparticles. J. Mol. Struct. 2021, 1239, 130496. [Google Scholar] [CrossRef]
- Du, X.; Oturan, M.A.; Zhou, M.; Belkessa, N.; Su, P.; Cai, J.; Trellu, C.; Mousset, E. Nanostructured Electrodes for Electrocatalytic Advanced Oxidation Processes: From Materials Preparation to Mechanisms Understanding and Wastewater Treatment Applications. Appl. Catal. B 2021, 296, 120332. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, Y.; Wu, Z.; Yao, G.; Lai, B. Synthesis Strategies and Emerging Mechanisms of Metal-Organic Frameworks for Sulfate Radical-Based Advanced Oxidation Process: A Review. Chem. Eng. J. 2021, 421, 127863. [Google Scholar] [CrossRef]
- Usman, M.; Cheema, S.A.; Farooq, M. Heterogeneous Fenton and Persulfate Oxidation for Treatment of Landfill Leachate: A Review Supplement. J. Clean. Prod. 2020, 256, 120448. [Google Scholar] [CrossRef]
- Liu, C.; Wu, B.; Chen, X. Sulfate Radical-Based Oxidation for Sludge Treatment: A Review. Chem. Eng. J. 2018, 335, 865–875. [Google Scholar] [CrossRef]
- Tang, S.; Zhao, M.; Yuan, D.; Li, X.; Zhang, X.; Wang, Z.; Jiao, T.; Wang, K. MnFe2O4 Nanoparticles Promoted Electrochemical Oxidation Coupling with Persulfate Activation for Tetracycline Degradation. Sep. Purif. Technol. 2021, 255, 117690. [Google Scholar] [CrossRef]
- Yang, G.; Dong, J.; Xing, B.; Mo, S.; Song, X.; Liu, X.; Yuan, J. Ni, Fe, and N-Tridoped Activated Carbon as a Highly Active Heterogeneous Persulfate Catalyst toward the Degradation of Organic Pollutant in Water. Sep. Purif. Technol. 2020, 252, 117440. [Google Scholar] [CrossRef]
- de Souza, W.F.; Guimarães, I.R.; Oliveira, L.C.A.; Guerreiro, M.C.; Guarieiro, A.L.N.; Carvalho, K.T.G. Natural and H2-Reduced Limonite for Organic Oxidation by a Fenton-like System: Mechanism Study via ESI-MS and Theoretical Calculations. J. Mol. Catal. A Chem. 2007, 278, 145–151. [Google Scholar] [CrossRef]
- Zou, X.; Chen, T.; Liu, H.; Zhang, P.; Chen, D.; Zhu, C. Catalytic Cracking of Toluene over Hematite Derived from Thermally Treated Natural Limonite. Fuel 2016, 177, 180–189. [Google Scholar] [CrossRef]
- Tao, H.-C.; Wei, X.-Y.; Zhang, L.-J.; Lei, T.; Xu, N. Degradation of P-Nitrophenol in a BES-Fenton System Based on Limonite. J. Hazard. Mater. 2013, 254–255, 236–241. [Google Scholar] [CrossRef]
- Li, X.; Cai, G.; Li, Y.; Zhu, X.; Qi, X.; Zhang, X.; Shu, B.; Li, K.; Wei, Y.; Wang, H. Limonite as a Source of Solid Iron in the Crystallization of Scorodite Aiming at Arsenic Removal from Smelting Wastewater. J. Clean. Prod. 2021, 278, 123552. [Google Scholar] [CrossRef]
- Eren Belgin, E.; Aycik, G.A.; Kalemtas, A.; Pelit, A.; Dilek, D.A.; Kavak, M.T. Preparation and Characterization of a Novel Ionizing Electromagnetic Radiation Shielding Material: Hematite Filled Polyester Based Composites. Radiat. Phys. Chem. 2015, 115, 43–48. [Google Scholar] [CrossRef]
- Abdou, M.I.; El-Sayed Ahmed, H.; Wahab Gaber, M.A.; Fadl, A.M. Enhancement of Anti-Corrosion and Mechanical Properties of Alkyd-Based Protective Paints for Steel Petroleum Structures Incorporating Natural Limonite Pigment. Cogent Eng. 2018, 5, 1427844. [Google Scholar] [CrossRef]
- Toda, K.; Tanaka, T.; Tsuda, Y.; Ban, M.; Koveke, E.P.; Koinuma, M.; Ohira, S.-I. Sulfurized Limonite as Material for Fast Decomposition of Organic Compounds by Heterogeneous Fenton Reaction. J. Hazard. Mater. 2014, 278, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Shahwan, T.; Abu Sirriah, S.; Nairat, M.; Boyacı, E.; Eroğlu, A.E.; Scott, T.B.; Hallam, K.R. Green Synthesis of Iron Nanoparticles and Their Application as a Fenton-like Catalyst for the Degradation of Aqueous Cationic and Anionic Dyes. Chem. Eng. J. 2011, 172, 258–266. [Google Scholar] [CrossRef]
- Zakria, H.S.; Othman, M.H.D.; Kamaludin, R.; Sheikh Abdul Kadir, S.H.; Kurniawan, T.A.; Jilani, A. Immobilization Techniques of a Photocatalyst into and onto a Polymer Membrane for Photocatalytic Activity. RSC Adv. 2021, 11, 6985–7014. [Google Scholar] [CrossRef]
- Zhu, L.; Ai, Z.; Ho, W.; Zhang, L. Core–Shell Fe–Fe2O3 Nanostructures as Effective Persulfate Activator for Degradation of Methyl Orange. Sep. Purif. Technol. 2013, 108, 159–165. [Google Scholar] [CrossRef]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of Sulfamonomethoxine with Fe3O4 Magnetic Nanoparticles as Heterogeneous Activator of Persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef]
- Shang, W.; Dong, Z.; Li, M.; Song, X.; Zhang, M.; Jiang, C.; Feiyun, S. Degradation of Diatrizoate in Water by Fe(II)-Activated Persulfate Oxidation. Chem. Eng. J. 2019, 361, 1333–1344. [Google Scholar] [CrossRef]
- Al-Shamsi, M.A.; Thomson, N.R. Treatment of Organic Compounds by Activated Persulfate Using Nanoscale Zerovalent Iron. Ind. Eng. Chem. Res. 2013, 52, 13564–13571. [Google Scholar] [CrossRef]
- Jonidi Jafari, A.; Kakavandi, B.; Jaafarzadeh, N.; Rezaei Kalantary, R.; Ahmadi, M.; Akbar Babaei, A. Fenton-like Catalytic Oxidation of Tetracycline by AC@Fe3O4 as a Heterogeneous Persulfate Activator: Adsorption and Degradation Studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Wu, S.; Liang, G.; Guan, X.; Qian, G.; He, Z. Precise Control of Iron Activating Persulfate by Current Generation in an Electrochemical Membrane Reactor. Environ. Int. 2019, 131, 105024. [Google Scholar] [CrossRef]
- Li, P.; Hu, H.; Luo, G.; Zhu, S.; Guo, L.; Qu, P.; Shen, Q.; He, T. Crystal Facet-Dependent CO2 Photoreduction over Porous ZnO Nanocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 56039–56048. [Google Scholar] [CrossRef] [PubMed]
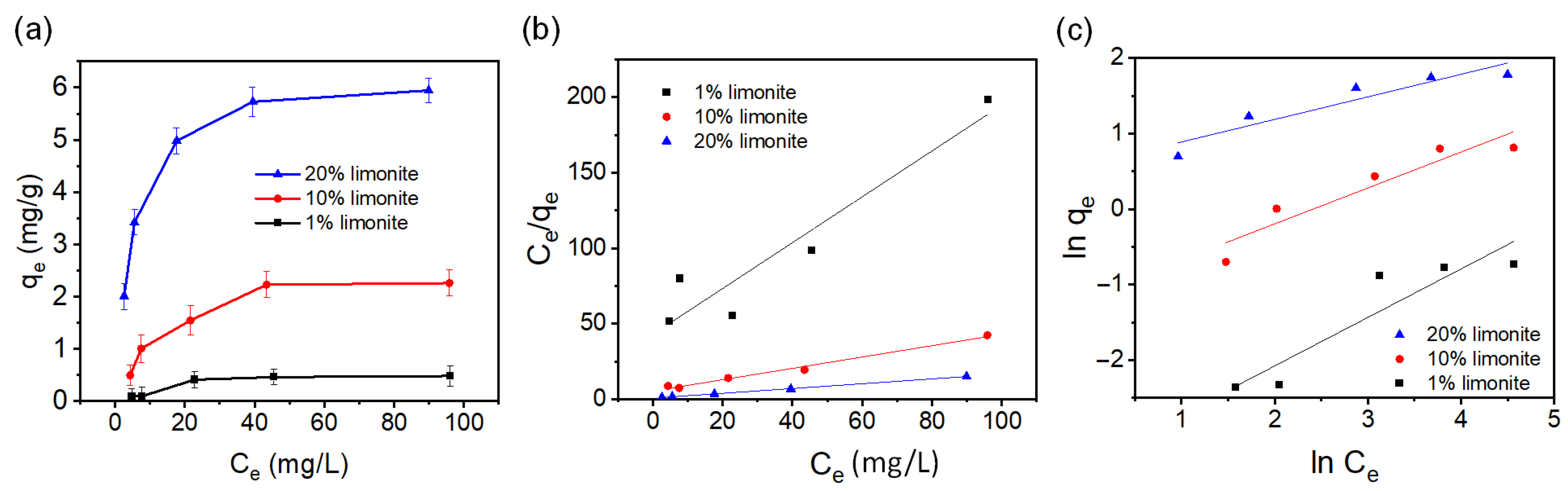



| Sample | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | KF ((mg/g) (L/mg)1/n) | n | R2 | |
| 1% limonite | 0.659 | 0.0353 | 0.90 | 0.0347 | 1.556 | 0.86 |
| 10% limonite | 2.660 | 0.0691 | 0.99 | 0.317 | 2.101 | 0.89 |
| 20% limonite | 6.291 | 0.2079 | 0.99 | 1.807 | 3.347 | 0.88 |
| Effect of Limonite Content | Effect of PS Concentration | Effect of MB Concentration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Limonite Content (wt%) | First-Order Reaction Kinetic Model | Second-Order Reaction Kinetic Model | PS Concentration (mM) | First-Order Reaction Kinetic Model | Second-Order Reaction Kinetic Model | MB Concentration (mg/L) | First-Order Reaction Kinetic Model | Second-Order Reaction Kinetic Model | ||||||
| k1 (min−1) | R2 | k2 (L mg−1 min−1) | R2 | k1 (min−1) | R2 | k2 (L mg−1 min−1) | R2 | k1 (min−1) | R2 | k2 (L mg−1 min−1) | R2 | |||
| 1 | 0.396 | 0.98 | 1.608 | 0.91 | 2 | 0.322 | 0.99 | 0.673 | 0.89 | 10 | 7.268 | 0.94 | 7.981 | 0.66 |
| 10 | 0.402 | 0.95 | 2.974 | 0.88 | 4 | 0.429 | 0.95 | 2.574 | 0.89 | 20 | 7.587 | 0.95 | 5.453 | 0.55 |
| 20 | 0.328 | 0.95 | 1.428 | 0.93 | 8 | 0.339 | 0.88 | 2.005 | 0.97 | 30 | 7.848 | 0.96 | 5.129 | 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, S.X.; Lau, K.S.; Zakaria, S.; Chia, C.H.; Wongchoosuk, C. Chitosan Fibers Loaded with Limonite as a Catalyst for the Decolorization of Methylene Blue via a Persulfate-Based Advanced Oxidation Process. Polymers 2022, 14, 5165. https://doi.org/10.3390/polym14235165
Chin SX, Lau KS, Zakaria S, Chia CH, Wongchoosuk C. Chitosan Fibers Loaded with Limonite as a Catalyst for the Decolorization of Methylene Blue via a Persulfate-Based Advanced Oxidation Process. Polymers. 2022; 14(23):5165. https://doi.org/10.3390/polym14235165
Chicago/Turabian StyleChin, Siew Xian, Kam Sheng Lau, Sarani Zakaria, Chin Hua Chia, and Chatchawal Wongchoosuk. 2022. "Chitosan Fibers Loaded with Limonite as a Catalyst for the Decolorization of Methylene Blue via a Persulfate-Based Advanced Oxidation Process" Polymers 14, no. 23: 5165. https://doi.org/10.3390/polym14235165