Highly Self-Healable Polymeric Coating Materials with Enhanced Mechanical Properties Based on the Charge Transfer Complex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CTC-Based Self-Healing PCMs
2.3. Preparation of Non-CTC-Based PCM
2.4. Characterizations
3. Results and Discussion
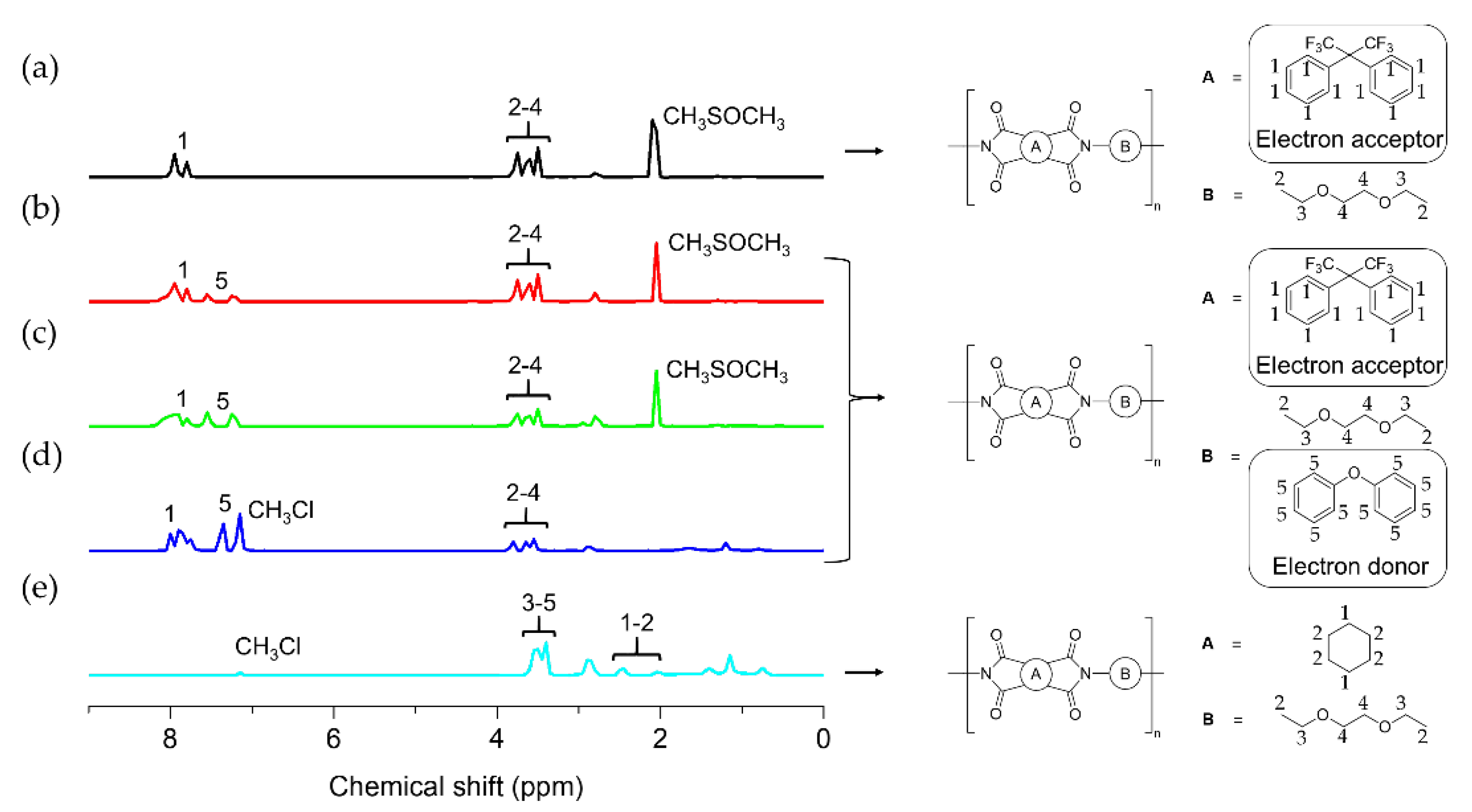
3.1. Synthesis
3.2. Thermal Properties
3.3. Self-Healing Properties
3.4. Mechanical Properties
3.5. Proposed Mechanism
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yaqoob, A.A.; Ahmad, A.; Khatoon, A.; Setapar, S.H.M. Polyaniline-based materials for supercapacitors. In Handbook of Supercapacitor Materials: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2021; pp. 113–130. ISBN 978-3-527-34687-5. [Google Scholar]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T.-U. Environmental applications of smart polymer composites. In Smart Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–312. ISBN 9780128199619. [Google Scholar]
- Diesendruck, C.E.; Sottos, N.R.; Moore, J.S.; White, S.R. Biomimetic self-healing. Angew. Chem. Int. Ed. Engl. 2015, 54, 10428–10447. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Seo, H.C.; Kim, Y.; Khil, M.; Cho, J.W.; Lee, J.; Jung, Y.C. Effects of hard segment of polyurethane with disulfide bonds on shape memory and self-healing ability. Macromol. Res. 2020, 28, 234–240. [Google Scholar] [CrossRef]
- Lee, W.; Oh, H.; Cha, S. A brief review of self-healing polyurethane based on dynamic chemistry. Macromol. Res. 2021, 29, 649–664. [Google Scholar] [CrossRef]
- Toohey, K.S.; Sottos, N.R.; Lewis, J.A.; Moore, J.S.; White, S.R. Self-healing materials with microvascular networks. Nat. Mater. 2007, 6, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; White, S.R.; Braun, P.V. Self-healing polymer coatings. Adv. Mater. 2009, 21, 645–649. [Google Scholar] [CrossRef]
- Hager, M.D.; Greil, P.; Leyens, C.; van der Zwaag, S.; Schubert, U.S. Self-healing materials. Adv. Mater. 2010, 22, 5424–5430. [Google Scholar] [CrossRef]
- Meng, L.M.; Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. A dual mechanism single-component self-healing strategy for polymers. J. Mater. Chem. 2010, 20, 6030–6038. [Google Scholar] [CrossRef]
- Murphy, E.B.; Bolanos, E.; Schaffner-Hamann, C.; Wudl, F.; Nutt, S.R.; Auad, M.L. Synthesis and characterization of a single-component thermally remendable polymer network: Staudinger and stille revisited. Macromolecules 2008, 41, 5203–5209. [Google Scholar] [CrossRef]
- Pratama, P.A.; Sharifi, M.; Peterson, A.M.; Palmese, G.R. Room temperature self-healing thermoset based on the Diels-Alder reaction. ACS Appl. Mater. Interfaces 2013, 5, 12425–12431. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chuo, T.-W. Self-healing polymers based on thermally reversible Diels–Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Canadell, J.; Goossens, H.; Klumperman, B. Self-healing materials based on disulfide links. Macromolecules 2011, 44, 2536–2541. [Google Scholar] [CrossRef]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-healing of covalently cross-linked polymers by reshuffling thiuram disulfide moieties in air under visible light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef]
- Kim, S.M.; Jeon, H.; Shin, S.H.; Park, S.A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior toughness and fast self-healing at room temperature engineered by transparent elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef]
- Takashima, Y.; Yonekura, K.; Koyanagi, K.; Iwaso, K.; Nakahata, M.; Yamaguchi, H.; Harada, A. Multifunctional stimuli-responsive supramolecular materials with stretching, coloring, and self-healing properties functionalized via host–guest interactions. Macromolecules 2017, 50, 4144–4150. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host-guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [Green Version]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Lee, S.; Hong, P.H.; Kim, J.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Ahn J., B.; Eom, W.; Ko, M.J.; et al. Highly self-healable polymeric blend synthesized using polymeric glue with outstanding mechanical properties. Macromolecules 2020, 53, 2279–2286. [Google Scholar] [CrossRef]
- Kim, J.; Hong P., H.; Choi, K.; Moon, G.; Kang, J.; Lee, S.; Lee, S.; Jung, H.W.; Ko, M.J.; Hong, S.W. A heterocyclic polyurethane with enhanced self-healing efficiency and outstanding recovery of mechanical properties. Polymers 2020, 12, 968. [Google Scholar] [CrossRef] [Green Version]
- Aboudzadeh, A.; Fernandez, M.; Munoz, M.E.; Santamaria, A.; Mecerreyes, D. Ionic supramolecular networks fully based on chemicals coming from renewable sources. Macromol. Rapid. Commun. 2014, 35, 460–465. [Google Scholar] [CrossRef]
- Cao, Y.; Morrissey, T.G.; Acome, E.; Allec, S.I.; Wong, B.M.; Keplinger, C.; Wang, C. A transparent, self-healing, highly stretchable ionic conductor. Adv. Mater. 2017, 29, 1605099. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Choi, K.; Hong, P.H.; Park, H.J.; Kim, K.; Kim, Y.K.; Moon, G.; Jeon, H.; Lee, S.; et al. A water-triggered highly self-healable elastomer with enhanced mechanical properties achieved using localized zwitterionic assemblies. Chem. Eng. J. 2021, 420, 127636. [Google Scholar] [CrossRef]
- Mozhdehi, D.; Ayala, S.; Cromwell, O.R.; Guan, Z. Self-healing multiphase polymers via dynamic metal-ligand interactions. J. Am. Chem. Soc. 2014, 136, 16128–16131. [Google Scholar] [CrossRef] [PubMed]
- Enke, M.; Bose, R.K.; Bode, S.; Vitz, J.; Schacher, F.H.; Garcia, S.J.; van der Zwaag, S.; Hager, M.D.; Schubert, U.S. A metal salt dependent self-healing response in supramolecular block copolymers. Macromolecules 2016, 49, 8418–8429. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Zhu, W.; Xu, Z.; Fuchs, S.; Hilborn, J.; Zhu, L.; Ma, Q.; Wang, Y.; Weng, X.; et al. Self-healing silk fibroin-based hydrogel for bone regeneration: Dynamic metal-ligand self-Assembly approach. Adv. Funct. Mater. 2017, 27, 1700591. [Google Scholar] [CrossRef]
- Burattini, S.; Greenland, B.W.; Merino, D.H.; Weng, W.; Seppala, J.; Colquhoun, H.M.; Hayes, W.; Mackay, M.E.; Hamley, I.W.; Rowan, S.J. A healable supramolecular polymer blend based on aromatic π−π stacking and hydrogen-bonding interactions. J. Am. Chem. Soc. 2010, 132, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Greenland, B.W.; Hayes, W.; Mackay, M.E.; Rowan, S.J.; Colquhoun, H.M. A supramolecular polymer based on tweezer-type π−π stacking interactions: Molecular design for healability and enhanced toughness. Chem. Mater. 2011, 23, 6–8. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Nilsson, B.L. Complementary pi-pi interactions induce multicomponent coassembly into functional fibrils. Langmuir 2011, 27, 11145–11156. [Google Scholar] [CrossRef]
- Park, S.A.; Jeon, H.; Kim, H.; Shin, S.H.; Choy, S.; Hwang, D.S.; Koo, J.M.; Jegal, J.; Hwang, S.Y.; Park, J.; et al. Sustainable and recyclable super engineering thermoplastic from biorenewable monomer. Nat. Commun. 2019, 10, 2601. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Kim, H.J.; Kim, M.H.; Lee, J. Preparation of poly(phenylene sulfide)/nylon 6 grafted graphene Oxide nanocomposites with enhanced mechanical and thermal properties. Macromol. Res. 2020, 28, 241–248. [Google Scholar] [CrossRef]
- Son, S.M.; Lee, J.; Jeon, J.; Lim, S.I.; Kwon, H.T.; Eom, Y.; Chae, H.G. Preparation of high-performance polyethersulfone/cellulose nanocrystal nanocomposite fibers via dry-jet wet spinning. Macromol. Res. 2021, 29, 33–39. [Google Scholar] [CrossRef]
- Clair, T.S. Structure-property relationships in linear aromatic polyimides. In Polyimides; Springer: Dordrecht, Netherlands, 1990; pp. 58–78. ISBN 9789401096638. [Google Scholar]
- Wakita, J.; Jin, S.; Shin, T.J.; Ree, M.; Ando, S. Analysis of molecular aggregation structures of fully aromatic and semialiphatic polyimide films with synchrotron grazing incidence wide-angle X-ray scattering. Macromolecules 2010, 43, 1930–1941. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, T.Y.; Hong, P.H.; Choi, S.; Lee, Y.; Kwon, H.; Jeon, H.; Ko, D.W.; Park, I.; Han, H.; et al. Highly transparent, colorless optical film with outstanding mechanical strength and folding reliability using mismatched charge-transfer complex intensification. Adv. Funct. Mater. 2022, 32, 2111040. [Google Scholar] [CrossRef]
- Feger, C.; Khojasteh, M.M.; Htoo, M.S. Advances in Polyimide: Science and Technology; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 1993; pp. 441–452. ISBN 0877629838. [Google Scholar]
- Ghosh, M.K.; Mittal, K.L. Polyimides: Fundamentals and Application; CRC Press: Boca Raton, FL, USA, 1996; pp. 279–308. ISBN 0824794664. [Google Scholar]
- Kim, Y.N.; Lee, J.; Kim, Y.; Kim, J.; Han, H.; Jung, Y.C. Colorless polyimides with excellent optical transparency and self-healing porperties based on multi-exchange dynamic network. Appl. Mater. Today 2021, 25, 101226. [Google Scholar] [CrossRef]
- Gedde, U.W. Polymer Physics; Kluwer Academic Publishers: Dordrecht, Netherlands, 1995; pp. 191–206. ISBN 9780412626401. [Google Scholar]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 557–612. ISBN 9780471706069. [Google Scholar]
- Chang, K.; Jia, H.; Gu, S. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013; pp. 5–60. ISBN 9783527334391. [Google Scholar]
- Thomas, S.; Surendran, A. Self-Healing Polymer-Based Systems; Elservier: Amsterdam, Netherlands, 2020; pp. 39–59. ISBN 9780128184509. [Google Scholar]
- Garcia, S.J. Effect of polymer architechture on the intrinsic self-healing character of polymers. Eur. Polym. J. 2014, 53, 118–125. [Google Scholar] [CrossRef]








| Sample Designations | Dianhydride [%] | Diamine [%] | Solid Contents [%] | Tda 2% [°C] | Tgb [°C] | ||
|---|---|---|---|---|---|---|---|
| 6FDA | HPMDA | ODA | EDBEA | ||||
| FE100 | 100 | - | - | 100 | 20 | 352.8 | 120.1 |
| FE75 | 100 | - | 25 | 75 | 20 | 391.7 | 151.3 |
| FE50 | 100 | - | 50 | 50 | 20 | 397.5 | 187.6 |
| FE25 | 100 | - | 75 | 25 | 20 | 402.0 | 240.0 |
| HE100 | - | 100 | - | 100 | 20 | - | 92.6 |
| Sample Designations | Tensile Strength [MPa] | Elongation at Break [%] | Young’s Modulus [GPa] | HIT a [MPa] | EIT b [GPa] | Tgc [°C] |
|---|---|---|---|---|---|---|
| FE75 | 81.7 ± 6.4 | 4.2 ± 0.4 | 2.0 ± 0.1 | - | - | 151.3 |
| FE50 | 106.2 ± 8.1 | 6.5 ± 0.9 | 2.0 ± 0.1 | 386.0 | 5.3 | 187.6 |
| PEEK | 90.1 ± 4.1 | 155.8 ± 15.1 | 2.3 ± 0.3 | 331.0 | 4.8 | 142.8 |
| PSU | 66.5 ± 0.4 | 24.3 ± 6.9 | 0.3 ± 0.1 | 383.3 | 4.9 | 190.0 |
| PES | 79.4 ± 0.6 | 27.5 ± 0.7 | 0.4 ± 0.2 | 85.9 | 1.1 | 180.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.; Hong, P.H.; Lee, J.; Kim, J.; Moon, G.; Lee, S.; Park, I.; Han, H.; Hong, S.W. Highly Self-Healable Polymeric Coating Materials with Enhanced Mechanical Properties Based on the Charge Transfer Complex. Polymers 2022, 14, 5181. https://doi.org/10.3390/polym14235181
Ahn C, Hong PH, Lee J, Kim J, Moon G, Lee S, Park I, Han H, Hong SW. Highly Self-Healable Polymeric Coating Materials with Enhanced Mechanical Properties Based on the Charge Transfer Complex. Polymers. 2022; 14(23):5181. https://doi.org/10.3390/polym14235181
Chicago/Turabian StyleAhn, Chanjae, Pyong Hwa Hong, Juhen Lee, Jinsil Kim, Gyeongmin Moon, Sungkoo Lee, In Park, Haksoo Han, and Sung Woo Hong. 2022. "Highly Self-Healable Polymeric Coating Materials with Enhanced Mechanical Properties Based on the Charge Transfer Complex" Polymers 14, no. 23: 5181. https://doi.org/10.3390/polym14235181





