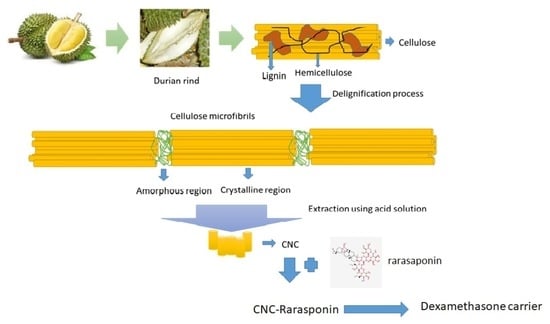
Cellulose Nanocrystals (CNCs) and Its Modified Form from Durian Rind as Dexamethasone Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pretreatment of Durian Rind
2.2.2. Extraction of Rarasaponin
2.2.3. CNCs Extraction
2.2.4. Modification to CNCs
2.2.5. Characterization of CNCs and CNCs-Rarasaponin
2.2.6. Drug Loading
2.2.7. Drug Release Study
2.2.8. Cells Viability Study
3. Results and Discussions
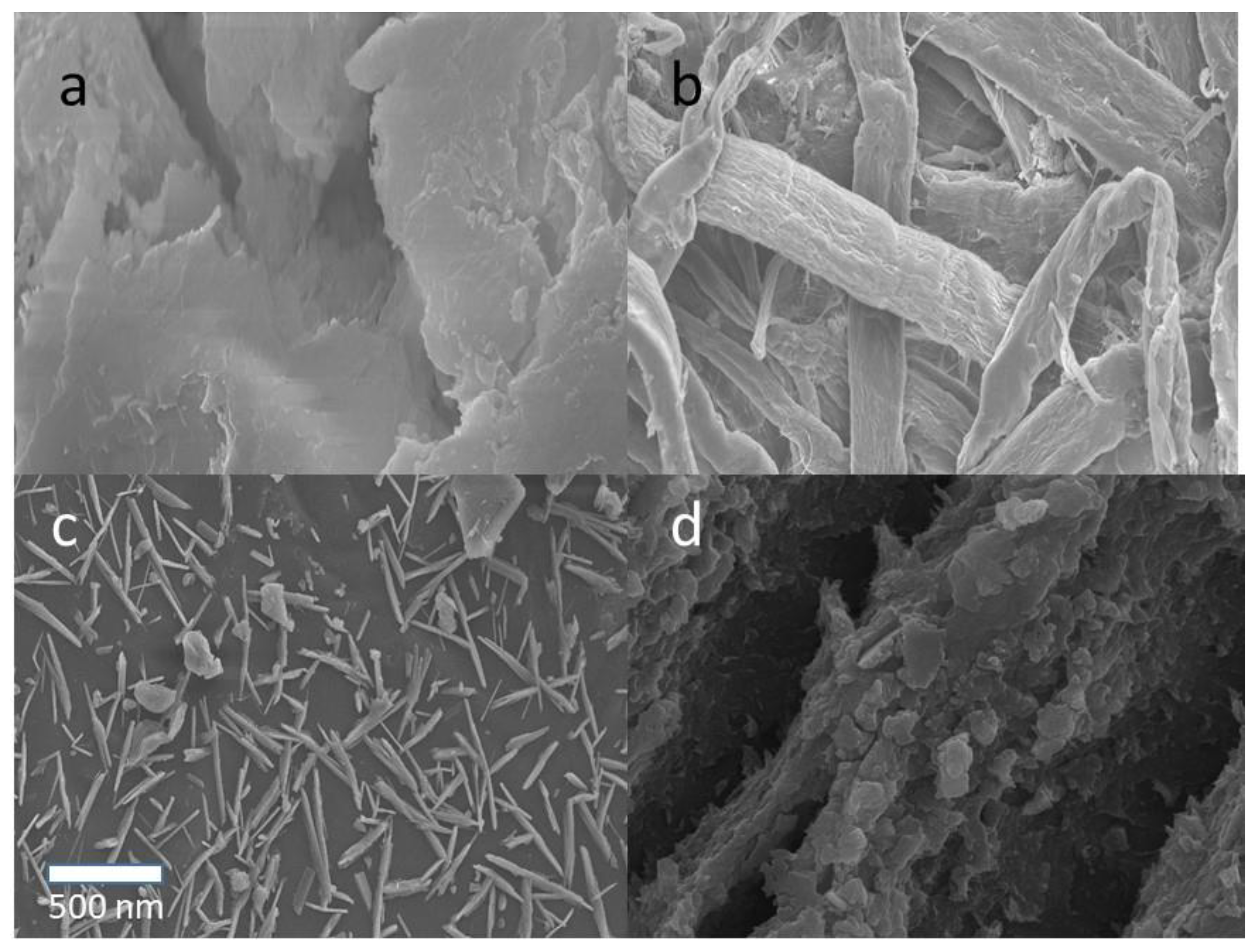
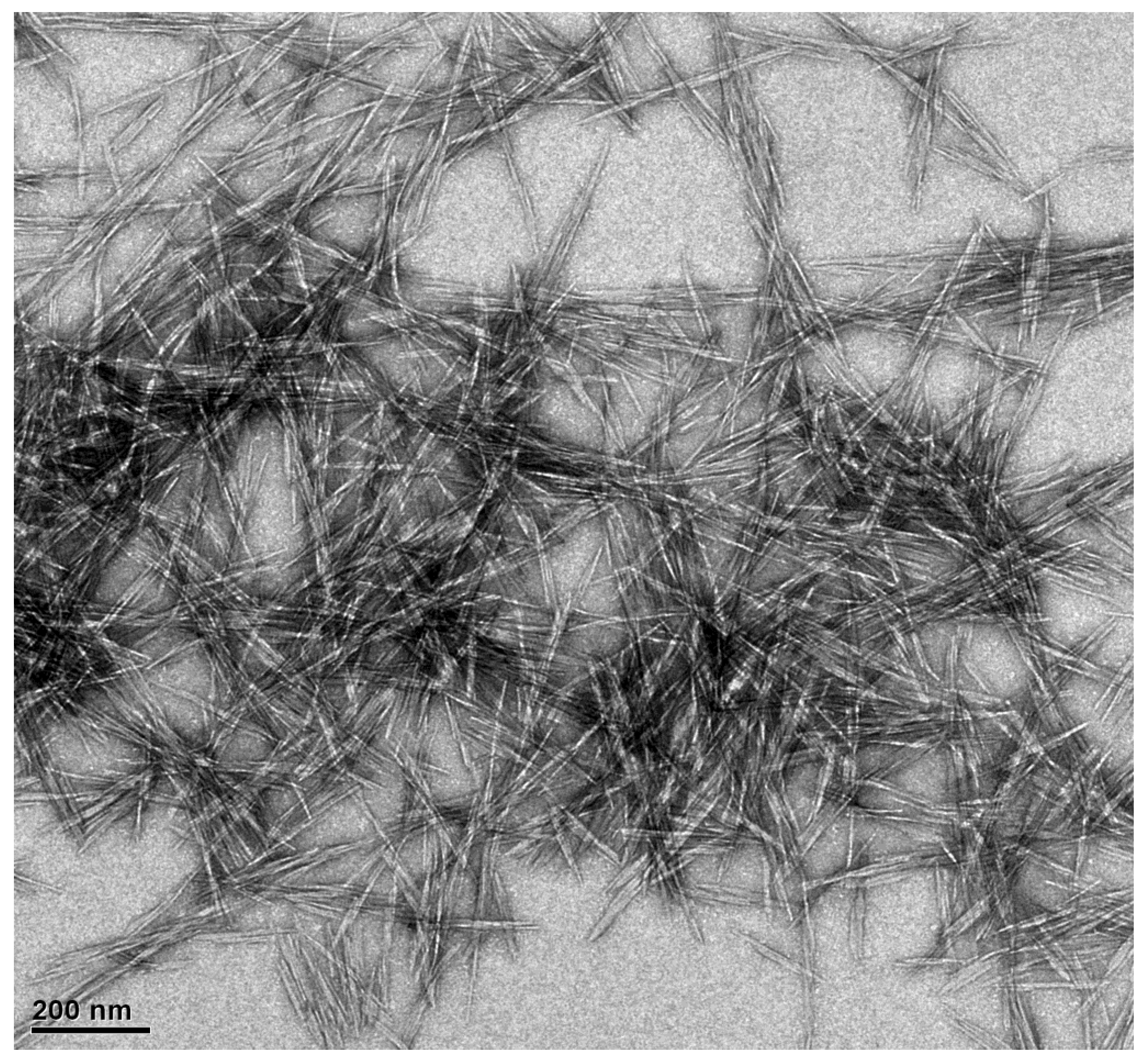
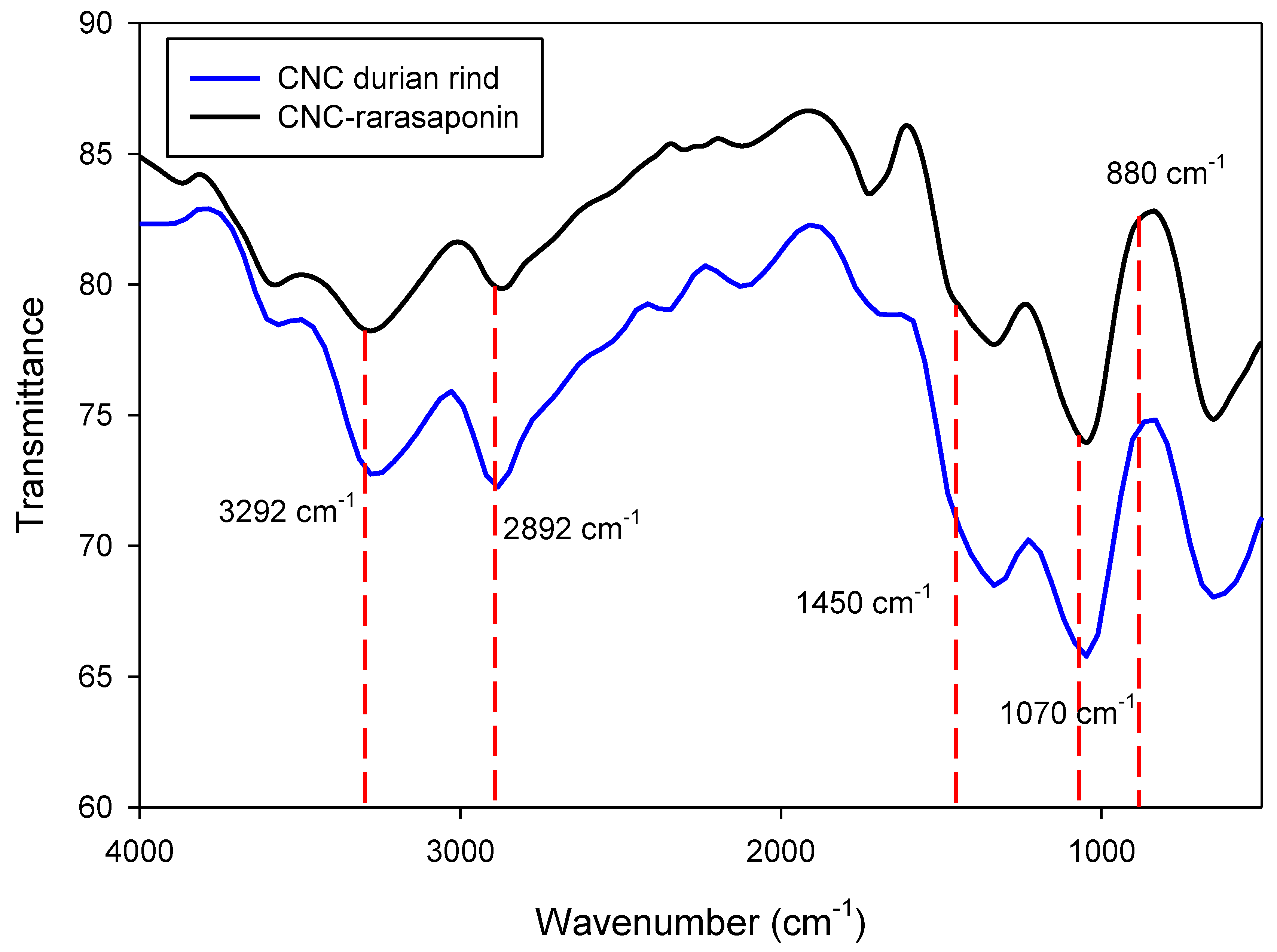
3.1. Characterization of CNCs and CNCs-Rarasaponin
3.2. Dexamethasone Loading
3.3. Dexamethasone Released Study
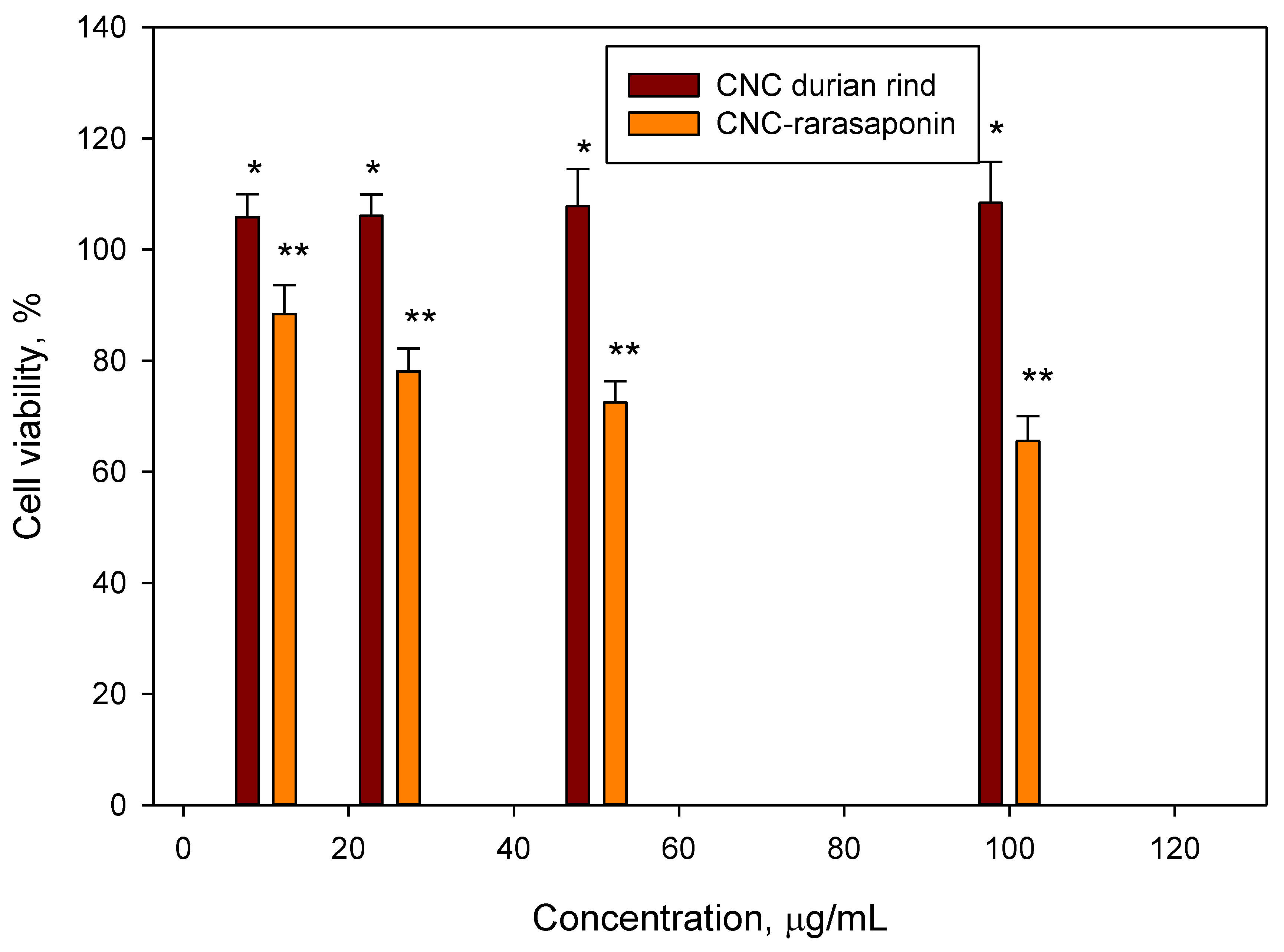
3.4. Biocompability of CNCs and CNCs-Rarasaponin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Shen, P.; Tang, Q.; Chen, X.; Li, Z. Nanocrystalline cellulose extracted from bast fibers: Preparation, characterization, and application. Carbohydr. Polym. 2022, 290, 119462. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline cellulose isolation via acid hydrolysis from non-woody biomass: Importance of hydrolysis parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Dehkhoda, S.; Bagheri, M.; Heydari, M.; Rabieh, S. Extraction of carboxylated nanocellulose from oat husk: Characterization, surface modification and in vitro evaluation of indomethacin drug release. Int. J. Biol. Macromol. 2022, 212, 165–171. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins. Planta Med. 1976, 2, 116–122. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef]
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. Effect of natural and synthetic surfactants on polysaccharide nanoparticles: Hydrophobic drug loading, release, and cytotoxic studies. Colloids Surf. A 2019, 578, 123618. [Google Scholar] [CrossRef]
- Jermy, B.R.; Ravinayagam, V.; Almohazey, D.; Alamoudi, W.A.; Daffala HAkhtar, S.; Tanimu, G. PEGylated green halloysite/spinel ferrite nanocomposites for pH-sensitive delivery of dexamethasone: A potential pulmonary drug delivery treatment option for COVID-19. Appl. Clay Sci. 2022, 216, 106333. [Google Scholar] [CrossRef]
- Ogundare, S.A.; Moodley, V.; Amaku, J.F.; Ogunmoye, A.O.; Atewolara-Odule, O.C.; Olubomehin, O.O.; Awokoya, K.N.; Sanyaolu, N.O.; Ibikunle, A.A.; van Zyl, W.E. Nanocrystalline cellulose derived from melon seed shell (Citrullus colocynthis L.) for reduction and stabilization of silver nanoparticles: Synthesis and catalytic activity. Carbohydr. Polym. Technol. Appl. 2021, 2, 100134. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Lin, C.X.; Qiao, S.Z.; Yu, C.Z.; Ismadji, S.; Lu, G.Q. Periodic mesoporous silica and organosilica with controlled morphologies as carriers for drug release. Microporous Mesoporous Mater. 2009, 117, 213–219. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of self-antibacterial chitosan/oxidized starch polyelectrolyte complex sponges for controlled delivery of curcumin. Food Hydrocoll. 2022, 135, 108147. [Google Scholar] [CrossRef]
- Tran Vo, T.M.; Piroonpan, T.; Preuksarattanawut, C.; Kobayashi, T.; Potiyaraj, P. Characterization of pH-responsive high molecular-weight chitosan/poly (vinyl alcohol) hydrogel prepared by gamma irradiation for localizing drug release. Bioresour. Bioprocess. 2022, 9, 89. [Google Scholar] [CrossRef]
- Dangi, D.; Mattoo, M.; Kumar, V.; Sharma, P. Synthesis and characterization of galactomannan polymer hydrogel and sustained drug delivery. Carbohydr. Polym. Technol. Appl. 2022, 4, 100230. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of betacyanin from dragon fruit peel by complex coacervation: Physicochemical characteristics, thermal stability, and release profile of microcapsules. Food Biosci. 2022, 49, 101882. [Google Scholar] [CrossRef]
- Alinavaz, S.; Jabbari, P.; Mahdavinia, G.; Jafari, H.; Sharifi, S.; Lighvan, Z.M.; Akbari, A. Novel magnetic carboxymethylcellulose/chitosan bio-nanocomposites for smart co-delivery of sunitinib malate anticancer compound and saffron extract. Polym. Int. 2022, 71, 1243–1251. [Google Scholar] [CrossRef]
- Milan, E.P.; Martins, V.C.A.; Horn, M.M.; Plepis, A.M.G. Influence of blend ratio and mangosteen extract in chitosan/collagen gels and scaffolds: Rheological and release studies. Carbohydr. Polym. 2022, 292, 119647. [Google Scholar] [CrossRef]
- Brazel, C.S.; Peppas, N.A. Modeling of drug release from swellable polymers. Eur. J. Pharm. Biopharm. 2000, 49, 47–58. [Google Scholar]
- Man, S.; Gao, W.; Zhang, Y.; Huang, I.; Liu, C. Chemical study and medical application of saponins as anticancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Podolak, L.; Galantry, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef]



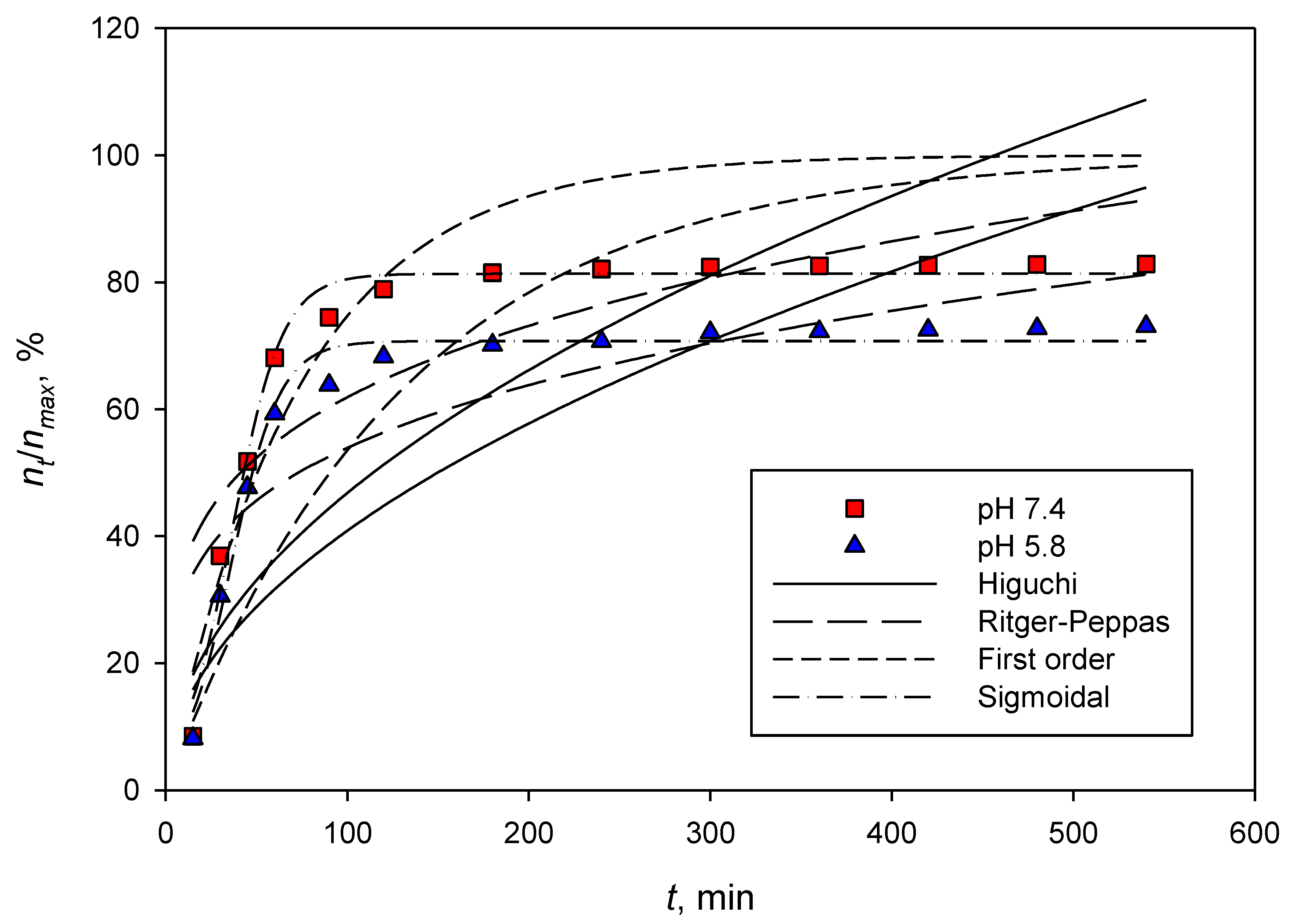
| pH | Drug Carrier | Parameters | |||
|---|---|---|---|---|---|
| Higuchi | Ritger–Peppas | First-order | Sigmoidal | ||
| 7.4 | CNCs-rarasaponin | KH = 5.2307 R2 = 0.0026 | KRP = 25.90 n = 0.2184 R2 = 0.6799 | KLa = 0.0202 R2 = 0.8822 | Rmax = 89.88 T50 = 32.18 ks = 12.59 R2 = 0.9739 |
| Durian rind CNCs | KH = 4.6792 R2 = 0.2107 | KRP = 20.42 n = 0.2409 R2 = 0.6968 | KLa = 0.0137 R2 = 0.6848 | Rmax = 81.36 T50 = 36.41 ks = 13.92 R2 = 0.9833 | |
| 5.8 | CNCs-rarasaponin | KH = 4.8576 R2 = 0.2298 | KRP = 20.99 n = 0.2426 R2 = 0.7117 | KLa = 0.0151 R2 = 0.7833 | Rmax = 84.13 T50 = 35.92 ks = 13.39 R2 = 0.9827 |
| Durian rind CNCs | KH = 4.0853 R2 = 0.2298 | KRP = 17.65 n = 0.2126 R2 = 0.7799 | KLa = 0.0077 R2 = 0.1644 | Rmax = 70.76 T50 = 35.91 ks = 13.39 R2 = 0.9827 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putro, J.N.; Edi Soetaredjo, F.; Irawaty, W.; Budi Hartono, S.; Santoso, S.P.; Lie, J.; Yuliana, M.; Widyarani; Shuwanto, H.; Wijaya, C.J.; et al. Cellulose Nanocrystals (CNCs) and Its Modified Form from Durian Rind as Dexamethasone Carrier. Polymers 2022, 14, 5197. https://doi.org/10.3390/polym14235197
Putro JN, Edi Soetaredjo F, Irawaty W, Budi Hartono S, Santoso SP, Lie J, Yuliana M, Widyarani, Shuwanto H, Wijaya CJ, et al. Cellulose Nanocrystals (CNCs) and Its Modified Form from Durian Rind as Dexamethasone Carrier. Polymers. 2022; 14(23):5197. https://doi.org/10.3390/polym14235197
Chicago/Turabian StylePutro, Jindrayani Nyoo, Felycia Edi Soetaredjo, Wenny Irawaty, Sandy Budi Hartono, Shella Permatasari Santoso, Jenni Lie, Maria Yuliana, Widyarani, Hardy Shuwanto, Christian Julius Wijaya, and et al. 2022. "Cellulose Nanocrystals (CNCs) and Its Modified Form from Durian Rind as Dexamethasone Carrier" Polymers 14, no. 23: 5197. https://doi.org/10.3390/polym14235197
APA StylePutro, J. N., Edi Soetaredjo, F., Irawaty, W., Budi Hartono, S., Santoso, S. P., Lie, J., Yuliana, M., Widyarani, Shuwanto, H., Wijaya, C. J., Gunarto, C., Puspitasari, N., & Ismadji, S. (2022). Cellulose Nanocrystals (CNCs) and Its Modified Form from Durian Rind as Dexamethasone Carrier. Polymers, 14(23), 5197. https://doi.org/10.3390/polym14235197