Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyalkanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
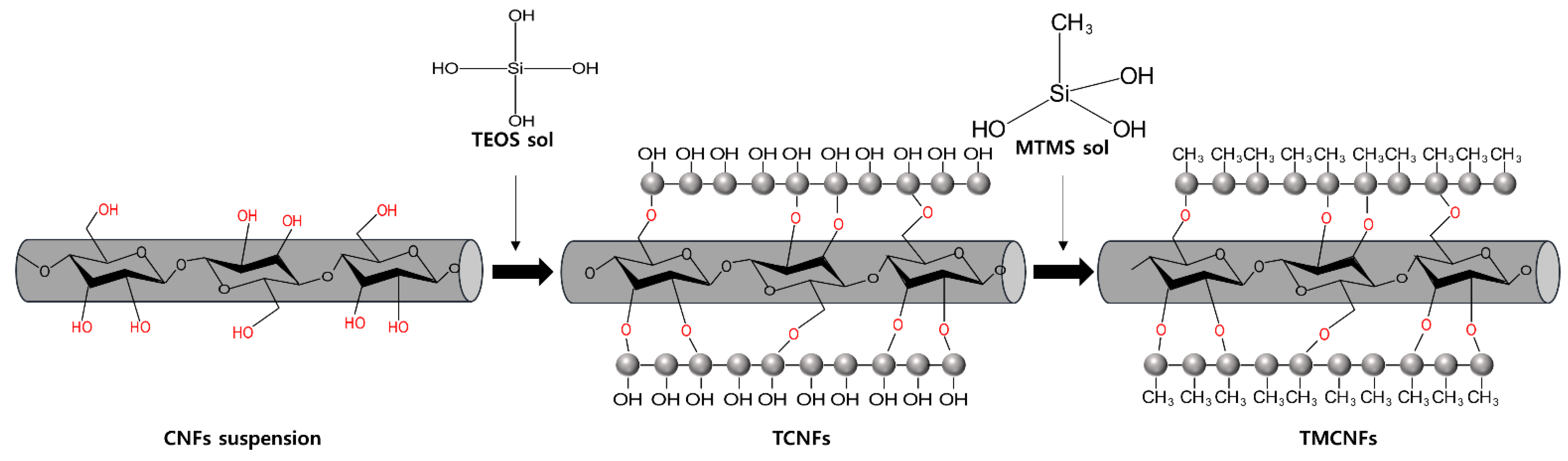
2.2. Hydrophobization of CNFs through Surface Modification
2.3. Characterizations of Modified CNFs
2.4. Preparation of PHA–CNFs Composites
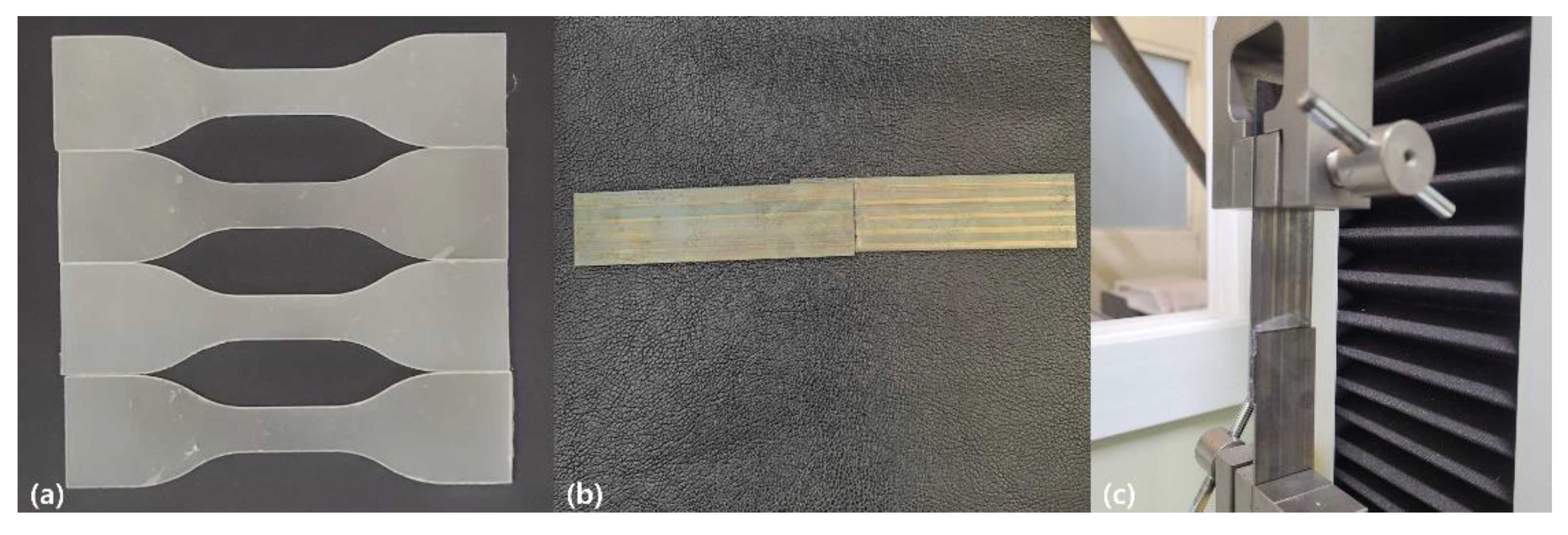
2.5. Characterizations of PHA–CNFs Composites
3. Results and Discussion
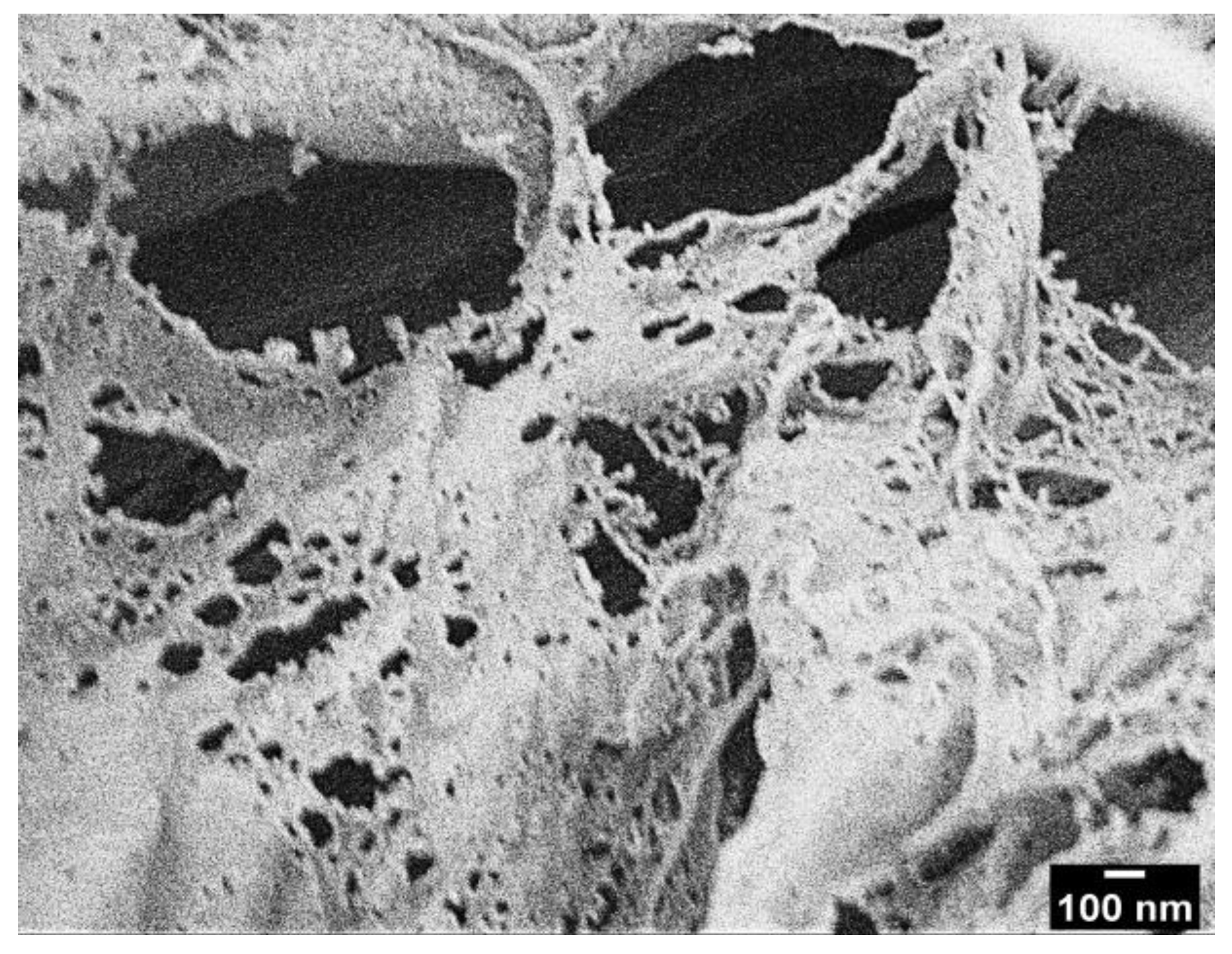
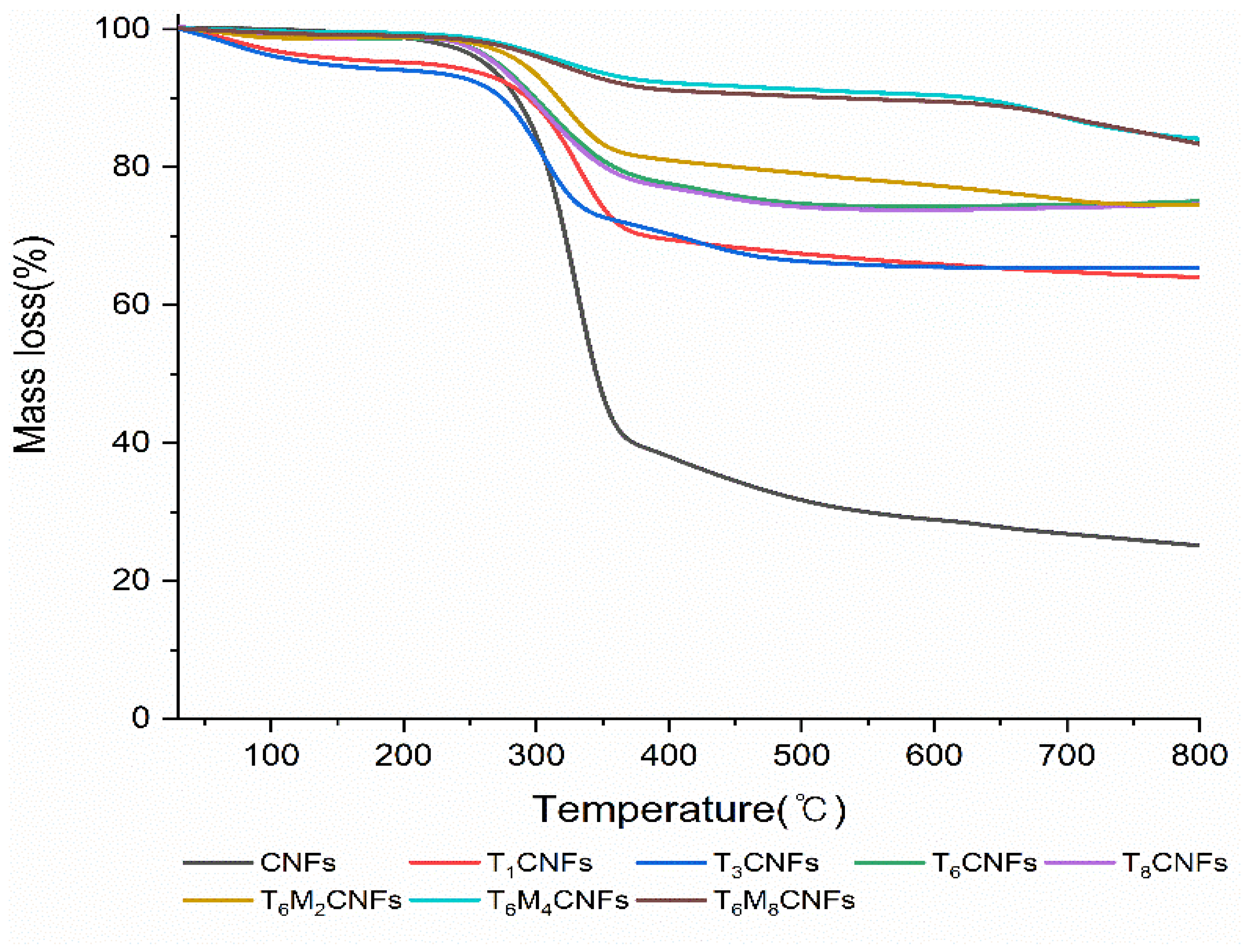
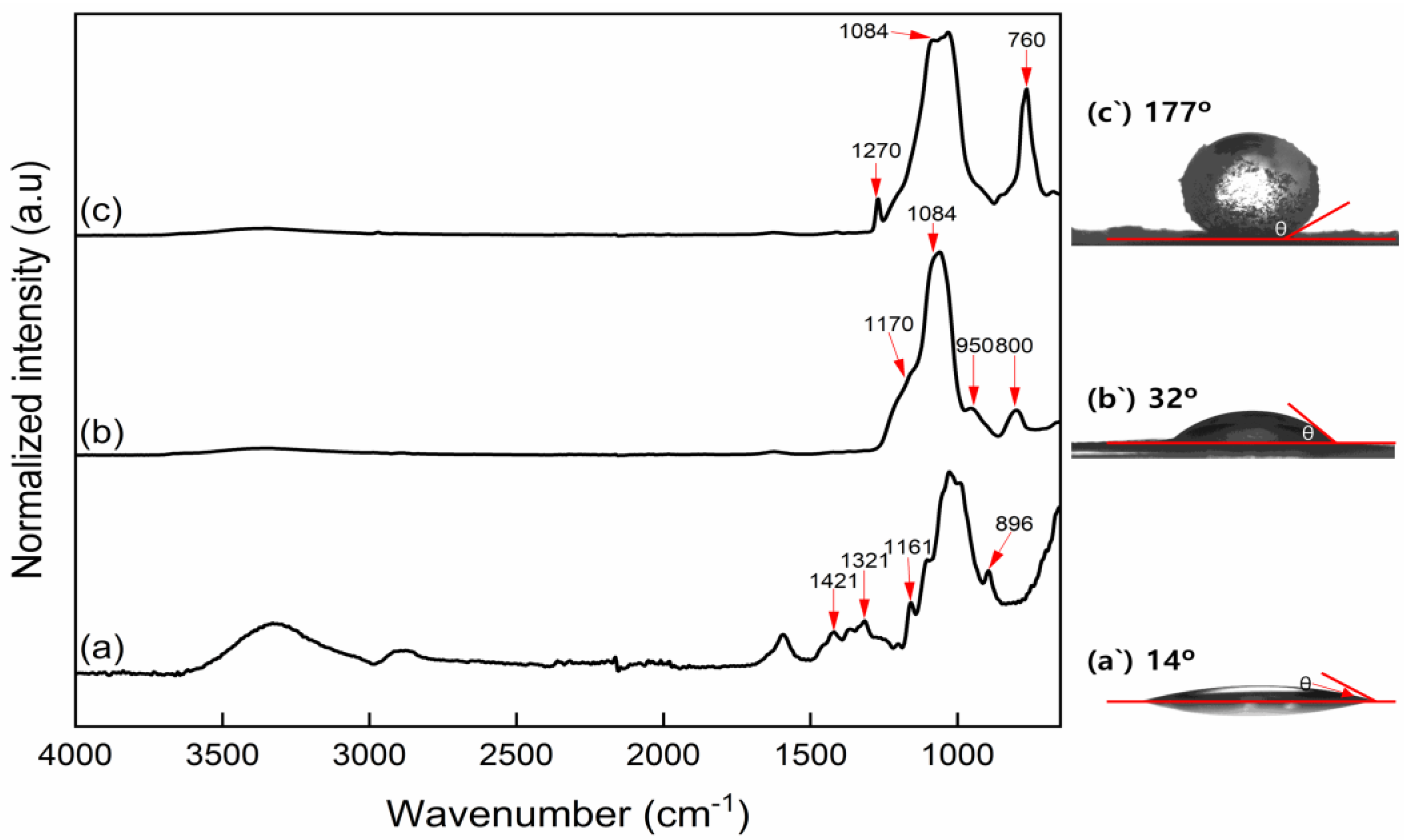
3.1. Characterizations of Modified CNFs
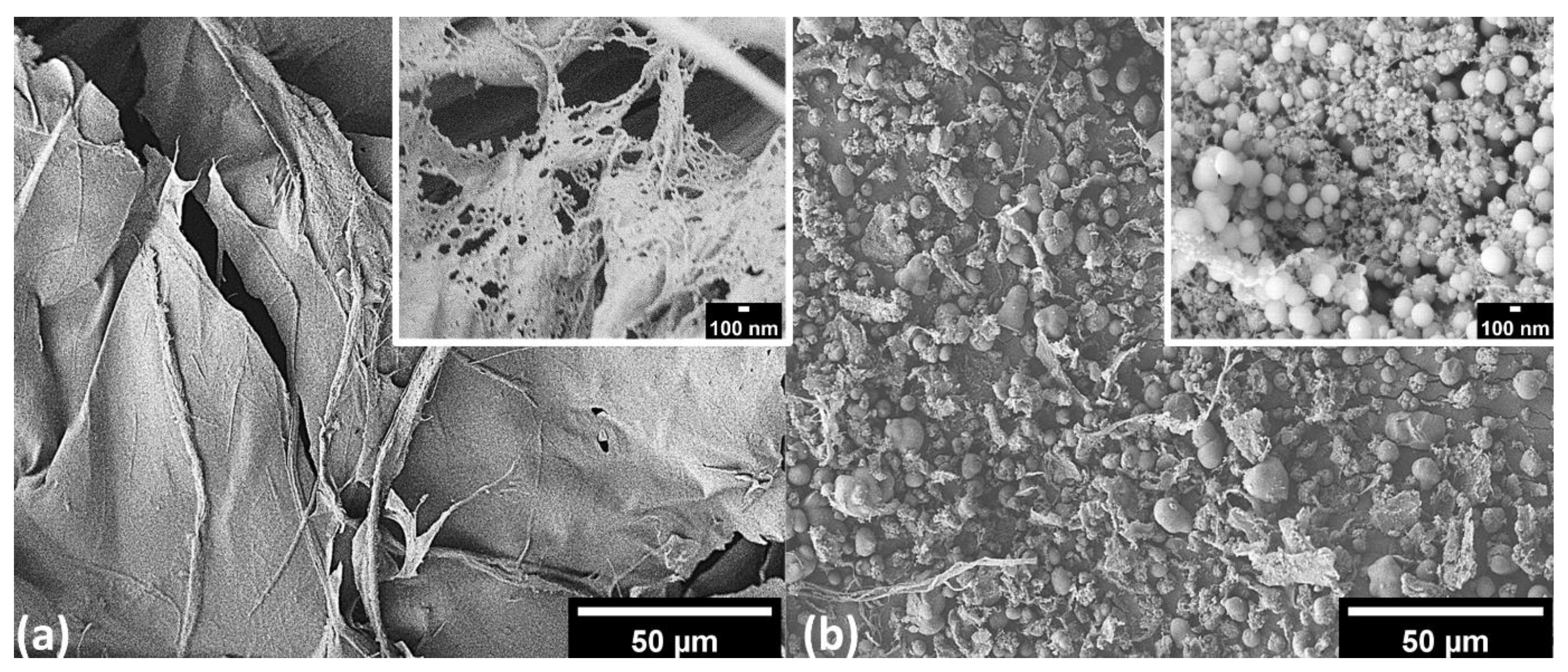
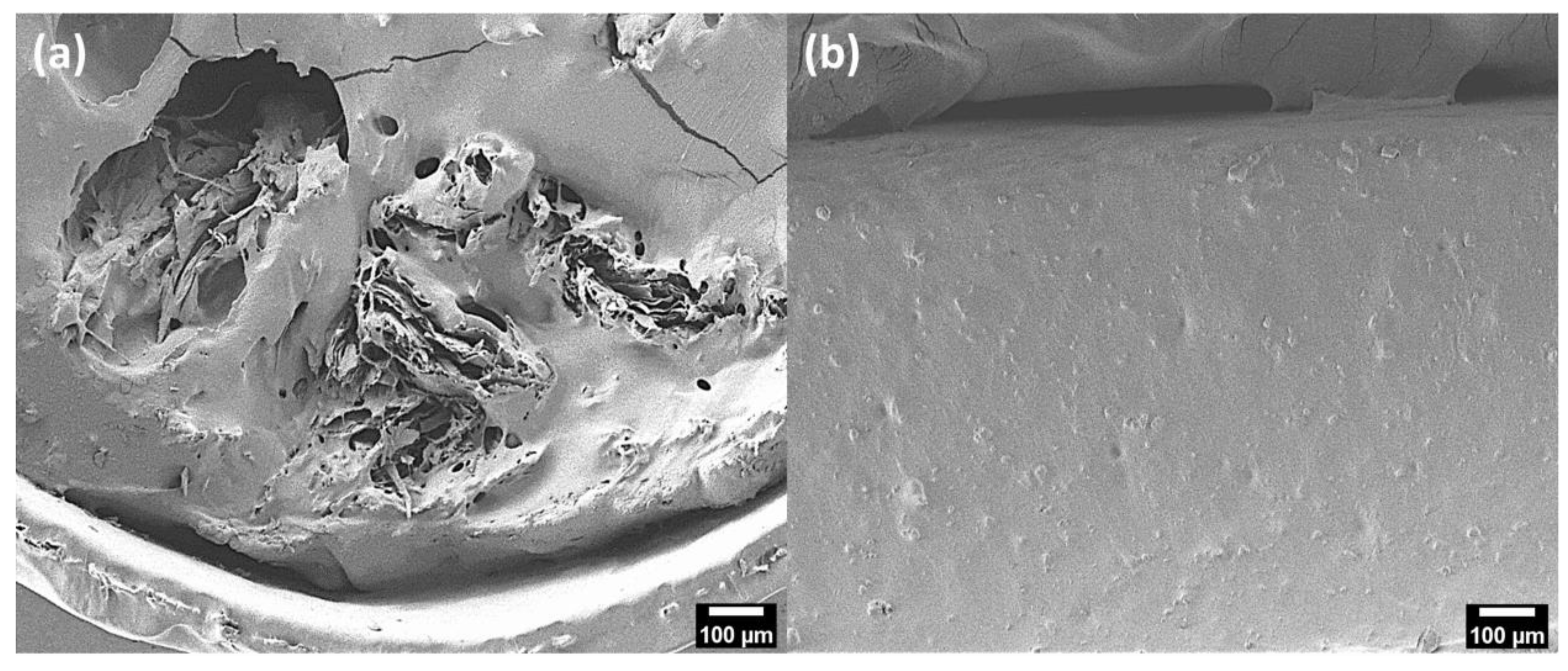
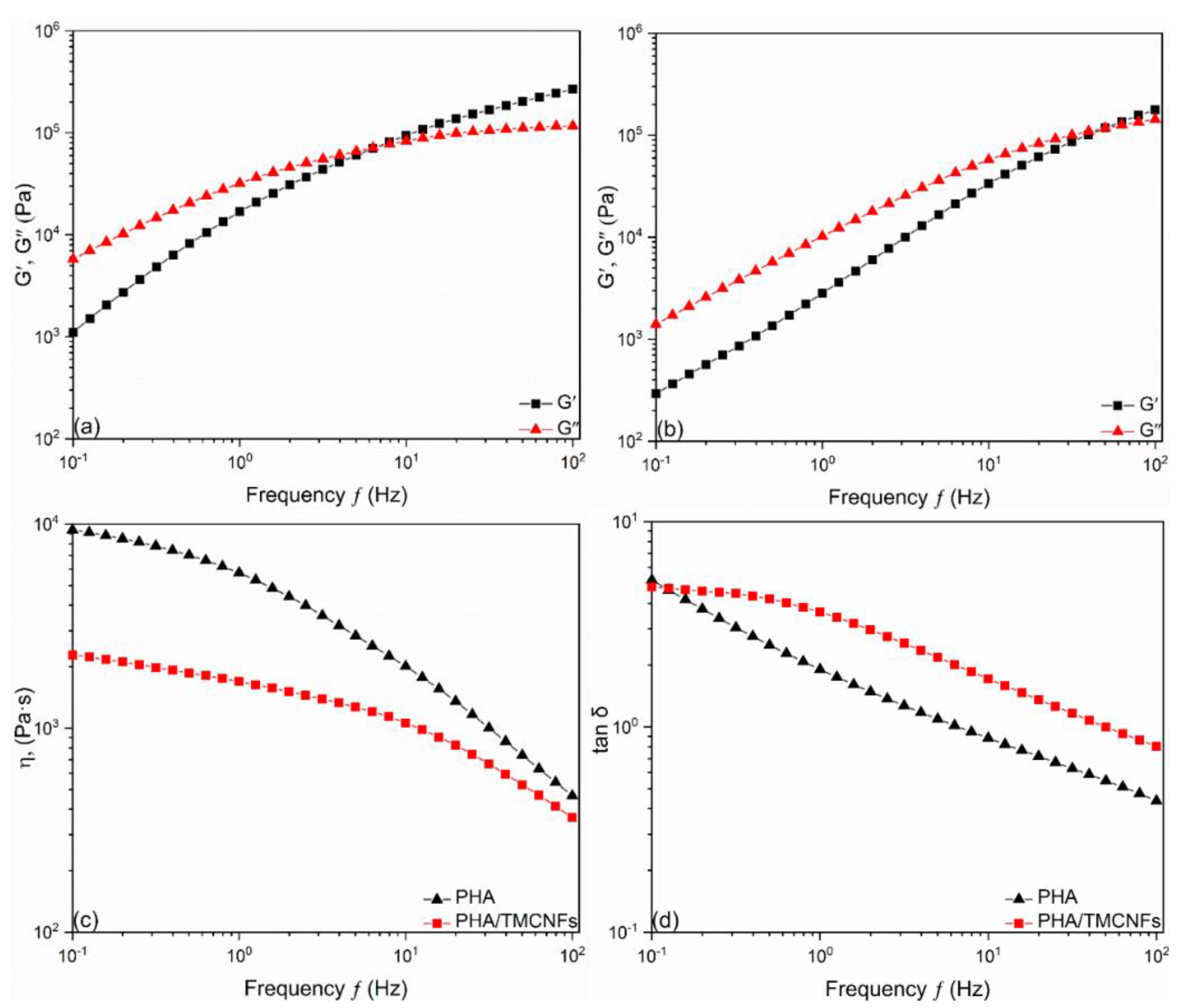
3.2. Characterizations of PHA–CNFs Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Napper, I.E.; Thompson, R.C. Environmental Deterioration of Biodegradable, Oxo-biodegradable, Compostable, and Conventional Plastic Carrier Bags in the Sea, Soil, and Open-Air over a 3-Year Period. Environ. Sci. Technol. 2019, 53, 4775–4783. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe Plastics Europe Association of Plastics Manufacturers Plastics—The Facts 2021 An analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 17 July 2022).
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic Pollution During COVID-19: Plastic Waste Directives and Its Long-term Impact on The Environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef]
- Bauer, A.S.; Tacker, M.; Uysal-Unalan, I.; Cruz, R.M.S.; Varzakas, T.; Krauter, V. Recyclability and redesign challenges in multilayer flexible food packaging—A review. Foods 2021, 10, 2701. [Google Scholar] [CrossRef] [PubMed]
- Viljanmaa, M.; Södergård, A.; Törmälä, P. Lactic acid based polymers as hot melt adhesives for packaging applications. Int. J. Adhes. Adhes. 2002, 22, 219–226. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly(4-Hydroxybutyrate): Current State and Perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257. [Google Scholar] [CrossRef]
- Feijoo, P.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Torres-Giner, S.; Lagaron, J.M.; Gamez-Perez, J.; Cabedo, L. Development and Characterization of Fully Renewable and Biodegradable Polyhydroxyalkanoate Blends with Improved Thermoformability. Polymers. 2022, 14, 2527. [Google Scholar] [CrossRef]
- Kafy, A.; Kim, H.C.; Zhai, L.; Kim, J.W.; Van Hai, L.; Kang, T.J.; Kim, J. Cellulose long fibers fabricated from cellulose nanofibers and its strong and tough characteristics. Sci. Rep. 2017, 7, 17683. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Sanandiya, N.D.; Vasudevan, J.; Das, R.; Lim, C.T.; Fernandez, J.G. Stimuli-responsive injectable cellulose thixogel for cell encapsulation. Int. J. Biol. Macromol. 2019, 130, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Raabe, J.; De Souza Fonseca, A.; Bufalino, L.; Ribeiro, C.; Martins, M.A.; Marconcini, J.M.; Tonoli, G.H.D. Evaluation of reaction factors for deposition of silica (SiO2) nanoparticles on cellulose fibers. Carbohydr. Polym. 2014, 114, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; Li, Y.H.; Zhang, L. Driving Forces for Accumulation of Cellulose Nanofibrils at the Oil/Water Interface. Langmuir 2018, 34, 10757–10763. [Google Scholar] [CrossRef] [PubMed]
- Scheuble, N.; Geue, T.; Windhab, E.J.; Fischer, P. Tailored interfacial rheology for gastric stable adsorption layers. Biomacromolecules 2014, 15, 3139–3145. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. A facile method for fabricating robust cellulose nanocrystal/SiO2 superhydrophobic coatings. J. Colloid Interface Sci. 2019, 536, 349–362. [Google Scholar] [CrossRef]
- Ma, H.; Ren, H.; Koshy, P.; Sorrell, C.C.; Hart, J.N. Enhancement of CeO2 Silanization by Spontaneous Breakage of Si-O Bonds through Facet Engineering. J. Phys. Chem. C 2020, 124, 2644–2655. [Google Scholar] [CrossRef]
- Vareda, J.P.; Maximiano, P.; Cunha, L.P.; Ferreira, A.F.; Simões, P.N.; Durães, L. Effect of different types of surfactants on the microstructure of methyltrimethoxysilane-derived silica aerogels: A combined experimental and computational approach. J. Colloid Interface Sci. 2018, 512, 64–76. [Google Scholar] [CrossRef]
- Park, H.; Yook, S.; Park, S.Y.; Youn, H.J. Hydrophobization of cellulose nanofibrils by silylation under an aqueous system. Palpu Chongi Gisul/Journal Korea Tech. Assoc. Pulp Pap. Ind. 2018, 50, 72–77. [Google Scholar] [CrossRef]
- Frank, B.P.; Durkin, D.P.; Caudill, E.R.; Zhu, L.; White, D.H.; Curry, M.L.; Pedersen, J.A.; Fairbrother, D.H. Impact of Silanization on the Structure, Dispersion Properties, and Biodegradability of Nanocellulose as a Nanocomposite Filler. ACS Appl. Nano Mater. 2018, 1, 7025–7038. [Google Scholar] [CrossRef]
- Bertsch, P.; Fischer, P. Adsorption and interfacial structure of nanocelluloses at fluid interfaces. Adv. Colloid Interface Sci. 2020, 276, 102089. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Andersson, R.L.; Hedenqvist, M.S.; Farris, S.; Olsson, R.T. Cellulose nanofibril core-shell silica coatings and their conversion into thermally stable nanotube aerogels. J. Mater. Chem. A 2015, 3, 15745–15754. [Google Scholar] [CrossRef]
- da Silva, L.F.M.; Rodrigues, T.N.S.S.; Figueiredo, M.A.V.; de Moura, M.F.S.F.; Chousal, J.A.G. Effect of adhesive type and thickness on the lap shear strength. J. Adhes. 2006, 82, 1091–1115. [Google Scholar] [CrossRef]
- Kadam, P.; Vaidya, P.; Mhaske, S. Synthesis and characterization of polyesteramide based hot melt adhesive obtained with dimer acid, castor oil and ethylenediamine. Int. J. Adhes. Adhes. 2014, 50, 151–156. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, P.; Lu, Y.; Nie, S. Effect of lignin on the thermal stability of cellulose nanofibrils produced from bagasse pulp. Cellulose 2019, 26, 7823–7835. [Google Scholar] [CrossRef]
- Soares, C.M.F.; Dos Santos, O.A.; Olivo, J.E.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Influence of the alkyl-substituted silane precursor on sol-gel encapsulated lipase activity. J. Mol. Catal. B Enzym. 2004, 29, 69–79. [Google Scholar] [CrossRef]
- Supian, M.A.F.; Amin, K.N.M.; Jamari, S.S.; Mohamad, S. Production of cellulose nanofiber (CNF) from empty fruit bunch (EFB) via mechanical method. J. Environ. Chem. Eng. 2020, 8, 103024. [Google Scholar] [CrossRef]
- Poyraz, B.; Tozluoğlu, A.; Candan, Z.; Demir, A.; Yavuz, M. Influence of PVA and silica on chemical, thermo-mechanical and electrical properties of Celluclast-treated nanofibrillated cellulose composites. Int. J. Biol. Macromol. 2017, 104, 384–392. [Google Scholar] [CrossRef]
- Jo, J.; Kim, H.; Jeong, S.Y.; Park, C.; Hwang, H.S.; Koo, B. Changes in mechanical properties of polyhydroxyalkanoate with double silanized cellulose nanocrystals using different organosiloxanes. Nanomaterials 2021, 11, 1542. [Google Scholar] [CrossRef]
- Posada, P.; Velásquez-Cock, J.; Gómez-Hoyos, C.; Serpa Guerra, A.M.; Lyulin, S.V.; Kenny, J.M.; Gañán, P.; Castro, C.; Zuluaga, R. Drying and redispersion of plant cellulose nanofibers for industrial applications: A review. Cellulose 2020, 27, 10649–10670. [Google Scholar] [CrossRef]
- Salmén, L.; Stevanic, J.S. Effect of drying conditions on cellulose microfibril aggregation and “hornification”. Cellulose 2018, 25, 6333–6344. [Google Scholar] [CrossRef]
- Cinelli, P.; Seggiani, M.; Mallegni, N.; Gigante, V.; Lazzeri, A. Processability and degradability of PHA-based composites in terrestrial environments. Int. J. Mol. Sci. 2019, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, M.; Raju, P.V.S.; Prasad, J. Optimization of physical schemes in WRF model on cyclone simulations over Bay of Bengal using one-way ANOVA and Tukey’s test. Sci. Rep. 2021, 11, 24412. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crops Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Delacre, M.; Leys, C.; Mora, Y.L.; Lakens, D. Taking parametric assumptions seriously: Arguments for the use of welch’s F-test instead of the classical f-test in one-way ANOVA. Int. Rev. Soc. Psychol. 2020, 32, 1–12. [Google Scholar] [CrossRef]
- Huang, T.; Kuboyama, K.; Fukuzumi, H.; Ougizawa, T. PMMA/TEMPO-oxidized cellulose nanofiber nanocomposite with improved mechanical properties, high transparency and tunable birefringence. Cellulose 2018, 25, 2393–2403. [Google Scholar] [CrossRef]
- Jamaluddin, N.; Kanno, T.; Asoh, T.A.; Uyama, H. Surface modification of cellulose nanofiber using acid anhydride for poly(lactic acid) reinforcement. Mater. Today Commun. 2019, 21, 100587. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Eects of cellulose nanocrystals and cellulose nanofibers on the structure and properties of polyhydroxybutyrate nanocomposites. Polymers. 2019, 11, 2063. [Google Scholar] [CrossRef]
- Yano, H.; Omura, H.; Honma, Y.; Okumura, H.; Sano, H.; Nakatsubo, F. Designing cellulose nanofiber surface for high density polyethylene reinforcement. Cellulose 2018, 25, 3351–3362. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Sadeghi, B.; Sadeghi, P.; Marfavi, Y.; Kowsari, E.; Zareiyazd, A.A.; Ramakrishna, S. Impacts of cellulose nanofibers on the morphological behavior and dynamic mechanical thermal properties of extruded polylactic acid/cellulose nanofibril nanocomposite foam. J. Appl. Polym. Sci. 2022, 139, 51673. [Google Scholar] [CrossRef]
- Dawidziuk, K.A. Peroxide-initiated Modification of Polylactic acid (PLA) and Poly(3-hydroxyalkanoates) (PHAs) in the Presence of Allylic and Acrylic Coagents. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2018. [Google Scholar]
- Corre, Y.M.; Bruzaud, S.; Audic, J.L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Antonov, S.V.; Ilyin, S.O. Rheological and Adhesion Properties of Hot-Melt Adhesives Based on Hydrocarbon Resins and Poly(ethylene-vinyl acetate). Polym. Sci., Ser. A 2021, 63, 283–295. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, U.; Pandey, C.; Mishra, P.; Pandey, G. Application of student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407. [Google Scholar] [CrossRef] [PubMed]

| Mechanical Properties | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Tensile strength (MPa) | Regression | 0.15 | 3 | 0.05 | 7.72 | 0.01 |
| Residual | 0.05 | 8 | 0.01 | |||
| Total | 0.20 | 11 | ||||
| Elongation at break (%) | Regression | 128,997.00 | 3 | 42,999.00 | 9.09 | 0.01 |
| Residual | 37,838.00 | 8 | 4730.00 | |||
| Total | 166,835.00 | 11 | ||||
| Sample Name | Tg ( °C) | Tm ( °C) | Hm (J/g) | Tc ( °C) | Hc (J/g) |
|---|---|---|---|---|---|
| PHA | −23 | 161 | 0.31 | - | - |
| TMCNFs 2% | −24 | 159 | 0.43 | 75 | 1.44 |
| TMCNFs 5% | −22 | 160 | 0.58 | 81 | 1.67 |
| TMCNFs 10% | −23 | 160 | 0.69 | 79 | 2.15 |
| Mechanical Properties | Group | N | Mean | Standard Deviation | t-Value | p-Value |
|---|---|---|---|---|---|---|
| Failure load (kN) | PHA | 3 | 409 | 41 | 1.48 | 0.213 |
| PHA–TMCNFs 10% | 3 | 340 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, J.; Jeong, S.-Y.; Lee, J.; Park, C.; Koo, B. Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyalkanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs). Polymers 2022, 14, 5284. https://doi.org/10.3390/polym14235284
Jo J, Jeong S-Y, Lee J, Park C, Koo B. Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyalkanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs). Polymers. 2022; 14(23):5284. https://doi.org/10.3390/polym14235284
Chicago/Turabian StyleJo, Jaemin, So-Yeon Jeong, Junhyeok Lee, Chulhwan Park, and Bonwook Koo. 2022. "Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyalkanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs)" Polymers 14, no. 23: 5284. https://doi.org/10.3390/polym14235284
APA StyleJo, J., Jeong, S.-Y., Lee, J., Park, C., & Koo, B. (2022). Green and Sustainable Hot Melt Adhesive (HMA) Based on Polyhydroxyalkanoate (PHA) and Silanized Cellulose Nanofibers (SCNFs). Polymers, 14(23), 5284. https://doi.org/10.3390/polym14235284







