Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines
Abstract
:1. Introduction
2. Materials and Methods
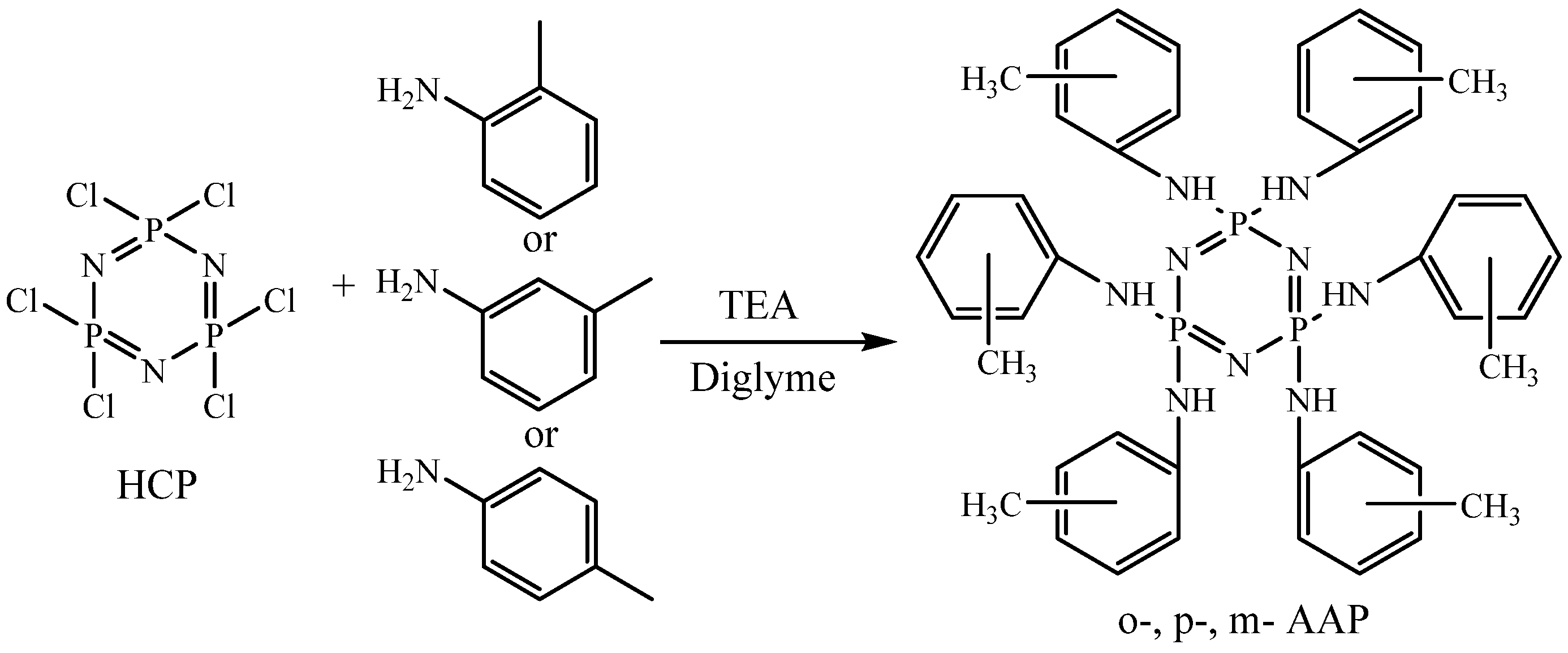
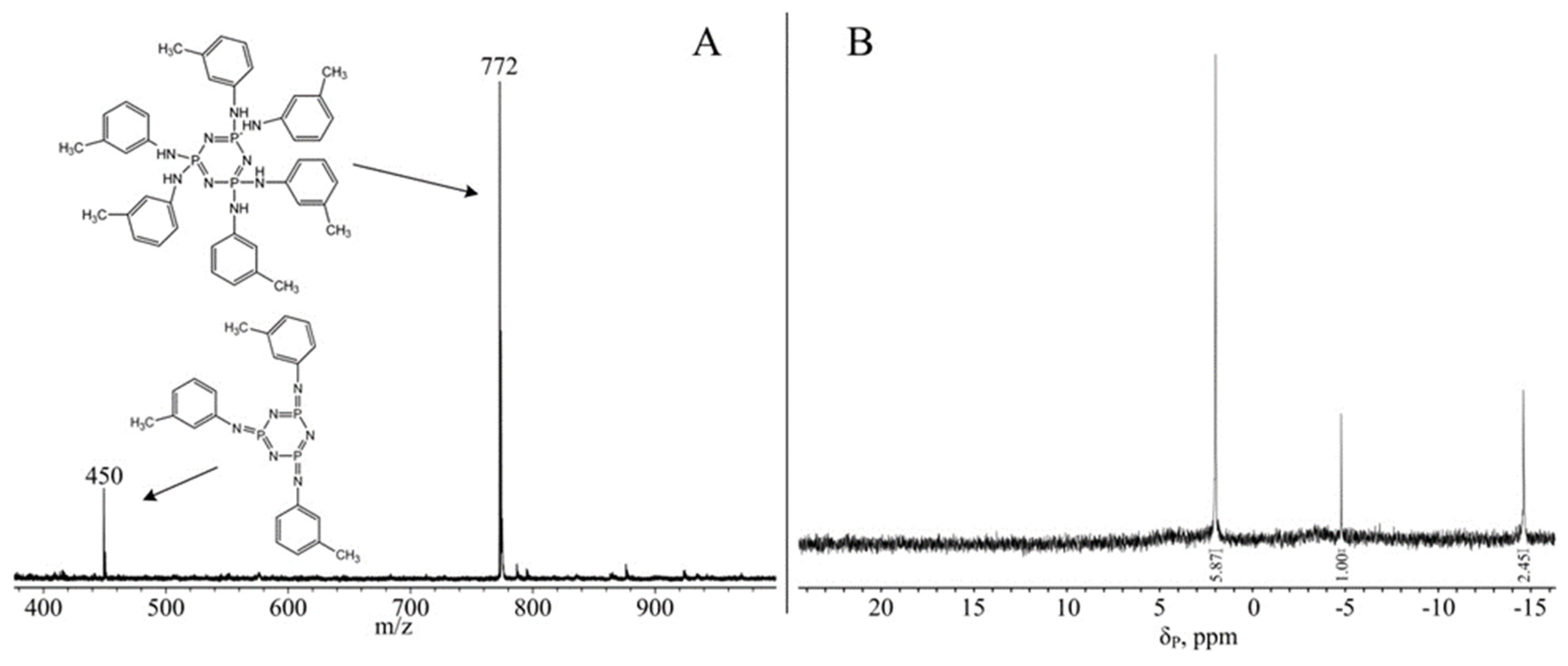
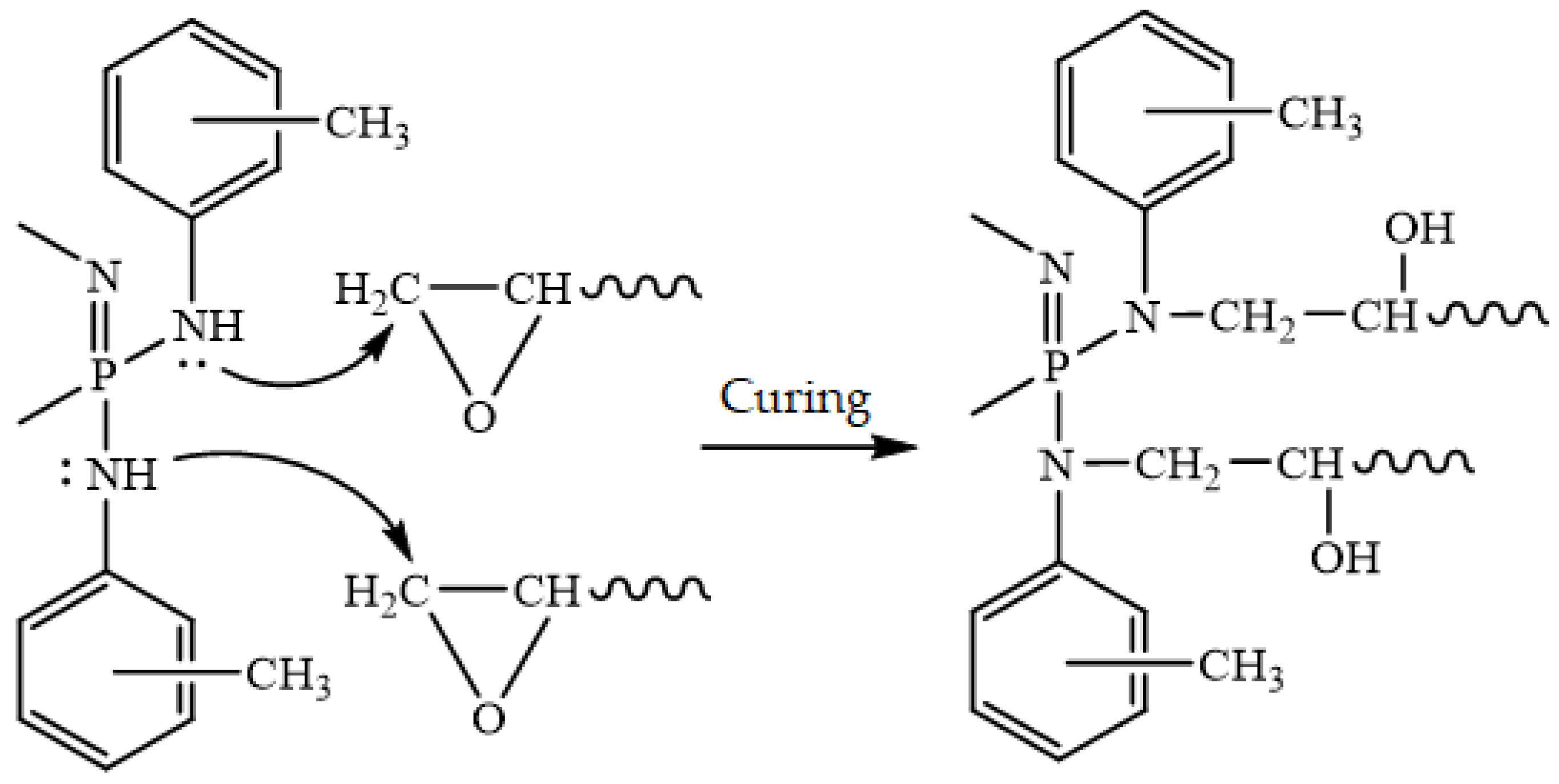
3. Results and Discussion
Reaction Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Lou, X.; Schotman, M.J.; Marín San Román, P.P.; Sijbesma, R.P. Photo-Crosslinked Coumarin-Containing Bis-Urea Amphiphile Hydrogels. Gels 2022, 8, 615. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Gorbunova, I.Y.; Kireev, V.V.; Onuchin, D.V.; Kerber, M.L.; Petrakova, V.V.; Kryuchkov, I.A.; Nevskiy, R.E.; Sokovishin, A.V.; Khammatova, V.V.; et al. The curing rheokinetics of epoxyphosphazene binders. Materials 2020, 13, 5685. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Abualnaja, M.; Abu Ali, O.A.; Abdel-Razik, H.H.; Albogami, S.M.; Fayad, E. Metallic Porphyrazine Networks: Synthesis, as Well as Thermal and Optical Properties for Accelerating the Oxidation of Thiols to Their Disulfides. Metals 2022, 12, 1523. [Google Scholar] [CrossRef]
- Dong, X.; Sun, J.; Huang, X.; Li, J.; Lv, K.; Zhang, P. Synthesis of a Low-Molecular-Weight Filtrate Reducer and Its Mechanism for Improving High Temperature Resistance of Water-Based Drilling Fluid Gel System. Gels 2022, 8, 619. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Shutov, V.V.; Sirotin, I.S.; Gorbunova, I.Y. Benzoxazine copolymers with mono-and difunctional epoxy active diluents with enhanced tackiness and reduced viscosity. J. Compos. Sci. 2021, 5, 250. [Google Scholar] [CrossRef]
- Costanza, G.; Del Ferraro, A.; Tata, M.E. Experimental Set-Up of the Production Process and Mechanical Characterization of Metal Foams Manufactured by Lost-PLA Technique with Different Cell Morphology. Metals 2022, 12, 1385. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Ariane, M.; Alexiadis, A. Fluid-Structure Interaction in Coronary Stents: A Discrete Multiphysics Approach. ChemEngineering 2021, 5, 60. [Google Scholar] [CrossRef]
- Zawani, M.; Maarof, M.; Tabata, Y.; Motta, A.; Fauzi, M.B. Quercetin-Embedded Gelastin Injectable Hydrogel as Provisional Biotemplate for Future Cutaneous Application: Optimization and In Vitro Evaluation. Gels 2022, 8, 623. [Google Scholar] [CrossRef]
- Zhang, L.; Song, G.; Zhao, Z.; Ma, L.; Xu, H.; Wu, G.; Song, Y.; Liu, Y.; Qiu, L.; Li, X. Structurally Stable, High-Strength Graphene Oxide/Carbon Nanotube/Epoxy Resin Aerogels as Three-Dimensional Skeletal Precursors for Wave-Absorbing Materials. Gels 2022, 8, 618. [Google Scholar] [CrossRef]
- Baş, G.S.; Sancaktar, E. Mechanical behavior of toughened epoxy structural adhesives for impact applications. ChemEngineering 2020, 4, 38. [Google Scholar] [CrossRef]
- Rubino, F.; Tucci, F.; Esperto, V.; Carlone, P. Filling Time Reduction in Liquid Composite Molding Processes. J. Compos. Sci. 2022, 6, 222. [Google Scholar] [CrossRef]
- Naik, N.; Shivamurthy, B.; Thimmappa, B.H.S.; Guo, Z.; Bhat, R. Bio-Based Epoxies: Mechanical Characterization and Their Applicability in the Development of Eco-Friendly Composites. J. Compos. Sci. 2022, 6, 294. [Google Scholar] [CrossRef]
- Samardžija, M.; Alar, V.; Špada, V.; Stojanović, I. Corrosion Behaviour of an Epoxy Resin Reinforced with Aluminium Nanoparticles. Coatings 2022, 12, 1500. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Zhu, J. Synergistic flame-retardant effect of aluminum hydroxide and ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2022, 139, e53168. [Google Scholar] [CrossRef]
- Lukina, Y.S.; Mishchenko, B.P.; Zaytsev, V.V.; Vasilev, M.G.; Selezneva, I.I. Osteoplastic Material Based on a Bone Matrix Resistant to Osteoclastic Resorption under Conditions of a Pronounced Regenerative Process as a Carrier for rhBMP. Inorg. Mater. Appl. Res. 2022, 13, 952–960. [Google Scholar] [CrossRef]
- Fedoseev, M.S.; Derzhavinskaya, L.F.; Scherban, R.V. Thermomechanical and Adhesion Properties of Polymers and Composites as Affected by Nature of Epoxy Isocyanate Binders. Inorg. Mater. Appl. Res. 2021, 12, 1104–1111. [Google Scholar] [CrossRef]
- Zarybnicka, L.; Machotova, J.; Kopecka, R.; Sevcik, R.; Hudakova, M.; Pokorny, J.; Sal, J. Effect of Cyclotriphosphazene-Based Curing Agents on the Flame Resistance of Epoxy Resins. Polymers 2020, 13, 8. [Google Scholar] [CrossRef]
- Bornosuz, N.V.; Korotkov, R.F.; Kolenchenko, A.A.; Shapagin, A.V.; Orlov, A.V.; Gorbunova, I.Y.; Kireev, V.V.; Sirotin, I.S. The Influence of Substituents in Phosphazene Catalyst-Flame Retardant on the Thermochemistry of Benzoxazine Curing. Polymers 2021, 13, 3111. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Q.; Hu, Y. Synthesis of a novel flame retardant containing phosphorus, nitrogen and boron and its application in flame-retardant epoxy resin. Polym. Degrad. Stab. 2016, 133, 358–366. [Google Scholar] [CrossRef]
- Allcock, H.R.; Morozowich, N.L. Bioerodible polyphosphazenes and their medical potential. Polym. Chem. 2012, 3, 578–590. [Google Scholar] [CrossRef]
- Ganapathiappan, S.; Krishnamurthy, S.S. Studies of phosphazenes. Part 30. Reactions of hexachlorocyclotriphosphazene with aromatic primary amines: Interplay of geminal and non-geminal modes of chlorine replacement. J. Chem. Soc. Dalton Trans. 1987, 3, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Chistyakov, E.M.; Terekhov, I.V.; Shapagin, A.V.; Filatov, S.N.; Chuev, V.P. Curing of Epoxy Resin DER-331 by Hexakis (4-acetamidophenoxy) cyclotriphosphazene and Properties of the Prepared Composition. Polymers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballesteros, B.; Santos, L. A reinvestigation of the molecular structures, vibrations and rotation of methyl group in o-methylaniline in S0 and S1 states studied by laser induced fluorescence spectroscopy and ab initio calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 1069–1081. [Google Scholar] [CrossRef] [PubMed]





| Curing Agent | Temperature, °C | Mass Loss, % |
|---|---|---|
| M-AAP | 5 | 1.77 |
| 10 | 11.65 | |
| 15 | 97.07 | |
| P-AAP | 5 | 4.48 |
| 10 | 43.93 | |
| 15 | 90.36 | |
| O-AAP | 5 | 0 |
| 10 | 2.76 | |
| 15 | 2.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybyan, A.A.; Bilichenko, J.V.; Kireev, V.V.; Kolenchenko, A.A.; Chistyakov, E.M. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers 2022, 14, 5334. https://doi.org/10.3390/polym14245334
Rybyan AA, Bilichenko JV, Kireev VV, Kolenchenko AA, Chistyakov EM. Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers. 2022; 14(24):5334. https://doi.org/10.3390/polym14245334
Chicago/Turabian StyleRybyan, Artem A., Julia V. Bilichenko, Vyacheslav V. Kireev, Alexander A. Kolenchenko, and Evgeniy M. Chistyakov. 2022. "Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines" Polymers 14, no. 24: 5334. https://doi.org/10.3390/polym14245334
APA StyleRybyan, A. A., Bilichenko, J. V., Kireev, V. V., Kolenchenko, A. A., & Chistyakov, E. M. (2022). Curing of DER-331 Epoxy Resin with Arylaminocyclotriphosphazenes Based on o-, m-, and p-methylanilines. Polymers, 14(24), 5334. https://doi.org/10.3390/polym14245334







