Effect of Water Immersion on Compressive Properties of Coir Fiber Magnesium Phosphate Cement
Abstract
:1. Introduction
2. Experimental Programs
2.1. Specimens and Raw Materials
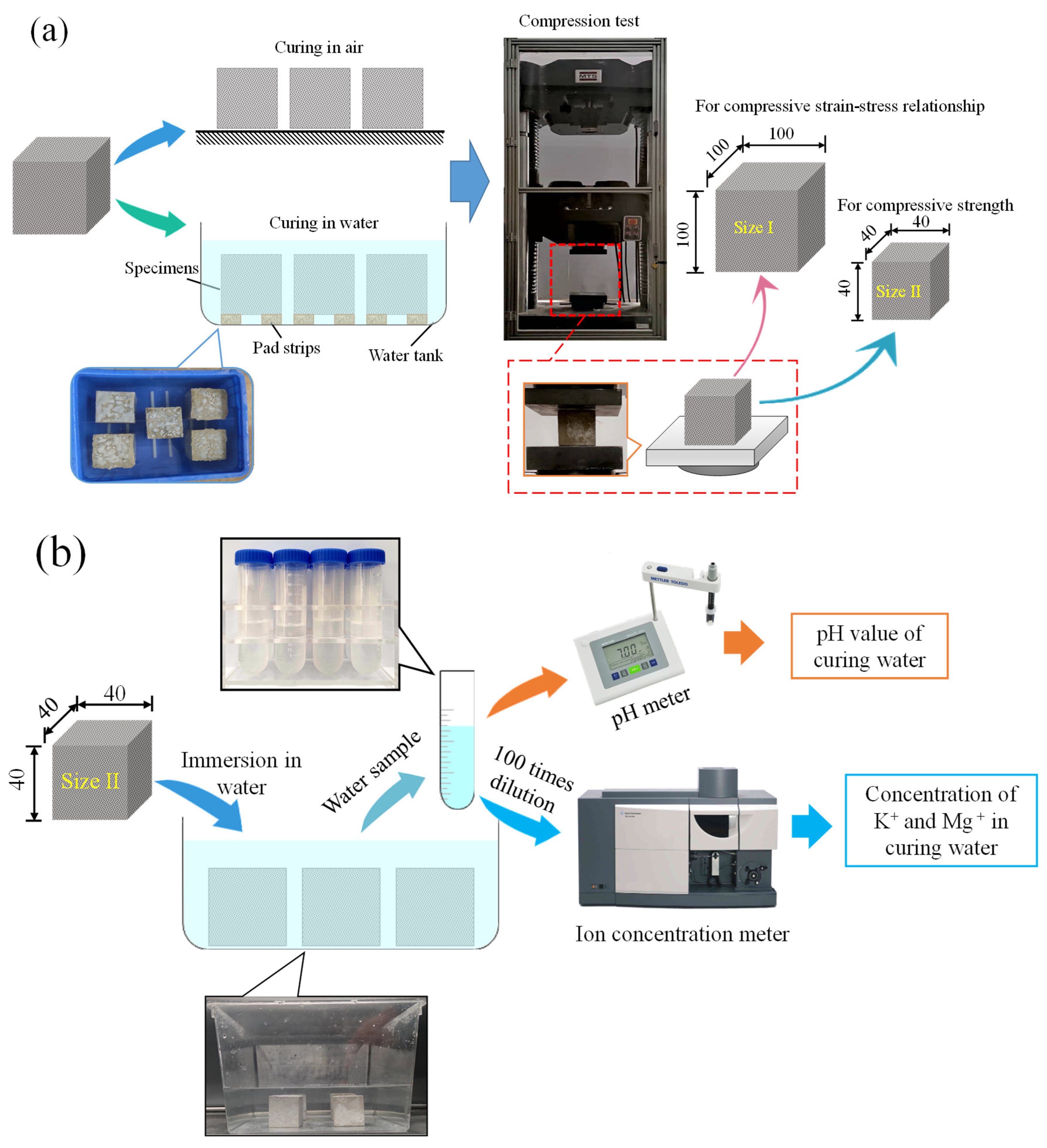
2.2. Test Setup
3. Compressive Behaviors
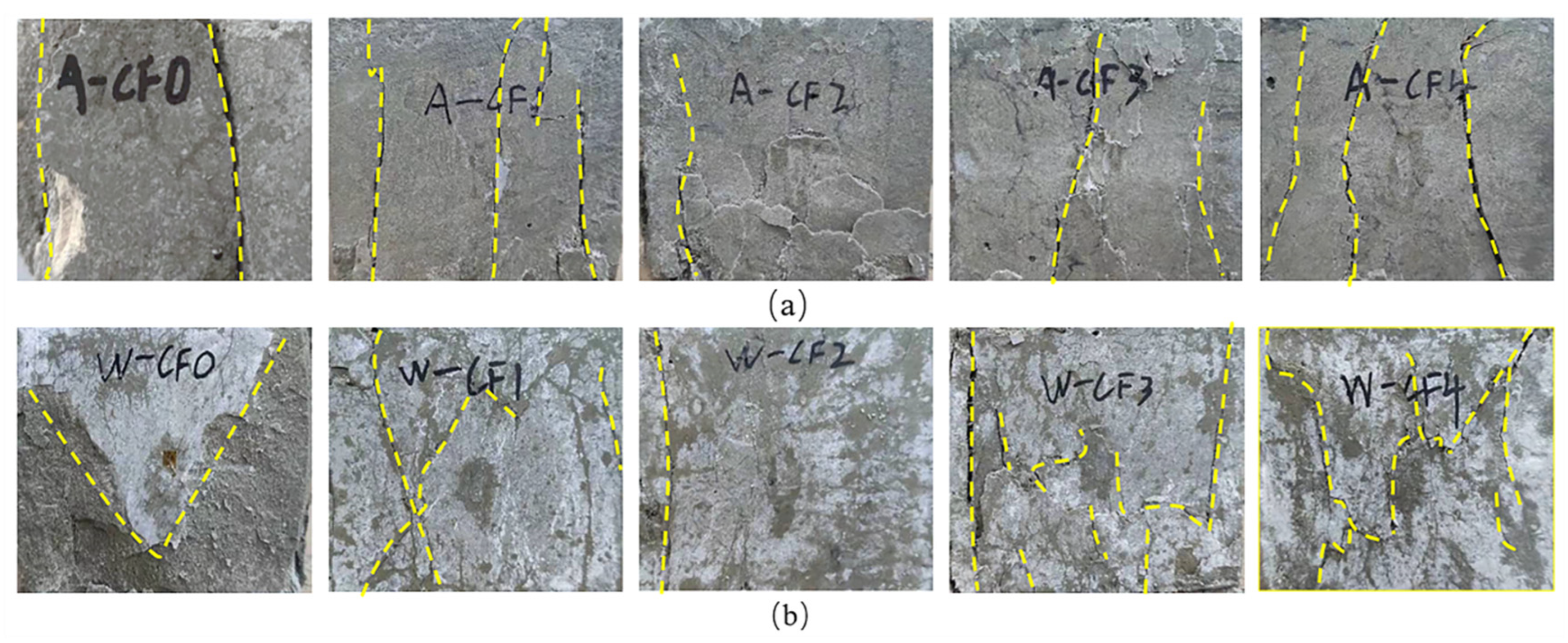
3.1. Failure Mode
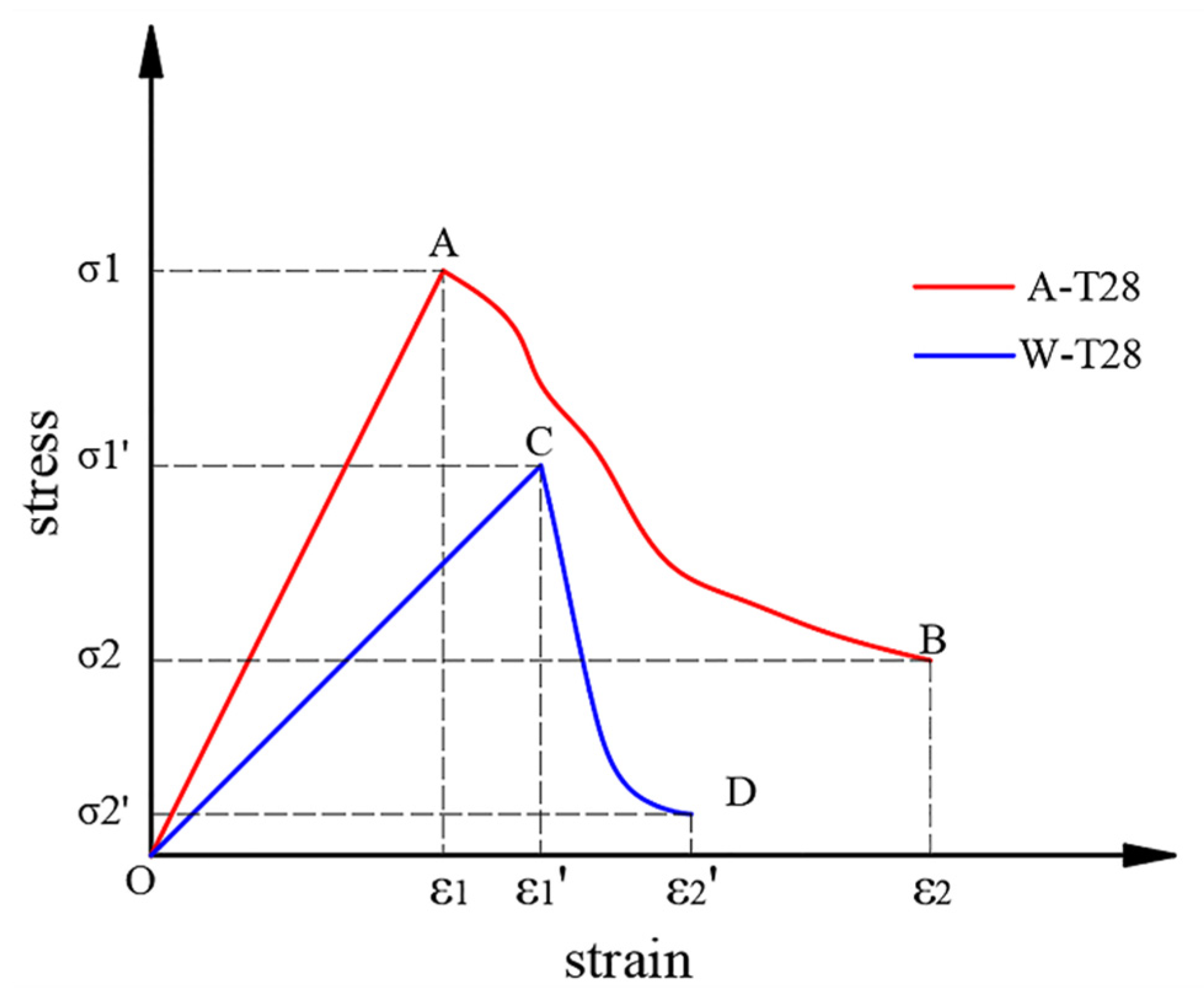
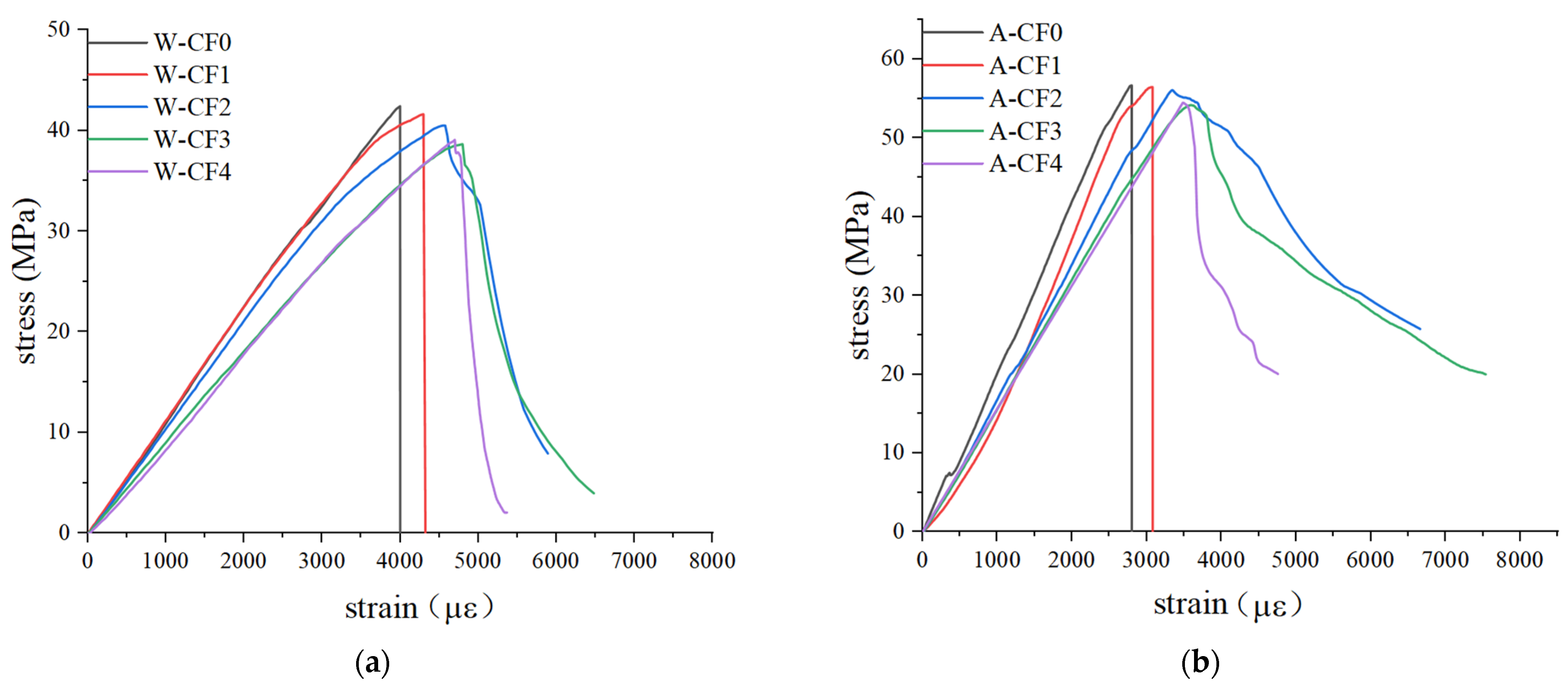
3.2. Stress–Strain Relationship
3.3. Elastic Modulus
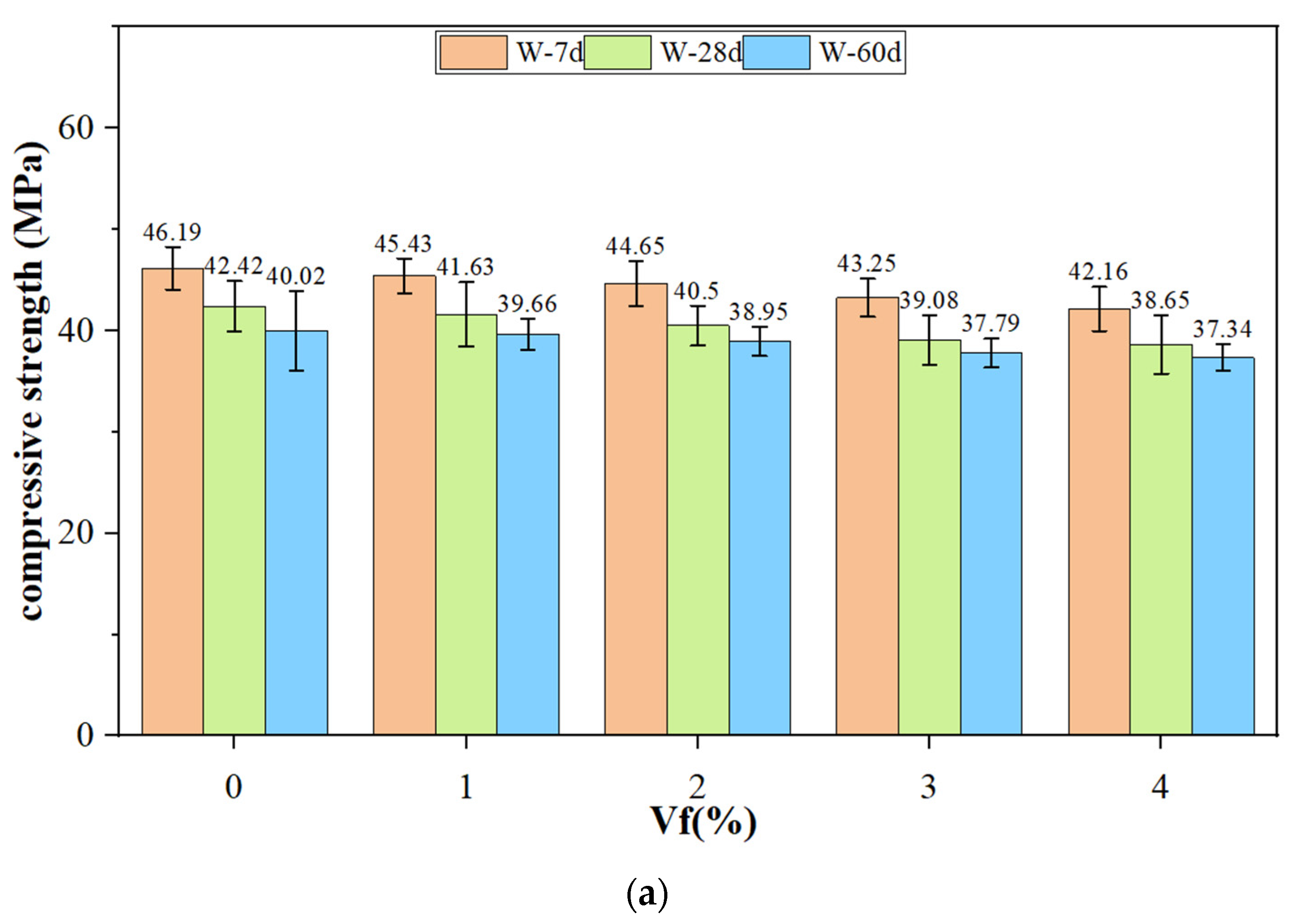
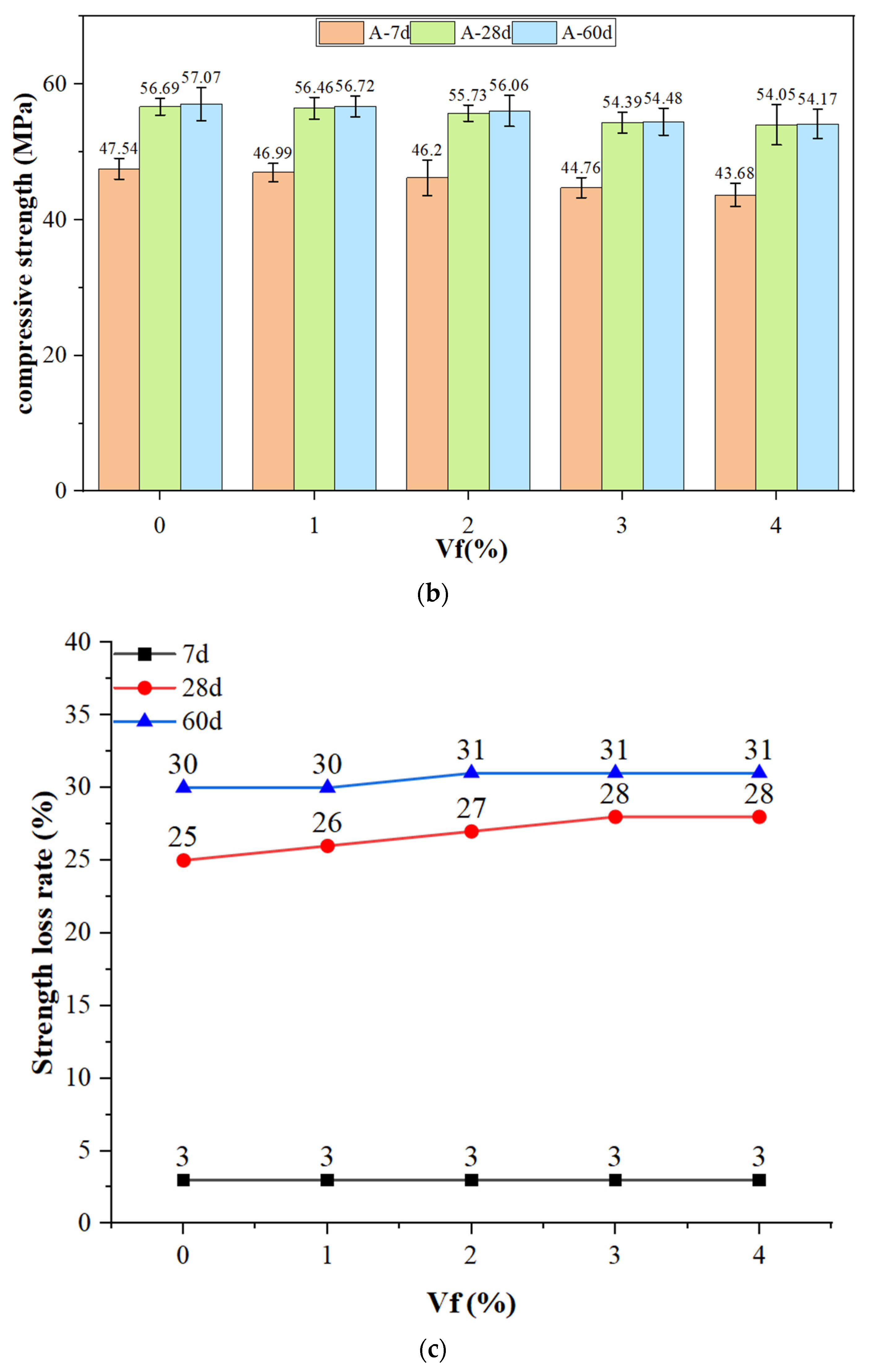
3.4. Compressive Strength
4. Microcosmic
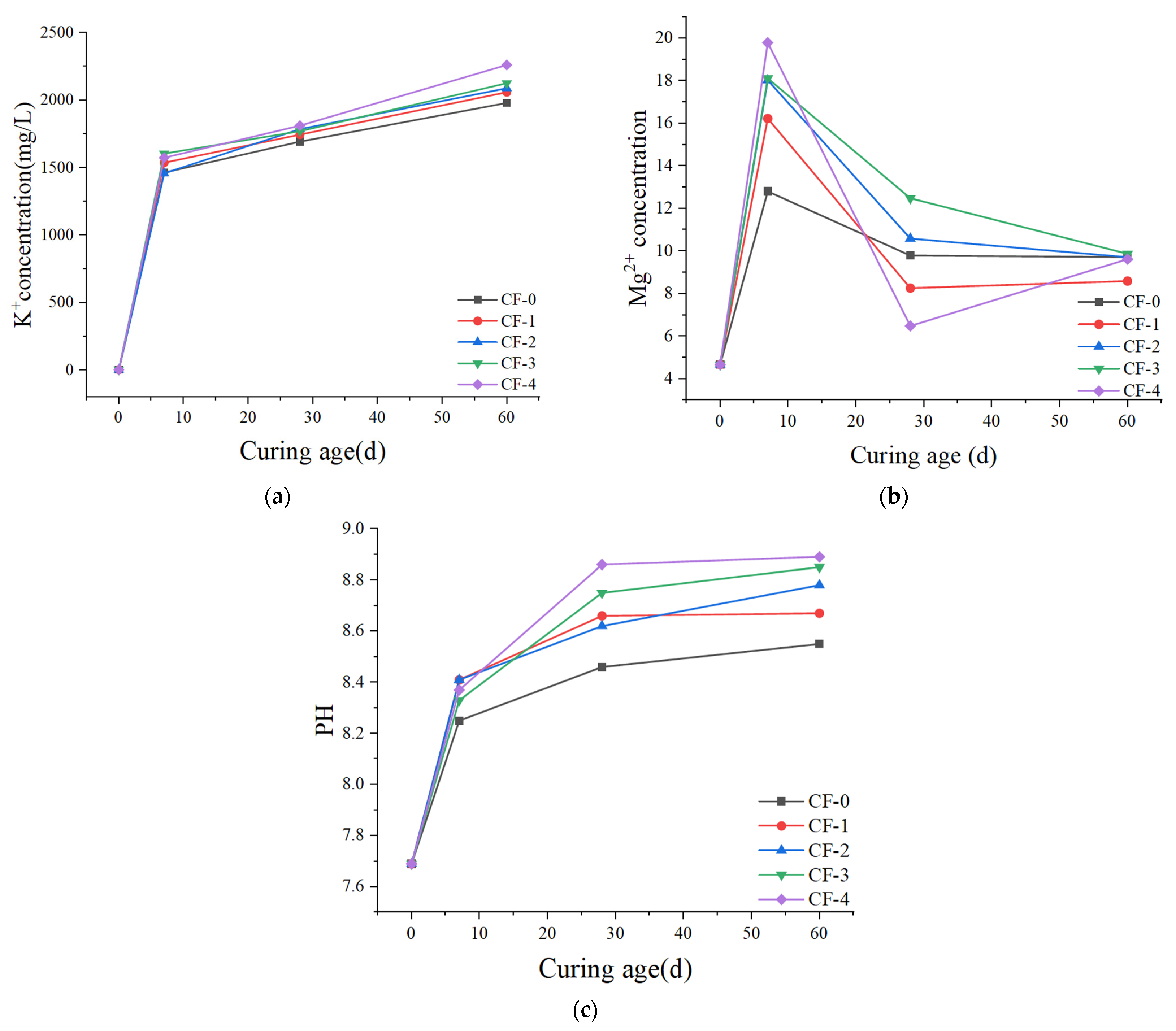
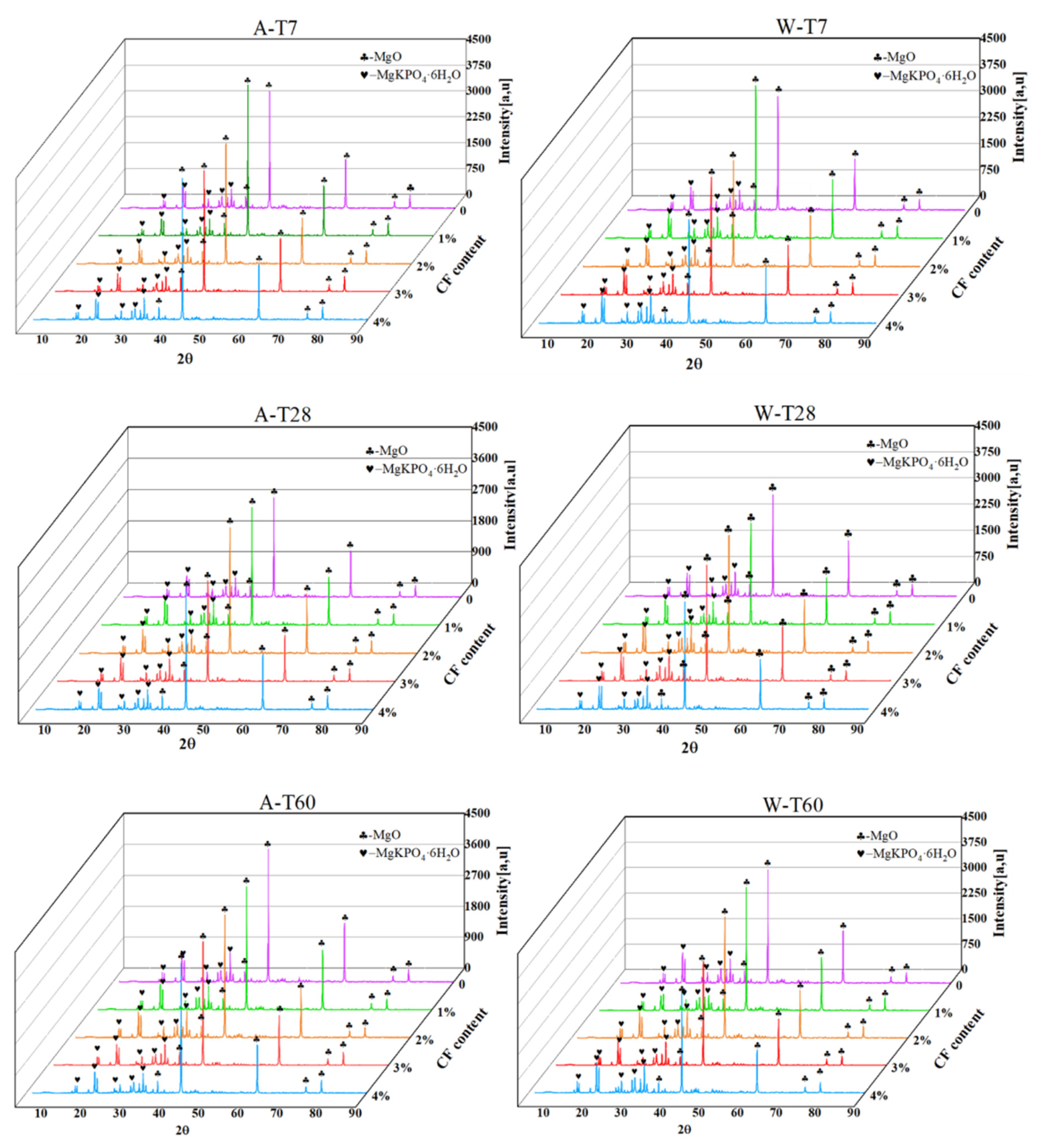
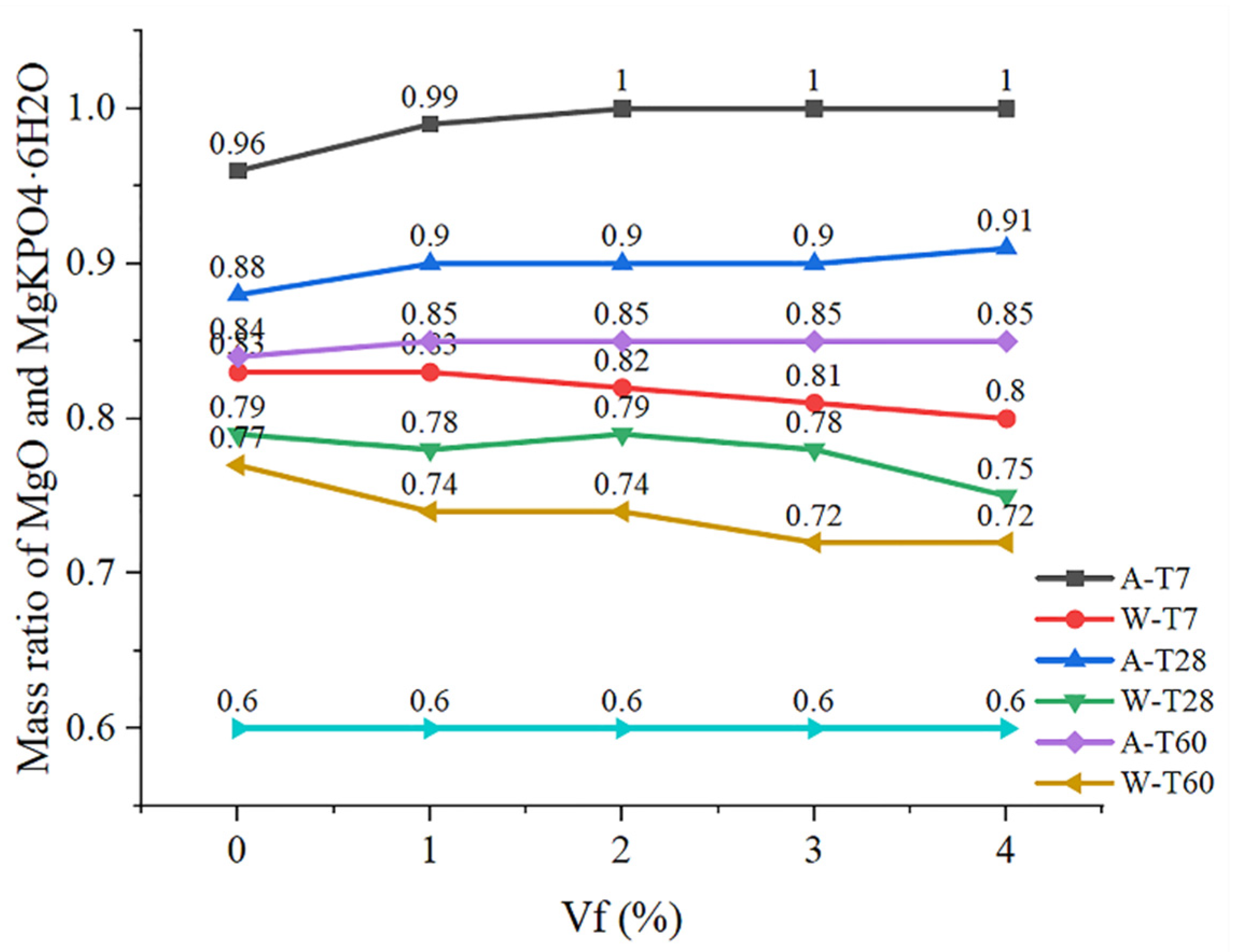
4.1. Solubility Analysis
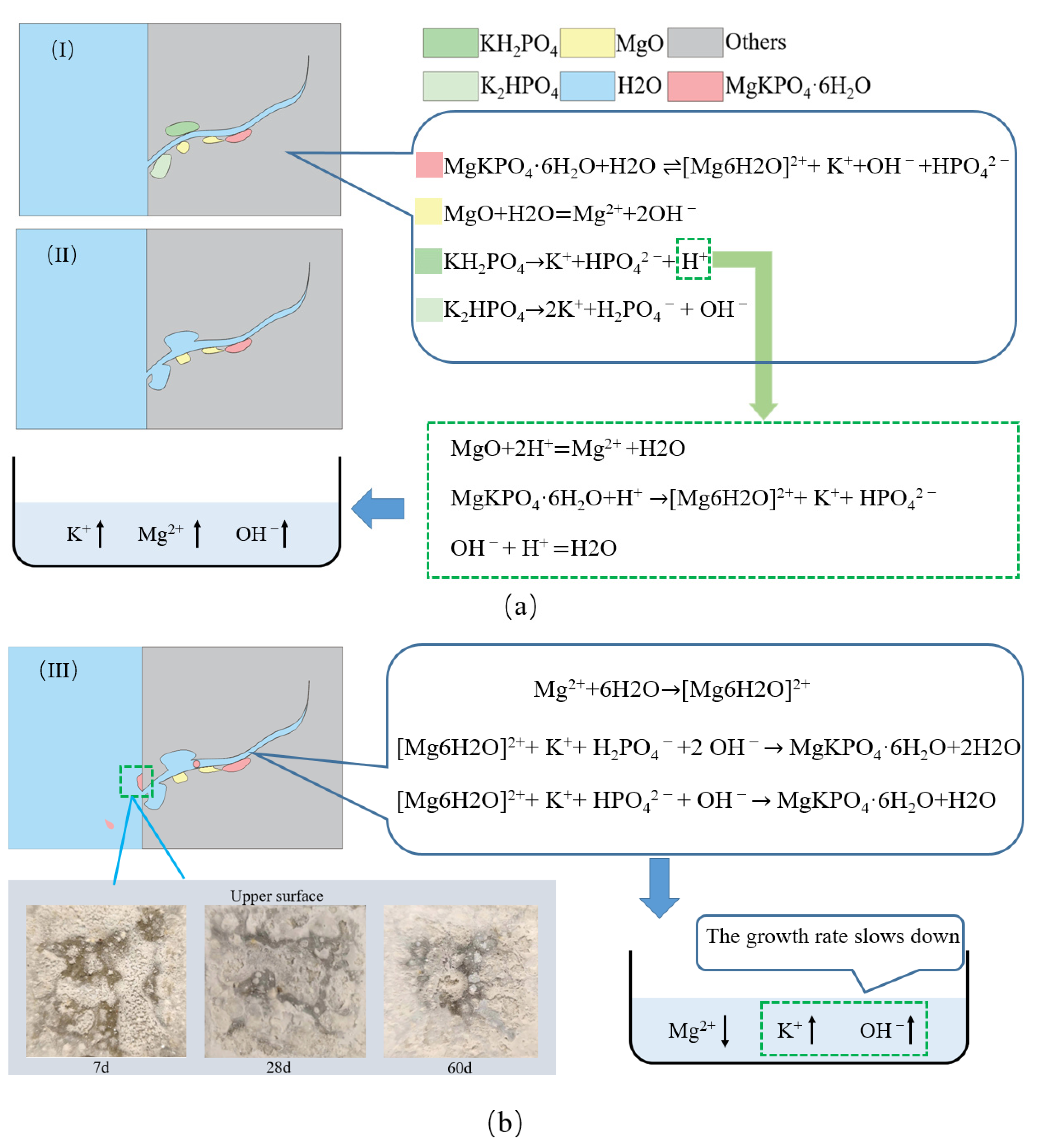
4.2. Hydration Analysis
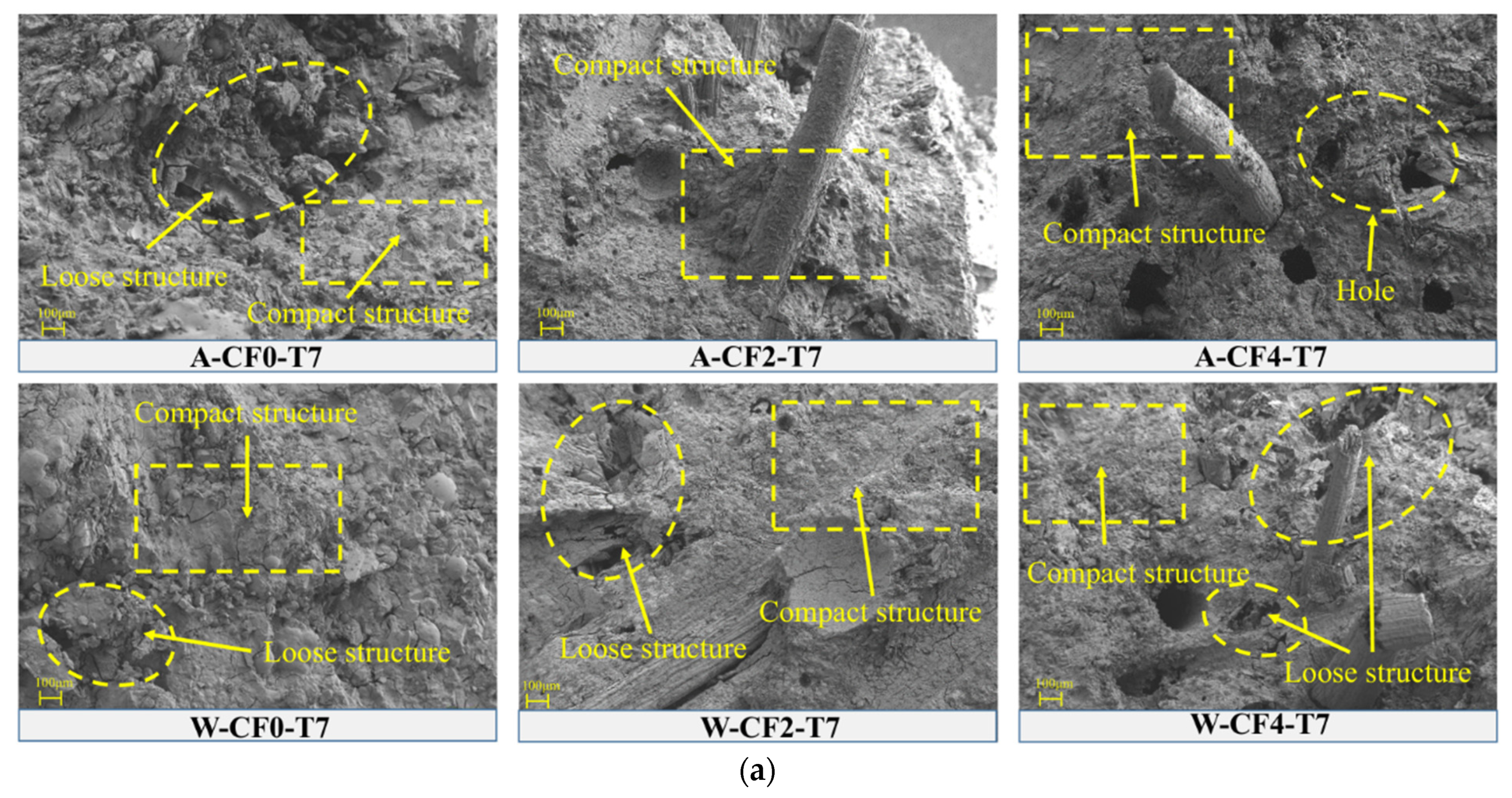
4.3. Microstructure
5. Conclusions
- (1)
- Water immersion will change the failure mode of CF-MPC, resulting in oblique development and cracks penetrating the matrix.
- (2)
- When CF-MPC is cured in water, the elastic modulus of the specimen will be greatly reduced, the peak stress will be reduced, and the peak strain will be increased.
- (3)
- During water curing, the K+ and Mg2+ concentration change diagram and PH change diagram portray the dissolution, migration, and recrystallization process of CF-MPC under water immersion curing, and they explain the change in MgO/MgKPO4·6H2O mass ratio and microstructure defects caused by water immersion.
- (4)
- XRD results show that the mass ratio of MgO to MgKPO4·6H2O in soaked CF-MPC will decrease with the increase in curing time, and the mass ratio of MgO to MgKPO4·6H2O will decrease with the increase in fiber content at the same soaking time.
- (5)
- The results of solubility testing and SEM show that the soaking environment leads to the dissolution and leaching of unreacted phosphate in water environments, which generates additional defects in CF-MPC dense structure. However, excessive fiber content will also lead to the increase in structural holes, and the fibers embedded in MPC will be damaged with the increase in immersion time and the corrosion of alkaline solution.
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Q.B.; Zhang, S.Q.; Wu, X.L. Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Qian, J.S.; You, C.; Wang, Q.Z.; Wang, H.T.; Jia, X.W. A method for assessing bond performance of cement-based repair materials. Constr. Build. Mater. 2014, 68, 307–313. [Google Scholar] [CrossRef]
- Wang, A.J.; Zhang, J.; Li, J.M.; Ma, A.B.; Liu, L.T. Effect of liquid-to-solid ratios on the properties of magnesium phosphate chemically bonded ceramics. Mater. Sci. Eng. C 2013, 33, 2508–2512. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Chen, B. Experimental study of magnesia and M/P ratio influencing properties of magnesium phosphate cement. Constr. Build. Mater. 2014, 65, 177–183. [Google Scholar] [CrossRef]
- Yang, Q.B.; Wu, X.L. Factors influencing properties of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 1999, 29, 389–396. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z.J. Property evaluation of magnesium phosphate cement mortar as patch repair material. Constr. Build. Mater. 2010, 24, 695–700. [Google Scholar] [CrossRef]
- Halla, D.A.; Stevens, R.; El-Jazairi, B. The effect of retarders on the microstructure and mechanical properties of magnesia-phosphate cement mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct./Mat6riaux Constr. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G. Characterisation and utilization of natural coconut fibres composites. Mater. Des. 2009, 30, 2741–2744. [Google Scholar] [CrossRef]
- Zaman, H.U.; Beg, M.D.H. Effect of Coir Fiber Content and Compatibilizer on the Properties of Unidirectional Coir Fiber/Polypropylene Composites. Fibers Polym. 2014, 15, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Bledzki, A.K.; Reihmane, S.; Gassan, J. Properties and modification methods for vegetable fibers for natural fiber composites. J. Appl. Polym. Sci. 1996, 59, 1329–1336. [Google Scholar] [CrossRef]
- Mukherjee, P.S.; Satyanarayana, K.G. Structure and properties of some vegetable fibres: Part 1 Sisal fibre. J. Mater. Sci. 1984, 19, 3925–3934. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, M.A. Surface treatment of coir (Cocos nucifera) fibers and its influence on the fibers’ physico-mechanical properties. Compos. Sci. Technol. 2007, 67, 2369–2376. [Google Scholar] [CrossRef]
- Zhang, L.W.; Jiang, Z.Q.; Wu, H.; Zhang, W.H.; Lai, Y.S.; Zheng, W.L.; Li, J. Flexural Properties of Renewable Coir Fiber Reinforced Magnesium Phosphate Cement. Considering Fiber Length. Mater. 2020, 13, 3692. [Google Scholar] [CrossRef]
- Zhang, L.W.; Jiang, Z.Q.; Zhang, W.H.; Peng, S.X.; Chen, P.F. Flexural Properties and Microstructure Mechanism of Renewable Coir-Fiber-Reinforced Magnesium Phosphate Cement-Based Composite Considering Curing Ages. Polymers 2020, 12, 2556. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Zhang, L.W.; Geng, T.; Lai, Y.S.; Zheng, W.L.; Huang, M. Study on the Compressive Properties of Magnesium Phosphate Cement Mixing with Eco-Friendly Coir Fiber Considering Fiber Length. Materials 2020, 13, 3194. [Google Scholar] [CrossRef]
- Seehra Ms, S.S.; Gupta, S.; Kumar, S. Rapid setting magnesium phosphate cement for quick repair of concrete pavements—Characterization and durability aspects. Cem. Concr. Res. 1993, 23, 254–266. [Google Scholar] [CrossRef]
- Sarkar, A.K. Phosphate cement-based fast-setting binders. Am. Ceram. Soc. Bull. 1990, 69, 234–238. [Google Scholar]
- Li, D.X.; Li, X.P.; Feng, C.H. Research on water resistance of magnesium phosphate cement. J. Build. Mater. 2009, 12, 505–510. [Google Scholar]
- Liu, K.; Jiang, F.; Zhang, C.; Zhang, B.B.; Li, D.X. Failure mechanism of magnesium phosphate cement modified by potassium hydrogen phosphate under water curing condition. J. Chin. Ceram. Soc. 2012, 40, 1693–1698. [Google Scholar]
- Yu, J.H.; Yan, L.W. Water resistance test of magnesium phosphate concrete pavement rapid repair material. J. Shenyang Archit. Univ. 2018, 34, 855–863. [Google Scholar]
- Fang, Y.; Chen, B.; Oderji, S.Y. Experimental research on magnesium phosphate cement mortar reinforced by glass fiber. Constr. Build. Mater. 2018, 188, 729–736. [Google Scholar] [CrossRef]
- Dong, P.; Ahmad, M.R.; Chen, B.; Munir, M.J.; Kazmi, S.M.S. A study on magnesium phosphate cement mortars reinforced by polyvinyl alcohol fibers. Constr. Build. Mater. 2021, 302, 124154. [Google Scholar] [CrossRef]
- GB/T 17671-1999; Method of Testing Cements-Determination of Strength. China National Standards: Beijing, China, 1999.



| Component | MgO | CaO | SiO₂ | Fe₂O₃ | Al₂O₃ | Other |
|---|---|---|---|---|---|---|
| Content (%) | 96.25 | 1.18 | 1.16 | 1.09 | 0.29 | 3.29 |
| Component | SiO₂ | Al₂O₃ | CaO | Fe₂O₃ | K₂O | Other |
|---|---|---|---|---|---|---|
| Content (%) | 54.94 | 34.86 | 2.63 | 2.52 | 1.76 | 3.29 |
| Length (mm) | Average Diameter (μm) | Density (g/cm3) | Elasticity (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| 20 | 250 | 1.2 | 3.86–5.6 | 128–157 | 21.2–40.7 |
| Element | MgO | KH2PO4 | Borax | Fly Ash | Water |
|---|---|---|---|---|---|
| Content (g/cm3) | 1.17 | 0.80 | 0.12 | 0.30 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Song, S.; Huang, M.; Xie, Z.; Zhang, L.; Zheng, W. Effect of Water Immersion on Compressive Properties of Coir Fiber Magnesium Phosphate Cement. Polymers 2022, 14, 5339. https://doi.org/10.3390/polym14245339
Wang S, Song S, Huang M, Xie Z, Zhang L, Zheng W. Effect of Water Immersion on Compressive Properties of Coir Fiber Magnesium Phosphate Cement. Polymers. 2022; 14(24):5339. https://doi.org/10.3390/polym14245339
Chicago/Turabian StyleWang, Shimin, Shaozhi Song, Mingyu Huang, Zhujian Xie, Liwen Zhang, and Wenzhi Zheng. 2022. "Effect of Water Immersion on Compressive Properties of Coir Fiber Magnesium Phosphate Cement" Polymers 14, no. 24: 5339. https://doi.org/10.3390/polym14245339






