Breathability and Moisture Permeability of Cellulose Nanocrystals Hollow Microsphere Coatings for PET Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
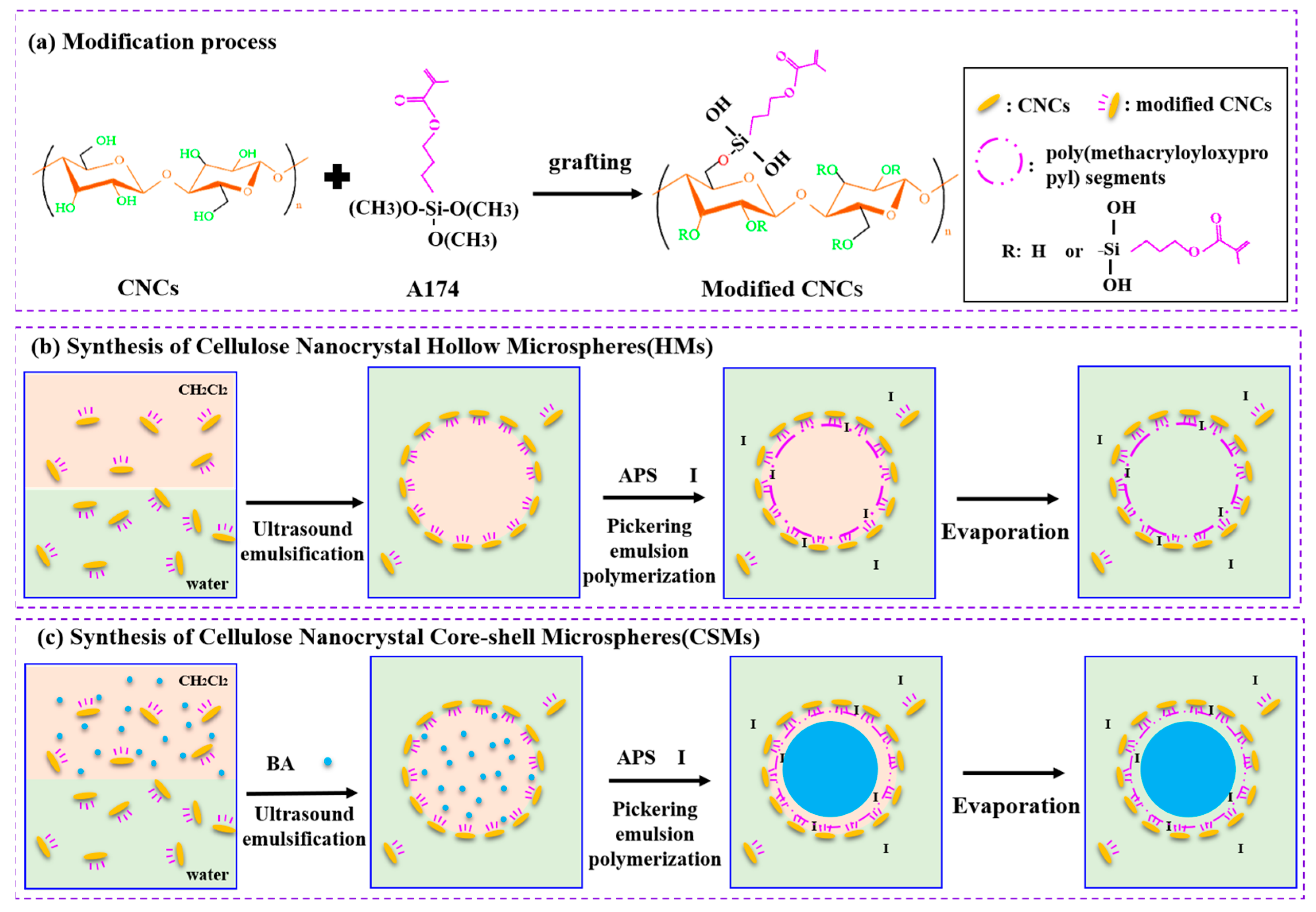
2.2. Preparation of Modified Cellulose Nanocrystals Particles
2.3. Synthesis of Cellulose Nanocrystals Hollow Microspheres
2.4. Fabrication Polyester Fabric with Cellulose Nanocrystals Hollow Microsphere Coatings
2.5. Characterization
3. Results and Discussion
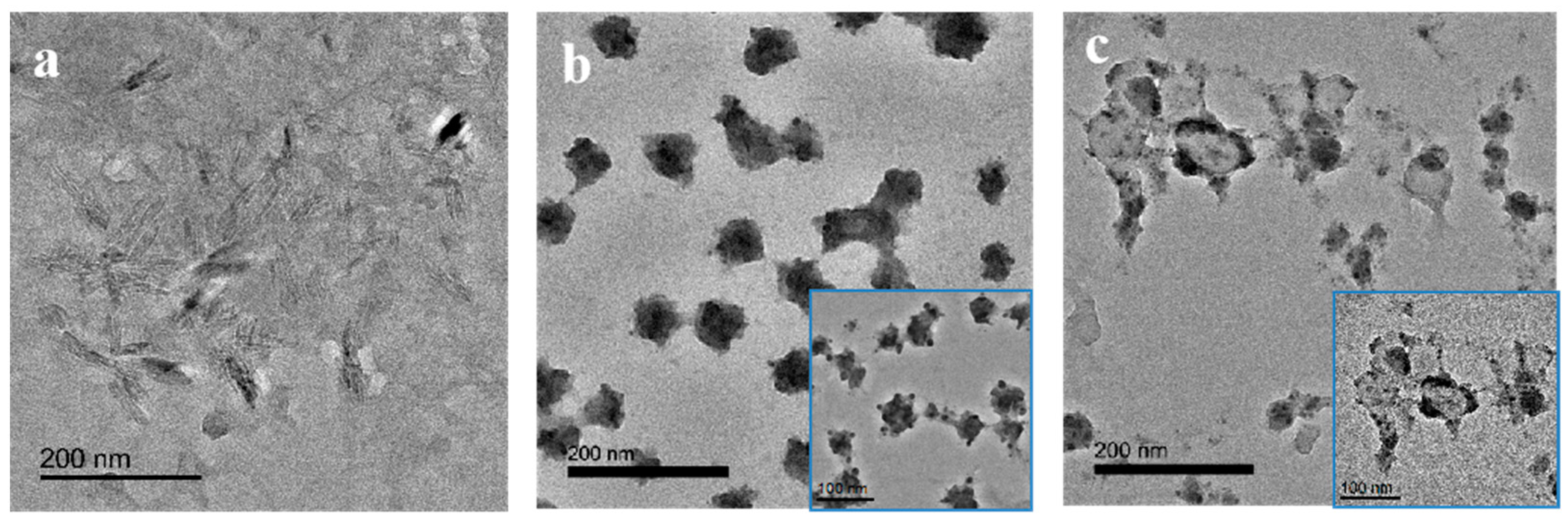
3.1. Preparation of Cellulose Nanocrystals Hollow Microspheres
3.2. Characterization of CNCs-Based Microspheres
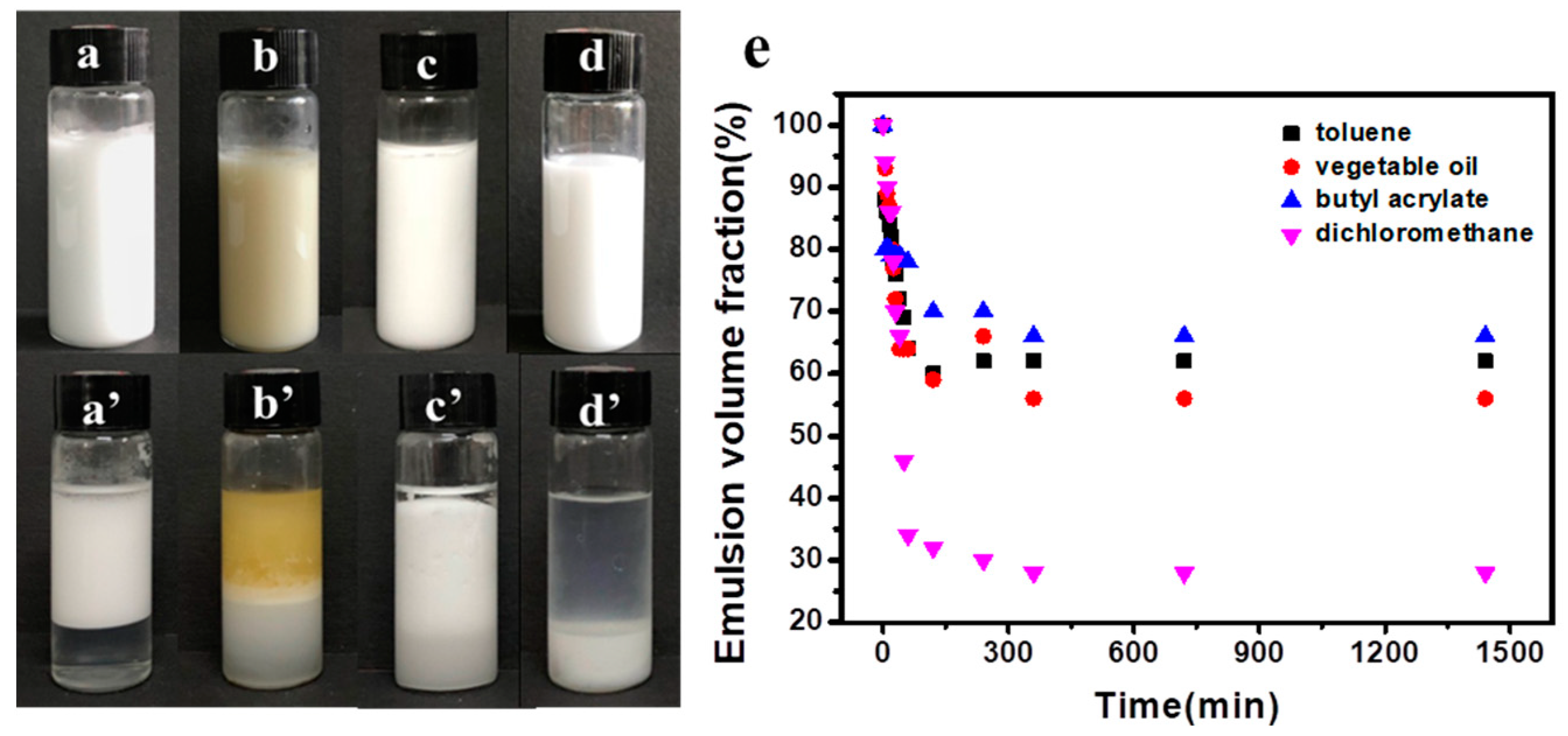
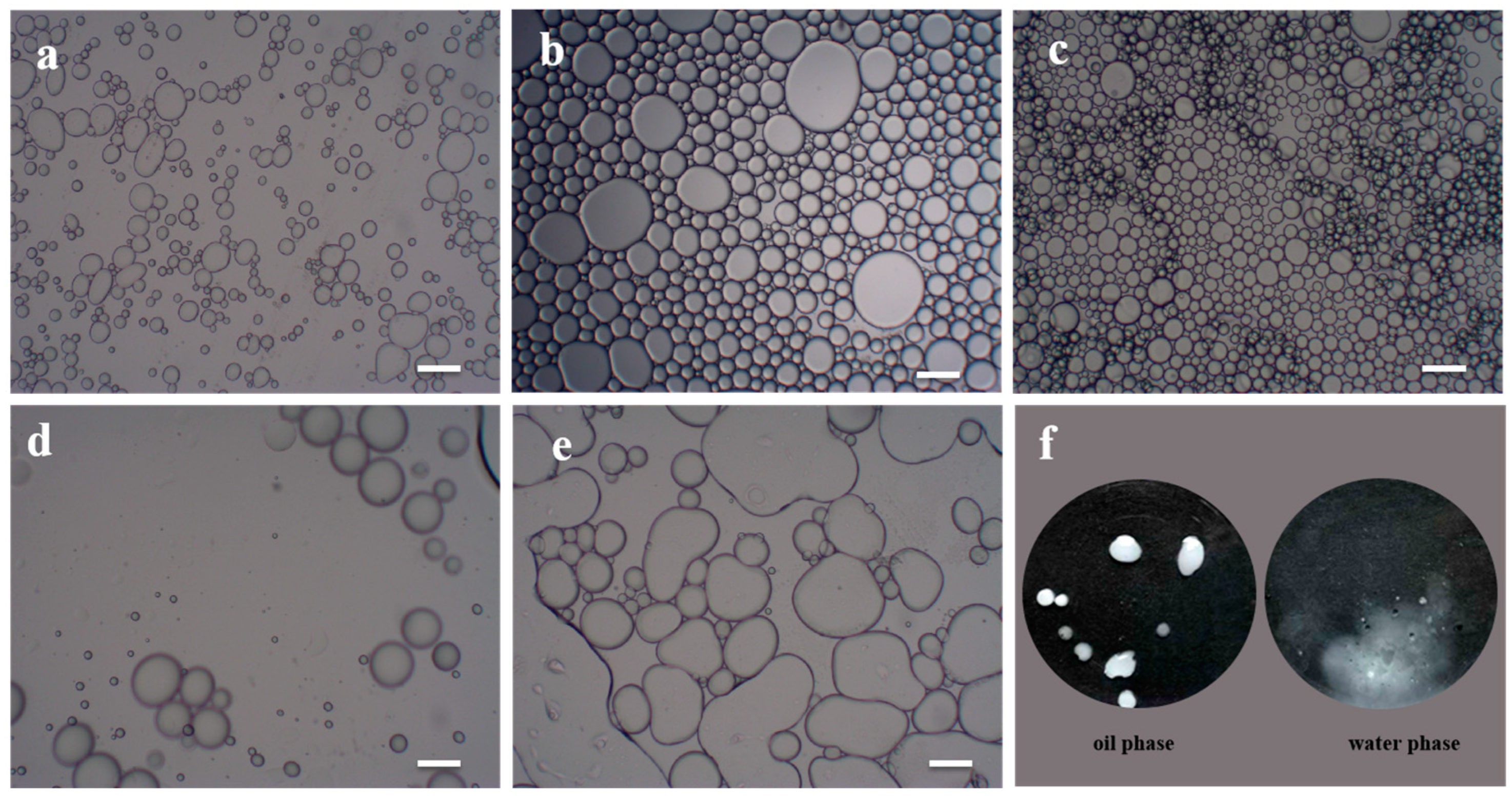
3.3. Modified Cellulose Nanocrystals as Pickering Emulsion Stabilizer
3.4. Cellulose Nanocrystals Hollow Microspheres Finished PET Fabrics
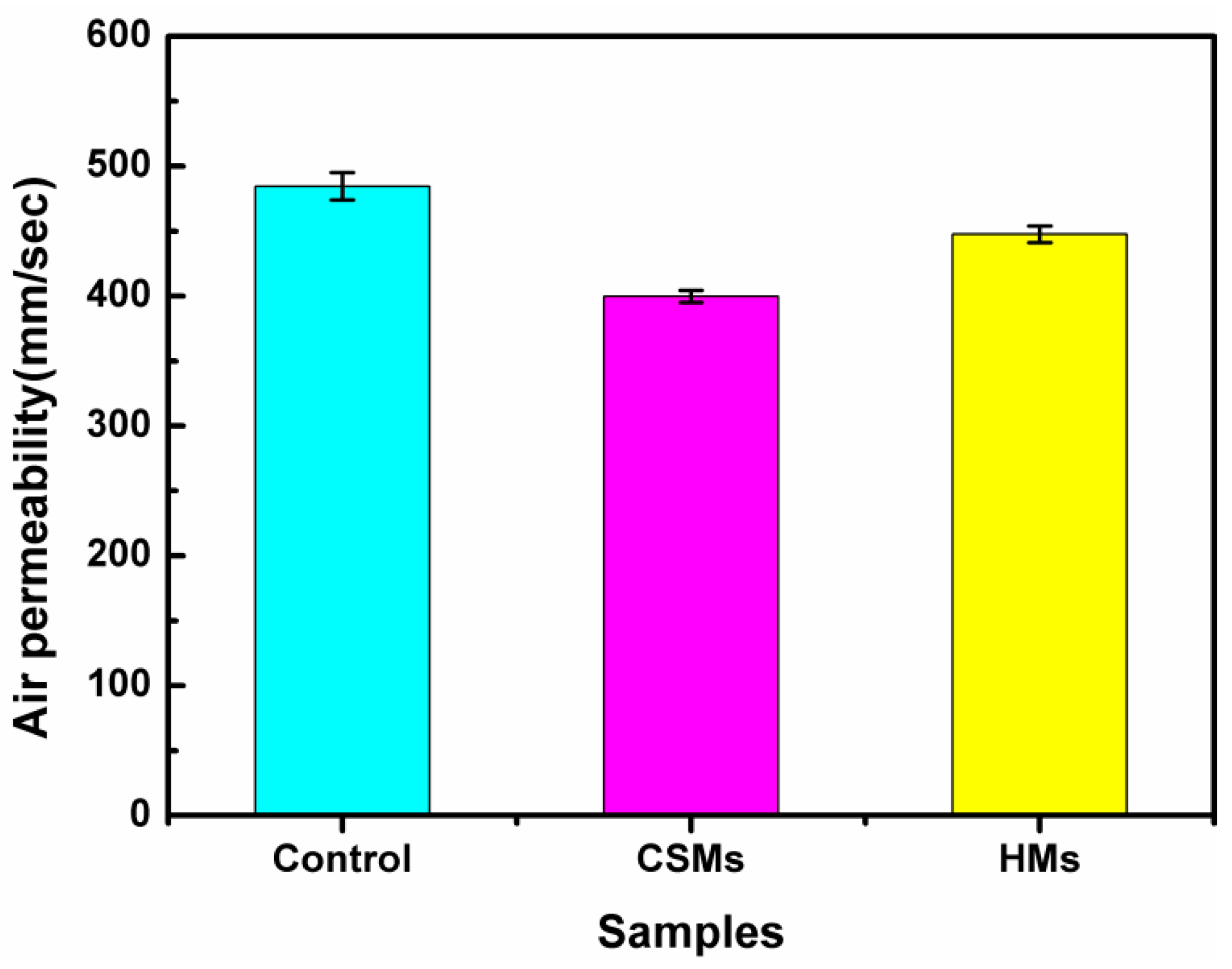
3.5. Breathability and Moisture Permeability of PET Fabrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Li, Y.; Jin, X.; He, Y.; Xiao, C.; Wang, W. Highly hydrophobic conductive polyester fabric based on homogeneous coating surface treatment. Polym.-Plast. Technol. Mater. 2018, 58, 246–254. [Google Scholar] [CrossRef]
- Kim, H.A. Water/moisture vapor permeabilities and thermal wear comfort of the Coolmax/bamboo/tencel included PET and PP composite yarns and their woven fabrics. J. Text. Inst. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Morton, W.E.; Hearle, J.W.S. Physical Properties of Textile Fibers; The Textile Institute, Butterworths: Manchester, UK, 1962; p. 165. [Google Scholar]
- Xu, W.; Liu, X. Surface modification of polyester fabric by corona discharge irradiation. Eur. Polym. J. 2003, 39, 199–202. [Google Scholar] [CrossRef]
- Kim, H.A. Moisture vapor permeability and thermal wear comfort of ecofriendly fiber-embedded woven fabrics for high-performance clothing. Materials 2021, 14, 6205. [Google Scholar] [CrossRef]
- Zaman, M.; Liu, H.; Xiao, H.; Chibante, F.; Ni, Y. Hydrophilic modification of polyester fabric by applying nanocrystalline cellulose containing surface. Carbohydr. Polym. 2013, 91, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Siriviriyanun, A.; O’Rear, E.A.; Yanumet, N. Modification of polyester fabric properties by surfactant-aided surface polymerization. J. Appl. Polym. Sci. 2007, 103, 4059–4064. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Barrier effectiveness and thermal comfort of protective clothing materials. J. Text. Inst. Proc. Abstr. 2007, 98, 87–98. [Google Scholar] [CrossRef]
- Mao, N.; Peng, H.; Quan, Z.; Zhang, H.; Wu, D.; Qin, X.; Wang, R.; Yu, J. Wettability control in tree structure-based 1D fiber assemblies for moisture wicking functionality. ACS Appl. Mater Interfaces 2019, 11, 44682–44690. [Google Scholar] [CrossRef]
- Miao, D.; Huang, Z.; Wang, X.; Yu, J.; Ding, B. Continuous, spontaneous, and directional water transport in the trilayered fibrous membranes for functional moisture wicking textiles. Small 2018, 14, 1801527. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, F.; Qiao, Y.; Xu, Q.; Zhou, J.; Zhang, J. Vi-PDMS incorporated with protein-based coatings designed for permeability-enhanced applications. J. Appl. Polym. Sci. 2018, 135, 46501. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Z.; Miao, D.; Zhao, J.; Yu, J.; Ding, B. Biomimetic fibrous murray membranes with ultrafast water transport and evaporation for smart moisture-wicking fabrics. ACS Nano 2019, 13, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, Y.; Ma, J. Reactive amphiphilic hollow SiO2 Janus nanoparticles for durable superhydrophobic coating. Nanoscale 2020, 12, 16443–16450. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, Y.; Liu, P.; Ma, J.; Zhang, W.; Liu, C.; Simion, D. Novel fabrication of stable Pickering emulsion and latex by hollow silica nanoparticles. J. Colloid Interface Sci. 2019, 553, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ogulata, R.; Mavruz, S. Investigation of porosity and air permeability values of plain knitted fabrics. Fibres Text. East. Eur. 2010, 18, 71–75. [Google Scholar]
- Mousa, H.; Alfadhel, H.; Nasr, E. Engineering and characterization of antibacterial coaxial nanofifiber membranes for oil/water separation. Polymers 2020, 12, 2597. [Google Scholar] [CrossRef]
- Bentz, K.C.; Savin, D.A. Hollow polymer nanocapsules: Synthesis, properties, and applications. Polym. Chem. 2018, 9, 2059–2081. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, J.; Xu, Q.; Zhou, J.; Simion, D.; Carmen, G.; Wang, J.; Li, Y. Hollow casein-based polymeric nanospheres for opaque coatings. ACS Appl. Mater. Interfaces 2016, 8, 11739–11748. [Google Scholar] [CrossRef]
- Hong, Z.; Bo, L.; Guangsu, H. Sound absorption behavior of multiporous hollow polymer micro-spheres. Mater. Lett. 2006, 60, 3451–3456. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P. pH-sensitive fluorescent hepatocyte-targeting multilayer polyelectrolyte hollow microspheres as a smart drug delivery system. Mol. Pharm. 2014, 11, 1599–1610. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, Y.; Ding, D.; Sun, M.; Zhang, L.; Jiang, X.; Yang, C. Hollow chitosan/poly(acrylic acid) nanospheres as drug carriers. Biomacromolecules 2007, 8, 1069–1076. [Google Scholar] [CrossRef]
- Akamatsu, K.; Chen, W.; Suzuki, Y.; Ito, T.; Nakao, A.; Sugawara, T.; Kikuchi, R.; Nakao, S. Preparation of monodisperse chitosan microcapsules with hollow structures using the SPG membrane emulsification technique. Langmuir 2010, 26, 14854–14860. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Wang, C.; Chen, M. Preparation of bowl-like and eggshell-like hollow carbon microspheres from potato starch. Mater. Lett. 2012, 70, 54–56. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Du, W.; Wu, C.; Zhao, J. Intelligent core-shell nanoparticles and hollow spheres based on gelatin and PAA via template polymerization. J. Colloid Interface Sci. 2009, 334, 153–160. [Google Scholar] [CrossRef]
- Reis, A.V.; Guilherme, M.R.; Paulino, A.T.; Muniz, E.C.; Mattoso, L.C.; Tambourgi, E.B. Synthesis of hollow-structured nano-and microspheres from pectin in a nanodroplet emulsion. Langmuir 2009, 25, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.J.; Daiguji, H.; Takemura, F. Factors affecting the size and uniformity of hollow poly(lactic acid) microcapsules fabricated from microbubble templates. J. Phys. Chem. B 2011, 115, 13828–13834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, J.; Xu, Q.; Zhou, J.; Simion, D.; Carmen, G. A facile method for fabricating room-temperature-film-formable casein-based hollow nanospheres. Colloids Surf. A 2015, 484, 329–335. [Google Scholar] [CrossRef]
- Liu, C.; Yao, W.; Zhang, L.; Qian, H.; Wu, W.; Jiang, X. Cell-penetrating hollow spheres based on milk protein. Chem. Commun. 2010, 46, 7566–7568. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yin, B.; Guo, J.; Wang, C. Biocompatible hollow magnetic supraparticles: Ultrafast microwave-assisted synthesis, casein-micelle-mediated cavity formation and controlled drug delivery. J. Mater. Chem. B 2013, 1, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Metaxa, A.F.; Efthimiadou, E.K.; Boukos, N.; Kordas, G. Polysaccharides as a source of advanced materials: Cellulose hollow microspheres for drug delivery in cancer therapy. J. Colloid Interface Sci. 2012, 384, 198–206. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Demina, T.S.; Kilyashova, L.A.; Popyrina, T.N.; Svidchenko, E.A.; Bhuniya, S.; Akopova, T.A.; Grandfifils, C. Polysaccharides as stabilizers for polymeric microcarriers fabrication. Polymers 2021, 13, 3045. [Google Scholar] [CrossRef] [PubMed]
- Hossen, J.M.; Sarkar, S.D.; Uddin, M.M.; Roy, C.K.; Azam, M.S. Mussel-inspired adhesive nano-filler for strengthening polyacrylamide hydrogel. ChemistrySelect 2020, 5, 8906–8914. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Yeo, J.C.C.; Koh, J.J.; Kong, J.; Thitsartarn, W.; Teo, W.S.; He, C. Synergistic toughening of poly(lactic acid)–cellulose nanocrystal composites through cooperative effect of cavitation and crazing deformation mechanisms. ACS Appl. Polym. Mater. 2019, 1, 509–518. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure-property relationship of cellulose nanocrystal-polyvinyl alcohol thin films for high barrier coating applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2019, 11, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A review on the modifification of cellulose and its applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hu, J.; Tang, Y.; Gu, H.; Jiang, M.; Zhang, J. In-situ preparation of hollow cellulose nanocrystals/zeolitic imidazolate framework hybrid microspheres derived from Pickering emulsion. J. Colloid Interface Sci. 2020, 572, 160–169. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, X.; Lou, T. Preparation of millimeter-sized chitosan/carboxymethyl cellulose hollow capsule and its dye adsorption properties. Carbohydr. Polym. 2020, 244, 116481. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, M.; Gabriel, M.S.; Teixeira Neto, A.A.; da Silva Bernardes, J.; Berry, R.; Tam, K.C. Polymeric hollow microcapsules (PHM) via cellulose nanocrystal stabilized Pickering emulsion polymerization. J. Colloid Interface Sci. 2019, 555, 489–497. [Google Scholar] [CrossRef]
- Jung, J.L.; Dong, S.J. Evaluation of liquid moisture management properties on hemp woven fabrics treated with liquid ammonia. Text. Res. J. 2017, 87, 1752–1764. [Google Scholar]
- Li, Y.; Xu, W.; Yeung, K.W. Moisture Management of Textiles. U.S. Patent 6499338B2, 2000. [Google Scholar]
- Huang, J.F.; New, J.; Tham, J.B.; Tok, A. Novel moisture management test of polyethylene terephthalate and nylon fabric under stretching and surface patterning. Text. Res. J. 2018, 88, 69–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Han, L.; Wang, B.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z.; Sui, X. Biodegradable regenerated cellulose-dispersed composites with improved properties via a pickering emulsion process. Carbohydr. Polym. 2018, 179, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhou, J.; Li, H.; Zhao, J. Nanocrystalline cellulose/fluorinated polyacrylate latex via RAFT-mediated surfactant-free emulsion polymerization and its application as waterborne textile finishing agent. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1305–1314. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, H.; Yao, H. Cellulose nanocrystals/fluorinated polyacrylate soap-free emulsion prepared via RAFT-assisted Pickering emulsion polymerization. Colloids Surf. B 2019, 177, 321–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Karimkhani, V.; Makowski, B.T.; Samaranayake, G.; Rowan, S.J. Nanoemulsions and nanolatexes stabilized by hydrophobically functionalized cellulose nanocrystals. Macromolecules 2017, 50, 6032–6042. [Google Scholar] [CrossRef]
- Yu, Q.; Yang, W.; Wang, Q.; Dong, W.; Du, M.; Ma, P. Functionalization of cellulose nanocrystals with gamma-MPS and its effect on the adhesive behavior of acrylic pressure sensitive adhesives. Carbohydr. Polym. 2019, 217, 168–177. [Google Scholar] [CrossRef]
- Xu, P.; Cao, Y.; Wu, B.; Ma, P.; Dong, W.; Bai, H.; Zhang, H.; Zhu, H.; Chen, M. Effects of modified nanocrystalline cellulose on the hydrophilicity, crystallization and mechanical behaviors of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). New J. Chem. 2018, 42, 11972–11978. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Pelegrini, B.L.; Fernandes, F.M.B.; Fernandes, T.; de Oliveira, J.H.; Rosseto, H.C.; Junior, A.G.O.; Reis, A.V.; Castelani, E.V.; Sobral, F.N.C.; Shirabayashi, W.V.I.; et al. Novel green strategy to improve the hydrophobicity of cellulose nanocrystals and the interfacial elasticity of Pickering emulsions. Cellulose 2021, 28, 6201–6238. [Google Scholar] [CrossRef]
- Tang, C.; Spinney, S.; Shi, Z.; Tang, J.; Peng, B.; Luo, J.; Tam, K.C. Amphiphilic cellulose nanocrystals for enhanced pickering emulsion stabilization. Langmuir 2018, 34, 12897–12905. [Google Scholar] [CrossRef]
- Chen, Q.H.; Liu, T.X.; Tang, C.H. Tuning the stability and microstructure of fine Pickering emulsions stabilized by cellulose nanocrystals. Ind. Crops Prod. 2019, 141, 11733. [Google Scholar] [CrossRef]
- He, Y.; Wu, F.; Sun, X.; Li, R.; Guo, Y.; Li, C.; Zhang, L.; Xing, F.; Wang, W.; Gao, J. Factors that affect Pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Wu, J.; Sun, G.; Nan, F.; Ngai, T.; Ma, G. Systematic studies of Pickering emulsions stabilized by uniform-sized PLGA particles: Preparation and stabilization mechanism. J. Mater. Chem. B 2014, 2, 7605–7611. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose processing properties and potential applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Binks, B.P.; Desforges, A. Synergistic stabilization of emulsions by a mixture of surface-active nanoparticles and surfactant. Langmuir 2007, 23, 1098–1106. [Google Scholar] [CrossRef]
- Holler, R.P.; Dulle, M.; Thoma, S.; Mayer, M.; Steiner, A.M.; Forster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-assisted assembly of modular 3d plasmonic raspberry-like core/satellite nanoclusters: Correlation of structure and optical properties. ACS Nano 2016, 10, 5740–5750. [Google Scholar] [CrossRef] [Green Version]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Li, Z.; Ngai, T. Microgel particles at the fluid-fluid interfaces. Nanoscale 2013, 5, 1399–1410. [Google Scholar] [CrossRef]
- Wang, K.; Fu, C.; Wang, R.; Tao, G.; Xia, Z. High-resilience cotton base yarn for anti-wrinkle and durable heat-insulation fabric. Compos. Part B. 2021, 212, 108663. [Google Scholar] [CrossRef]
- Ahmad, F.; Mushtaq, B.; Butt, F.A.; Zafar, M.S.; Ahmad, S.; Afzal, A.; Nawab, Y.; Rasheed, A.; Ulker, Z. Synthesis and characterization of nonwoven cotton-reinforced cellulose hydrogel for wound dressings. Polymers 2021, 13, 4098. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.J.; Chiang, M.Y.; Wang, E.T.; Wu, T.M. Synthesis, characterization, and physical properties of maleic acid-grafted poly (butylene adipate-coterephthalate)/cellulose nanocrystal composites. Polymers 2022, 14, 2742. [Google Scholar] [CrossRef] [PubMed]
- McQueen, R.H.; Batcheller, J.C.; Mah, T.; Hooper, P.M. Development of a protocol to assess fabric suitability for testing liquid moisture transport properties. J. Text. Inst. 2013, 104, 900–905. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, Y.; Wang, W.; Yu, D. Moisture absorption, perspiration and thermal conductive polyester fabric prepared by thiol–ene click chemistry with reduced graphene oxide finishing agent. J. Mater. Sci. 2018, 53, 14262–14273. [Google Scholar] [CrossRef]




| Index | Pristine PET | CSMs Finished PET | HMs Finished PET | |
|---|---|---|---|---|
| Wetting time (s) | Top | 8.16 | 9.75 | 6.38 |
| Bottom | 16.41 | 10.88 | 7.59 | |
| Absorption rate (%/s) | Top | 35.21 | 47.79 | 43.66 |
| Bottom | 8.50 | 18.56 | 38.92 | |
| Max wetted radius manufacture breathability and manufacture breathability and (mm) | Top | 5.0 | 10.0 | 10.0 |
| Bottom | 5.0 | 10.0 | 25.0 | |
| Spreading speed (mm/s) | Top | 0.59 | 0.90 | 1.34 |
| Bottom | 0.30 | 0.85 | 2.71 | |
| One-way transported index (%) | - | −445.60 | −378.11 | 936.33 |
| The overall moisture management capability | - | 0 | 0.024 | 0.723 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Song, B.; Li, Y.; Zhou, Y.; Wang, Y.; Xu, Q.; Ma, J. Breathability and Moisture Permeability of Cellulose Nanocrystals Hollow Microsphere Coatings for PET Fabrics. Polymers 2022, 14, 5345. https://doi.org/10.3390/polym14245345
Zhang F, Song B, Li Y, Zhou Y, Wang Y, Xu Q, Ma J. Breathability and Moisture Permeability of Cellulose Nanocrystals Hollow Microsphere Coatings for PET Fabrics. Polymers. 2022; 14(24):5345. https://doi.org/10.3390/polym14245345
Chicago/Turabian StyleZhang, Fan, Bingyao Song, Yilin Li, Yingying Zhou, Yanbing Wang, Qunna Xu, and Jianzhong Ma. 2022. "Breathability and Moisture Permeability of Cellulose Nanocrystals Hollow Microsphere Coatings for PET Fabrics" Polymers 14, no. 24: 5345. https://doi.org/10.3390/polym14245345
APA StyleZhang, F., Song, B., Li, Y., Zhou, Y., Wang, Y., Xu, Q., & Ma, J. (2022). Breathability and Moisture Permeability of Cellulose Nanocrystals Hollow Microsphere Coatings for PET Fabrics. Polymers, 14(24), 5345. https://doi.org/10.3390/polym14245345







