Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Synthesis of PANI Electrode
2.2. Characterization of PANI Electrode
3. Results and Discussion
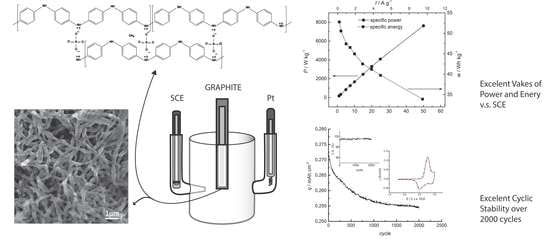
3.1. Electrochemical Synthesis of PANI Electrodes
3.2. Electrochemical Characterization
3.3. Electrical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef]
- Qu, K.; Bai, Y.; Gao, X.; Deng, M. Application of poly (aniline-co-o-methoxyaniline) as energy storage material. Synth. Met. 2020, 26, 116346. [Google Scholar] [CrossRef]
- Mahdavi, H.; Shahalizade, T. Investigation of the pseudocapacitive properties of polyaniline nanostructures obtained from scalable chemical oxidative synthesis routes. Ionics 2019, 25, 1331–1340. [Google Scholar] [CrossRef]
- Gvozdenović, M.M.; Jugović, B.Z.; Jokić, B.M.; Džunuzović, E.S.; Grgur, B.N. Electrochemical synthesis and characterization of poly(o-toluidine) as high energy storage material. Electrochim. Acta 2019, 317, 746–752. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application progress of polyaniline, polypyrrole and polythiophene in lithium-sulfur batteries. Polymers 2020, 12, 331. [Google Scholar] [CrossRef]
- Han, X.; Xiao, G.; Wang, Y.; Chen, X.; Duan, G.; Wu, Y.; Gong, X.; Wang, H. Design and fabrication of conductive polymer hydrogels and their applications in flexible supercapacitors. J. Mater. Chem. A 2020, 8, 23059–23095. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Z. Redox electrode materials for supercapatteries. J. Power Sources 2016, 326, 604–612. [Google Scholar] [CrossRef]
- Brousse, T.; Daniel, B. To Be or Not to Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, 5185–5189. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Yang, W.; Jian, S.; Zhang, C.; Duan, G. One step activation by ammonium chloride toward N-doped porous carbon from camellia oleifera for supercapacitor with high specific capacitance and rate capability. Diam. Relat. Mater. 2022, 130, 109526. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, L.; Chen, L.; Wang, F.; He, S.; Jiang, S.; Zhang, Q. ZnCl2 regulated flax-based porous carbon fibers for supercapacitors with good cycling stability. New J. Chem. 2021, 45, 22602–22609. [Google Scholar] [CrossRef]
- Contractor, A.Q.; Juvekar, V.A. Estimation of Equilibrium Capacitance of Polyaniline Films Using Step Voltammetry. J. Electrochem. Soc. 2015, 162, 1175–1181. [Google Scholar] [CrossRef]
- Alguail, A.A.; Al-Eggiely, A.H.; Grgur, B.N. Polyaniline–lead sulfate based cell with supercapattery behavior. J. Saudi Chem. Soc. 2017, 21, 575–582. [Google Scholar] [CrossRef]
- Grgur, B.N.; Gvozdenović, M.M.; Jugović, B.Z.; Trišović, T.L. Characteristics of the citrate-based zinc–polyaniline secondary cell with supercapattery behaviour. J. Serbian Chem. Soc. 2019, 84, 1261–1270. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of conducting polymers–persistent models and new concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef]
- Soni, R.; Kashyap, V.; Nagaraju, D.; Kurungot, S. Realizing High Capacitance and Rate Capability in Polyaniline by Enhancing the Electrochemical Surface Area through Induction of Superhydrophilicity. ACS Appl. Mater. Interfaces 2018, 10, 676–686. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Luo, X.; Cao, Y.; Gong, X.; Xu, W. Molecular Engineering of Polyaniline with Ultrathin Polydopamine and Monolayer Graphene for All-Solid-State Flexible Microsupercapacitors. ACS Appl. Energy Mater. 2021, 4, 1069–10080. [Google Scholar] [CrossRef]
- He, Y.; Han, X.; Du, Y.; Zhang, B.; Xu, P. Heteroatom-doped carbon nanostructures derived from conjugated polymers for energy applications. Polymers 2016, 8, 366. [Google Scholar] [CrossRef]
- Vega-Rios, A.; Rentería-Baltiérrez, F.Y.; Hernández-Escobar, C.A.; Zaragoza-Contreras, E.A. A new route toward graphene nanosheet/polyaniline composites using a reactive surfactant as polyaniline precursor. Synth. Met. 2013, 184, 52–60. [Google Scholar] [CrossRef]
- Mitchell, E.; Candler, J.; De Souza, F.; Gupta, R.K.; Gupta, B.K.; Dong, L.F. High performance supercapacitor based on multilayer of polyaniline and graphene oxide. Synth. Met. 2015, 199, 214–218. [Google Scholar] [CrossRef]
- Tang, W.; Peng, L.; Yuan, C.; Wang, J.; Mo, S.; Zhao, C.; Yu, Y.; Min, Y.; Epstein, A.J. Facile synthesis of 3D reduced graphene oxide and its polyaniline composite for super capacitor application. Synth. Met. 2015, 202, 140–146. [Google Scholar] [CrossRef]
- Li, K.; Guo, D.; Chen, J.; Kong, Y.; Xue, H. Oil-water interfacial synthesis of graphene-polyaniline-MnO hybrids using binary oxidant for high performance supercapacitor. Synth. Met. 2015, 209, 555–560. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhong, Q.; Qin, Y.; Xu, A.; Li, W.; Shi, J. In Situ Synthesis of Trifluoroacetic Acid-Doped Polyaniline/Reduced Graphene Oxide Composites for High-Performance All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 8774–8785. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, D.M.; Jung, J.Y.; Kim, M.J.; Khil, M.-S.; Jeong, S.H.; Kim, N.D. Hybrid Polyaniline/Liquid Crystalline CNT Fiber Composite for Ultimate Flexible Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 1130–1142. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, B.; Luo, J.; Gu, Y.; Liu, X. Synthesis of Polyaniline@MnO2/Graphene Ternary Hybrid Hollow Spheres via Pickering Emulsion Polymerization for Electrochemical Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 7721–7730. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, H.; Tian, D.; Li, Q.; Zhong, M.; Chen, J.; Hu, C.; Ji, H. Controllable Synthesis, Core-Shell Nanostructures, and Supercapacitor Performance of Highly Uniform Polypyrrole/Polyaniline Nanospheres. ACS Appl. Energy Mater. 2021, 4, 3701–3711. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Han, J.; Wang, T.; Leng, Y.; Wang, Y.; Li, T.; Han, Y. Preparation of Polyaniline onto dl -Tartaric Acid Assembled MXene Surface as an Electrode Material for Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 9326–9336. [Google Scholar] [CrossRef]
- Zotti, G.; Cattarin, S.; Comisso, N. Electrodeposition of polythiophene, polypyrrole and polyaniline by the cyclic potential sweep method. J. Electroanal. Chem. Interfacial Electrochem. 1987, 235, 259–273. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Jugović, B.; Gvozdenović, M.; Stevanović, J.; Trišović, T.; Grgur, B. Characterization of electrochemically synthesized PANI on graphite electrode for potential use in electrochemical power sources. Mater. Chem. Phys. 2009, 114, 939–942. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chu, Q.; Wang, Z.; Zhang, F.; Wang, S. Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J. Power Sources 2009, 190, 578–586. [Google Scholar] [CrossRef]
- Grgur, B.N. On the Question of Energy and Power Potentials of the Electrode Materials in the Rechargeable Cells. Int. J. Electrochem. Sci. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, W.; Du, G.; Wang, D.; Zhu, J.; Zhu, X.; Pezzotti, G. Electrochimica Acta Two-step method for synthesizing polyaniline with bimodal nanostructures for high performance supercapacitors. Electrochim. Acta 2018, 282, 286–294. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.; Huang, Y.; Bu, F.; Xu, Y. Integration of ultrathin graphene/polyaniline composite nanosheets with a robust 3D graphene framework for highly flexible all-solid-state supercapacitors with superior energy density and exceptional cycling stability. J. Mater. Chem. A 2017, 5, 5466–5474. [Google Scholar] [CrossRef]
- Ding, J.; Chen, P.; Chen, X.; Guo, K. Self-Assemble Strategy to Fabricate High Polyaniline Loading Nanocarbon Hydrogels for Flexible All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 3766–3776. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; He, S.; Du, H.; Liu, K.; Zhang, C.; Jiang, S. Facile Electrodeposition of NiCo2O4 Nanosheets on Porous Carbonized Wood for Wood-Derived Asymmetric Supercapacitors. Polymers 2022, 14, 2521. [Google Scholar] [CrossRef]












| Qp/mAh | mPANI/mg | Cg,tot/F g−1 | Cg/F g−1 | (Cg/Cg,tot)/% |
|---|---|---|---|---|
| 0.125 | 0.22 | 481 | 378 | 78 |
| 0.250 | 0.43 | 290 | 248 | 85 |
| 0.500 | 0.87 | 513 | 375 | 73 |
| 0.750 | 1.30 | 436 | 318 | 73 |
| 1.00 | 1.73 | 550 | 404 | 73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gojgić, J.; Petrović, M.; Jugović, B.; Jokić, B.; Grgur, B.; Gvozdenović, M. Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior. Polymers 2022, 14, 5365. https://doi.org/10.3390/polym14245365
Gojgić J, Petrović M, Jugović B, Jokić B, Grgur B, Gvozdenović M. Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior. Polymers. 2022; 14(24):5365. https://doi.org/10.3390/polym14245365
Chicago/Turabian StyleGojgić, Jelena, Miloš Petrović, Branimir Jugović, Bojan Jokić, Branimir Grgur, and Milica Gvozdenović. 2022. "Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior" Polymers 14, no. 24: 5365. https://doi.org/10.3390/polym14245365
APA StyleGojgić, J., Petrović, M., Jugović, B., Jokić, B., Grgur, B., & Gvozdenović, M. (2022). Electrochemical and Electrical Performances of High Energy Storage Polyaniline Electrode with Supercapattery Behavior. Polymers, 14(24), 5365. https://doi.org/10.3390/polym14245365