Molecular Dynamics Simulation of Poly(Ether Ether Ketone) (PEEK) Polymer to Analyze Intermolecular Ordering by Low Wavenumber Raman Spectroscopy and X-ray Diffraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Approximate Raman Signal from MD Simulation
2.2. Computation Details
2.3. Experiments
3. Results and Discussion
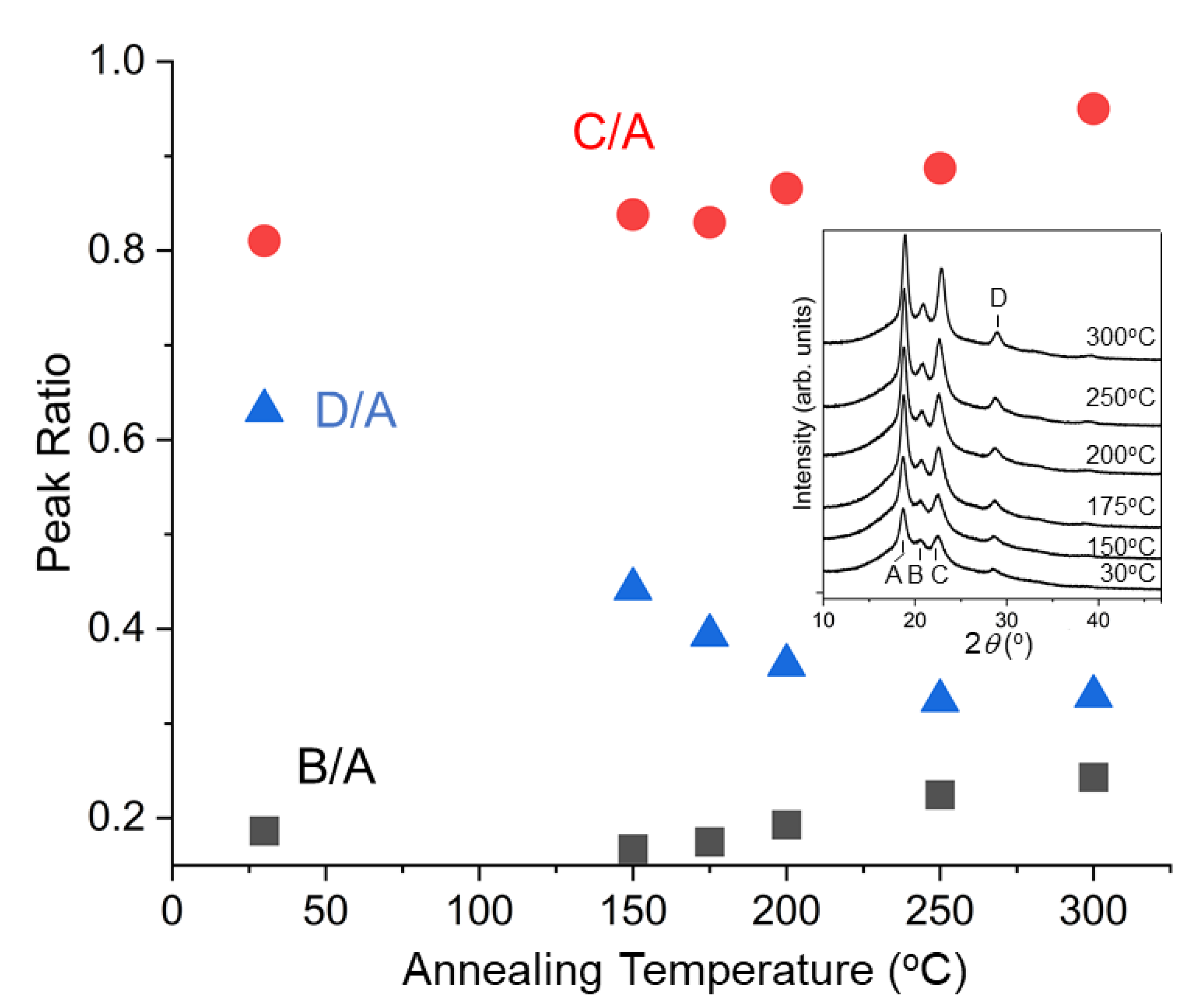
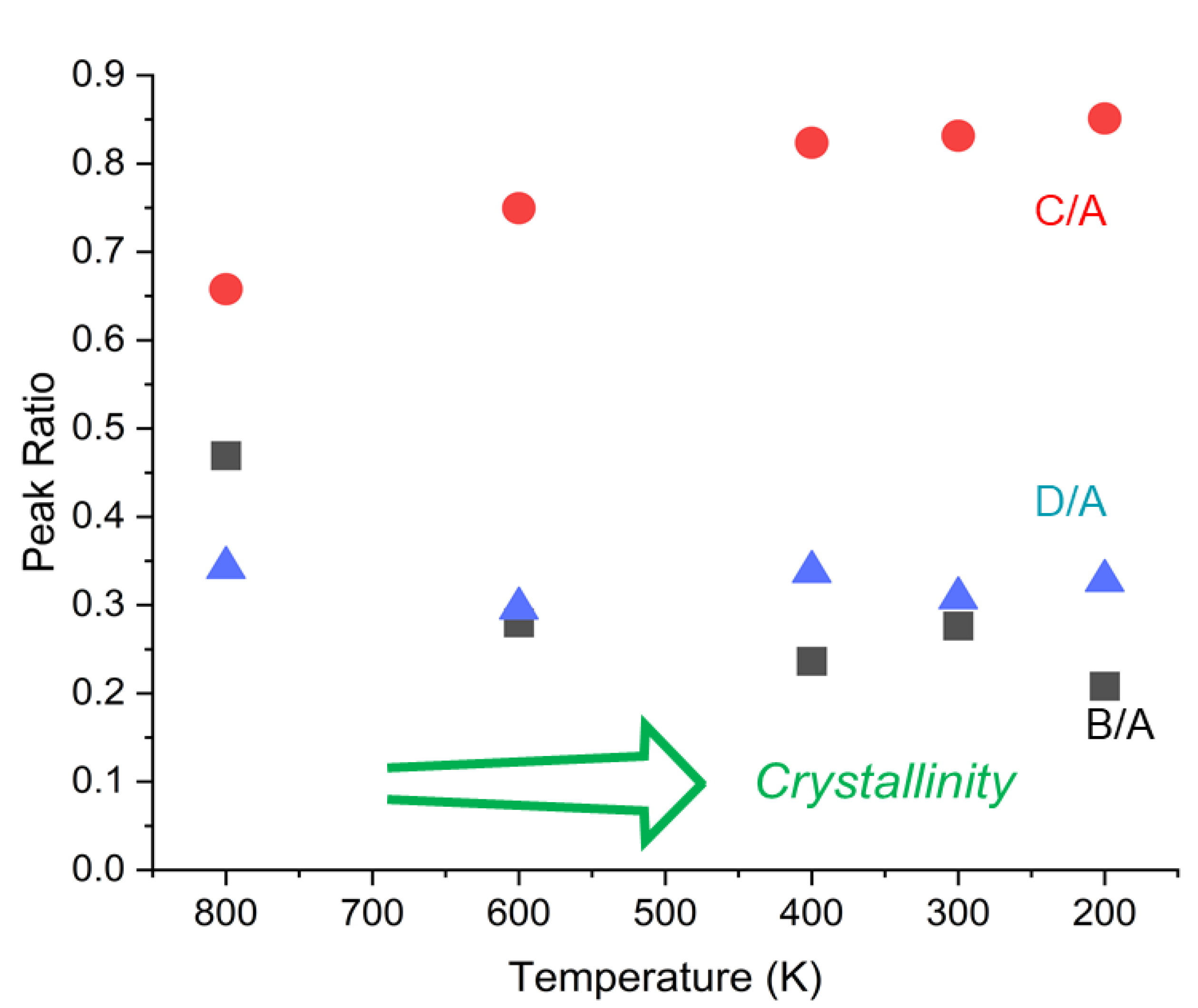
3.1. Experimental Results
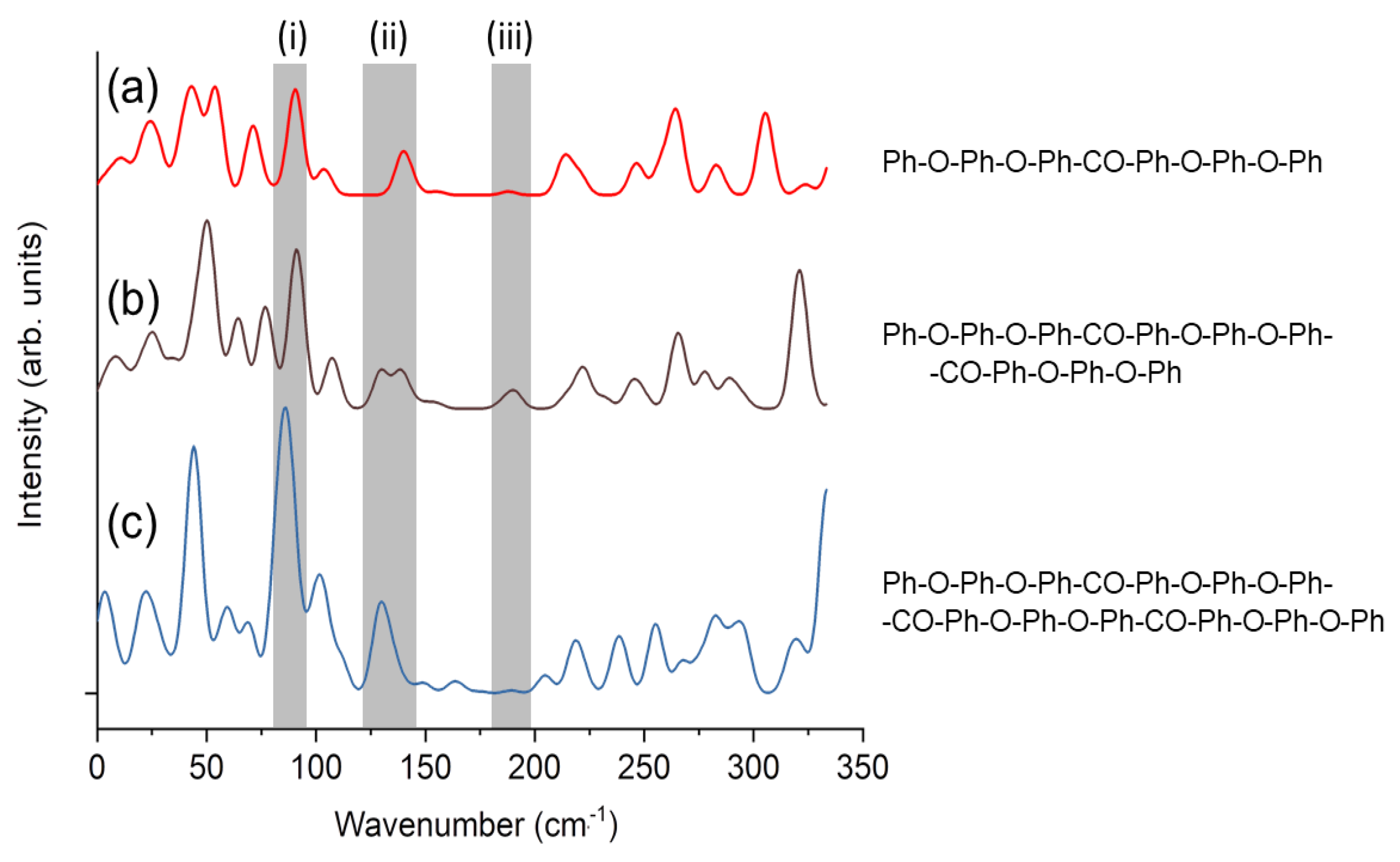
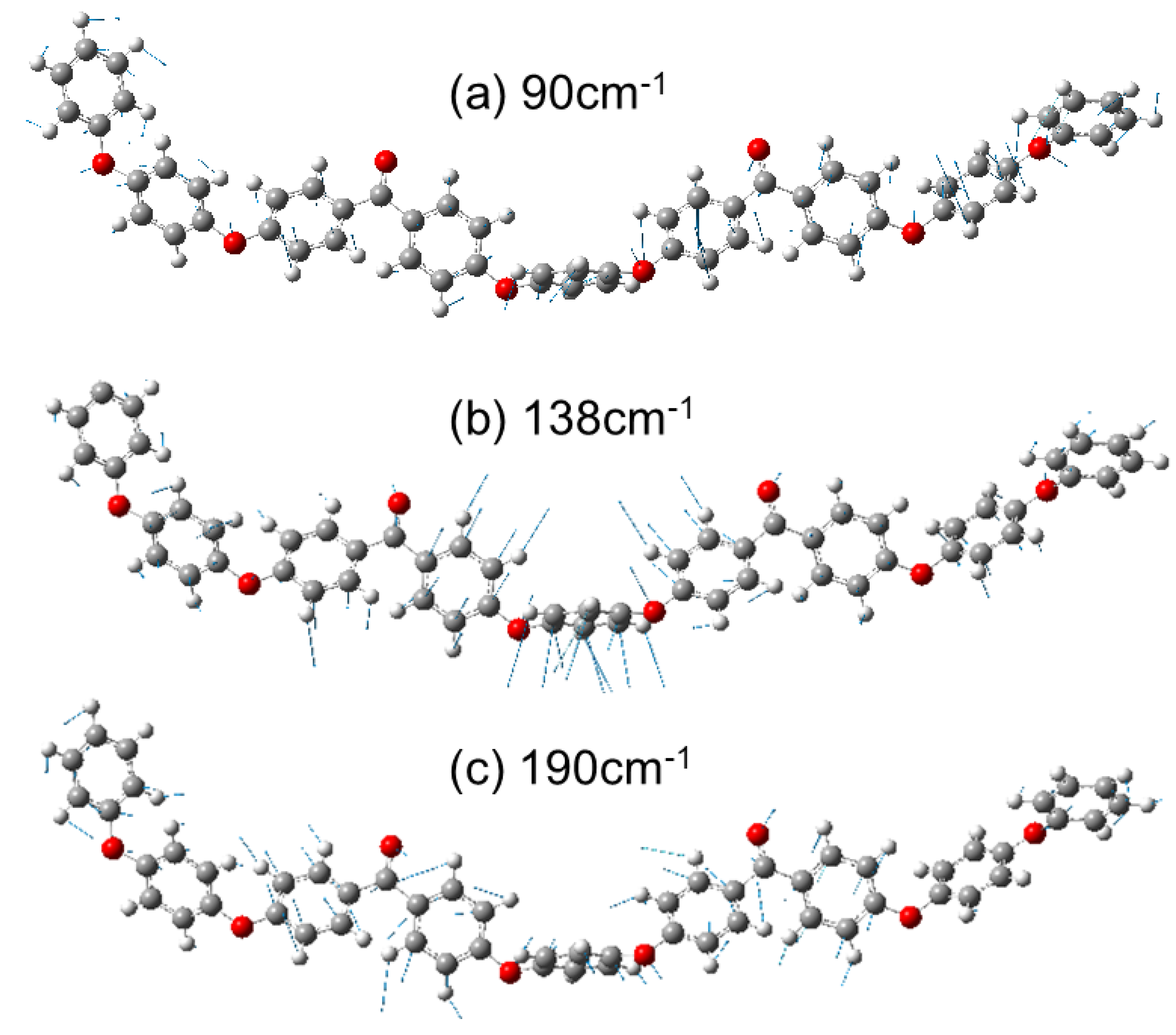
3.2. Calculated Raman Spectroscopy of a Single Molecule PEEK Chain
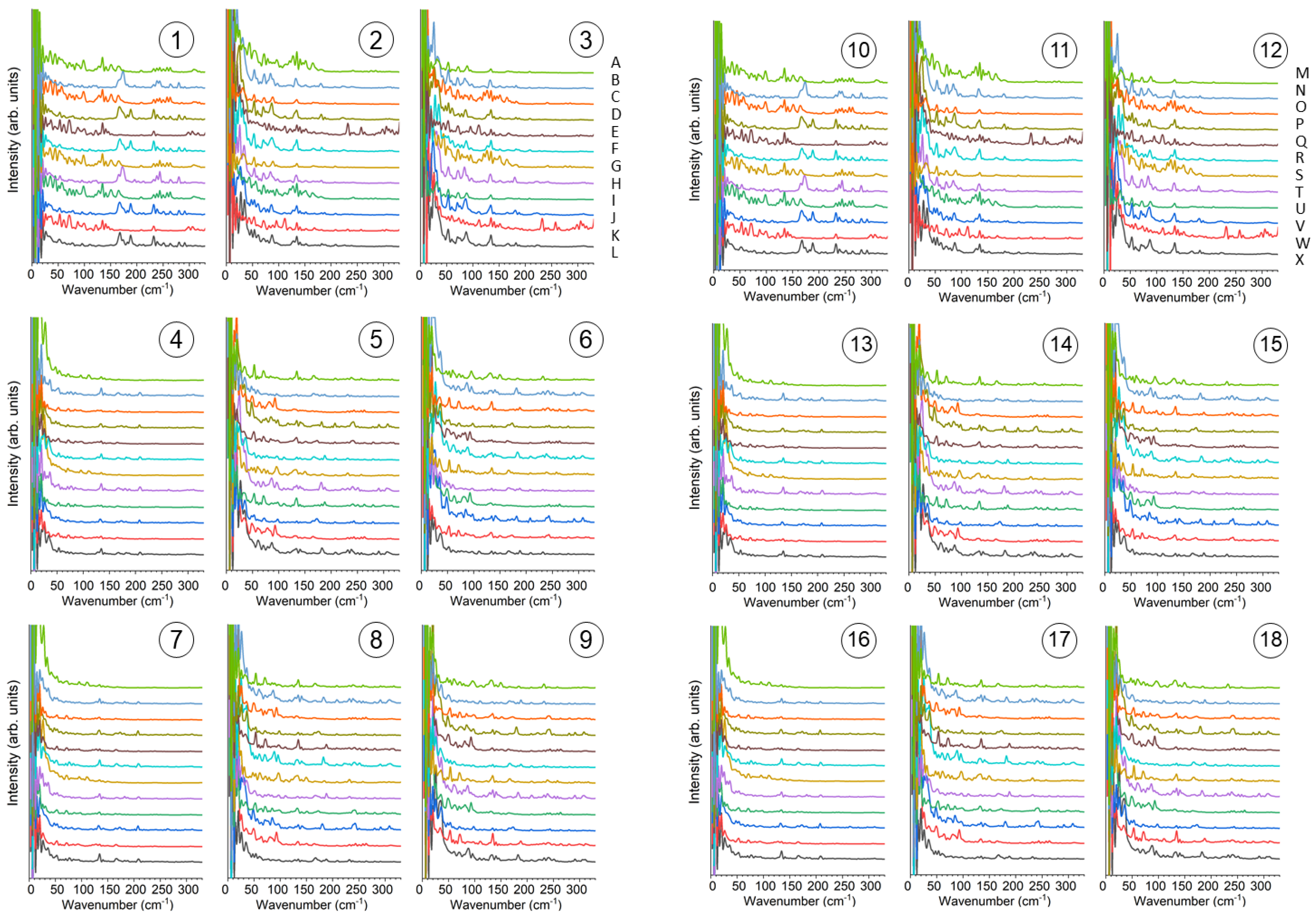
3.3. MD Simulations
3.4. Local Vibrational Spectra Simulated from MD
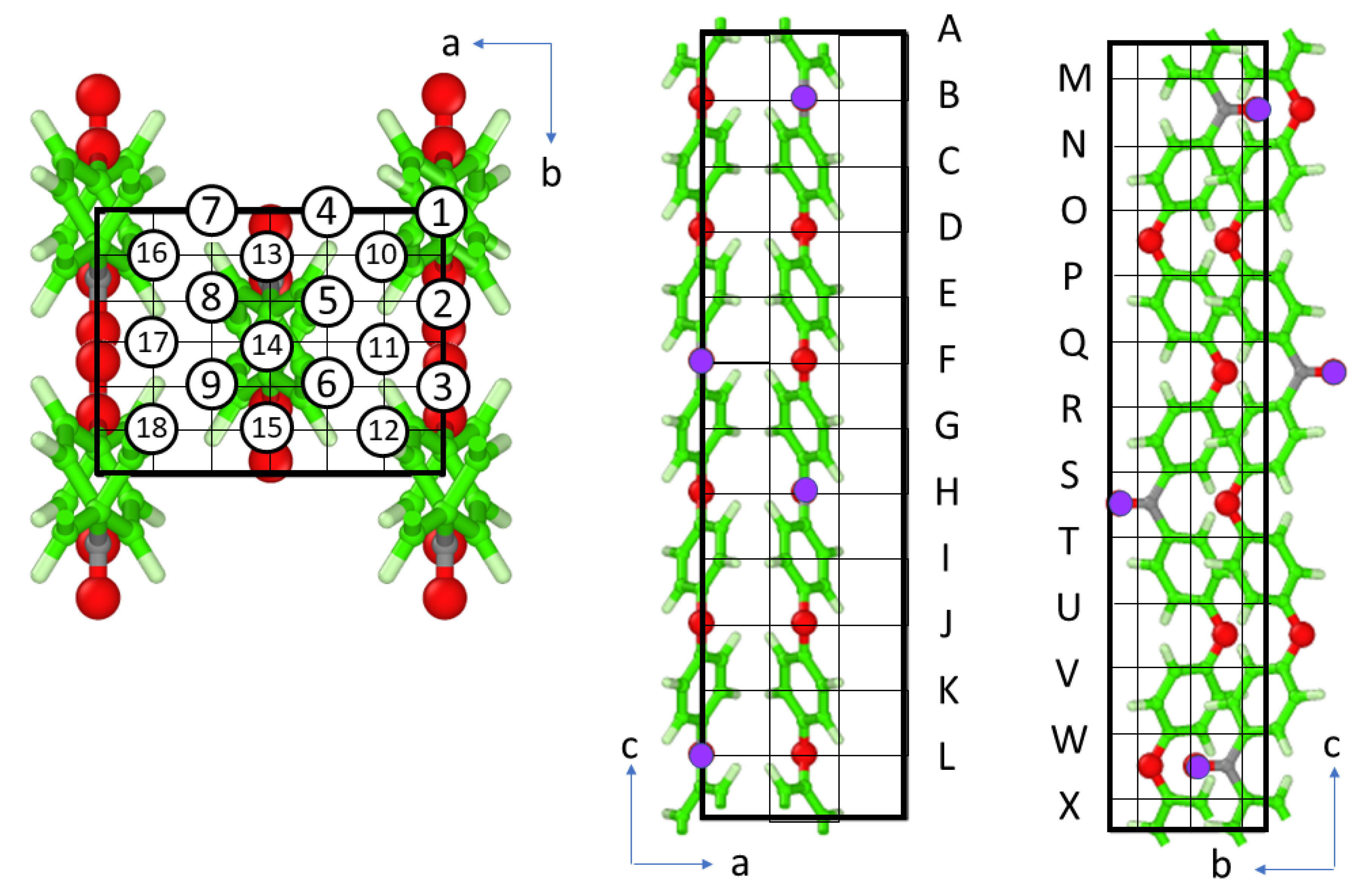
3.5. WAXRD Pattern from MD Simulation
3.6. Future Improvement of the Present Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attwood, T.E.; Dawson, P.C.; Freeman, J.L.; Hoy, L.R.J.; Rose, J.B.; Staniland, P.A. Synthesis and properties of polyaryletherketones. Polymer 1981, 22, 1096–1103. [Google Scholar] [CrossRef]
- Biron, M. Thermoplastics and Thermoplastic Composites, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Regis, M.; Bellare, A.; Pascolini, T.; Bracco, P. Characterization of thermally annealed PEEK and CFR-PEEK composites: Structure-properties relationships. Polym. Degrad. Stab. 2017, 136, 121. [Google Scholar] [CrossRef]
- Regis, M.; Zanetti, M.; Pressacco, M.; Bracco, P. Opposite role of different carbon fiber reinforcements on the non-isothermal crystallization behavior of poly(etheretherketone). Mater. Chem. Phys. 2016, 179, 223. [Google Scholar] [CrossRef]
- Lee, Y.; Porter, R.S. Crystallization of poly(etheretherketone) (PEEK) in carbon fiber composites. Polym. Eng. Sci. 1986, 25, 633. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delb, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kobayashi, S.; Numata, T.; Kamihara, N.; Shimada, T.; Jikei, M.; Muraoka, M.; Barnsley, J.E.; Fraser-Miller, S.J.; Gordon, K.C. Evaluation of crystallinity in carbon fiber-reinforced poly(ether ether ketone) by using infrared low frequency Raman spectroscopy. J. Appl. Polym. Sci. 2021, 139, e51677. [Google Scholar] [CrossRef]
- Hsu, S.L.; Patel, J.; Zhao, W. Chapter 10-vibrational spectroscopy of polymers. In Molecular Characterization of Polymers: A Fundamental Guide; Elsevier: Amsterdam, The Netherlands, 2021; pp. 369–407. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Morina, C.; Zhang, X.; Araki, T.; Dynesa, J.; Stövera, H.; Brash, J.; Lawrence, J.R.; Leppard, G.G. Soft X-ray spectromicroscopy of biological and synthetic polymer systems. J. Electron Spectrosc. Relat. Phenom. 2005, 144, 259–269. [Google Scholar] [CrossRef]
- Mortensen, K.; Talmon, Y. Cryo-TEM and SANS microstructural study of pluronic polymer solutions. Macromoelcules 1995, 28, 8829–8834. [Google Scholar] [CrossRef]
- Ellis, G.; Naffakh, M.; Marco, C.; Hendra, P.J. Fourier transform Raman spectroscopy in the study of technological polymers Part 1: Poly(aryl ether ketones), their composites and blends. Spectrochim. Acta A 1997, 53, 2279–2294. [Google Scholar] [CrossRef]
- Louden, J.D. Crystallinity in poly(aryl ether ketone) films studied by Raman spectroscopy. Polym. Commun. 1986, 27, 82–84. [Google Scholar]
- Agbenyega, J.K.; Ellis, G.; Hendra, P.J.; Maddams, W.F.; Passingham, C.; Willis, H.A.; Chalmers, J. Applications of Fourier Transform Raman spectroscopy in the synthetic polymer field. Spectrochim. Acta A 1990, 46, 197–216. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Stuart, B.H.; Thomas, P.S.; Williams, D.R. A comparison of thermal- and solvent-induced relaxation of poly(ether ether ketone) using Fourier transform Raman spectroscopy. Spectrochim. Acta A 1991, 47, 1299–1303. [Google Scholar] [CrossRef]
- Stuart, B.H.; Briscoe, B.J. A Fourier transform Raman spectroscopy study of poly (ether ether ketone)/polytetrafluoroethylene (PEEK/PTFE) blends. Spectrochim. Acta A 1994, 50, 2005–2009. [Google Scholar] [CrossRef]
- Lipiäinen, T.; Fraser-Miller, S.J.; Gordon, K.C.; Strachan, C.J. Direct comparison of low- and mid-frequency Raman spectroscopy for quantitative solid-state pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 149, 343. [Google Scholar] [CrossRef]
- Bouř, P.; Sopková, J.; Bednárová, L.; Malon, P.; Keiderling, T.A. Transfer of molecular property tensors in cartesian coordinates: A new algorithm for simulation of vibrational spectra. J. Comp. Chem. 1997, 18, 646–659. [Google Scholar] [CrossRef]
- Yamamoto, S.; Miyada, M.; Sato, H.; Hoshina, H.; Ozaki, Y. Low-Frequency vibrational modes of poly(glycolic acid) and thermal expansion of crystal lattice assigned on the basis of DFT-spectral simulation aided with a fragment method. J. Phys. Chem. B 2017, 121, 1128. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohnishi, E.; Sato, H.; Hoshina, H.; Ishikawa, D.; Ozaki, Y. Low-frequency vibrational modes of nylon 6 studied by using infrared and Raman spectroscopies and density functional theory calculations, J. Phys. Chem. B 2019, 123, 5368. [Google Scholar] [CrossRef]
- Placzek, G. Handuch der Radiologie; Marx, E., Ed.; Generis: Chisinau, Moldova, 1934; Volume 6, p. 205. [Google Scholar]
- Barron, L.D. Molecular Light Scattering and Optical Activity, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Nie, S.; Emery, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef]
- Cordero, B.; Gomez, V.; Platero-Prats, A.E.; Reves, M.; Echeverria, J.; Cremades, E.; Barragan, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2008, 2832–2838. [Google Scholar] [CrossRef]
- Wen, T.; Altman, R.B. 3D deep convolutional neural networks for amino acid environment similarity analysis. BMC Bioinform. 2017, 18, 302. [Google Scholar] [CrossRef]
- Shroff, R.; Cole, A.W.; Diaz, D.J.; Morrow, B.R.; Donnell, I.; Annapareddy, A.; Gollihar, J.; Ellington, A.D.; Thyer, R. Discovery of novel gain-of-function mutations guided by structure-based deep learning. ACS Synth. Biol. 2020, 9, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 16. Available online: https://www.gaussian.com (accessed on 6 December 2022).
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in ‘t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS-a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 10817. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.M.; Kollman, P.A.; Case, D.A. Development and testing of a general AMBER force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Kikutani, T.; Ookoshi, Y.; Takaku, A. The crystal structure and the refractive index of drawn and annealed poly(ether-ether-ketone) (PEEK) fibers. Sen’i Gakkaishi 1985, 41, T461–T467. [Google Scholar] [CrossRef]
- Jewett, A.I.; Stelter, D.; Lambert, J.; Saladi, S.M.; Roscioni, O.M.; Ricci, M.; Autin, L.; Maritan, M.; Bashusqeh, S.M.; Keyes, T.; et al. Moltemplate: A tool for coarse-grained modeling of complex biological matter and soft condensed matter physics. J. Mol. Biol. 2021, 433, 166841. [Google Scholar] [CrossRef]
- Dawson, P.C.; Blundell, D.J. X-ray data for poly(aryl ether ketones). Polymer 1980, 21, 577–578. [Google Scholar] [CrossRef]
- Kumar, S.; Anderson, D.P.; Adams, W.W. Crystallization and morphology of poly (aryl-ether-ether-ketone). Polymer 1986, 27, 329–336. [Google Scholar] [CrossRef]
- Hay, J.N.; Langford, J.I.; Lloyd, J.R. Variation in unit cell parameters of aromatic polymers with crystallization temperature. Polymer 1989, 30, 489–493. [Google Scholar] [CrossRef]
- Wakelyn, N.T. Variation of unit cell parameters of poly(arylene ether ether ketone) film with annealing temperature. J. Polym. Sci. Part C 1987, 25, 25–28. [Google Scholar] [CrossRef]
- Hay, J.N.; Kemmish, D.J.; Langford, J.I.; Rae, A.I.M. The structure of crystalline PEEK. Polym. Commun. 1984, 25, 175–178. [Google Scholar]
- Rueda, D.R.; Ania, F.; Richardson, A.; Ward, I.M.; Balta Calleja, F.J. X-Ray diffraction study of die drawn poly(aryletherketone)(PEEK). Polym. Commun. 1983, 24, 258–260. [Google Scholar]
- Fratini, A.V.; Cross, E.M.; Whitaker, R.B.; Adams, W.W. Refinement of the structure of PEEK fibre in an orthorhombic unit cell. Polymer 1986, 27, 861–865. [Google Scholar] [CrossRef]
- Pisani, W.A.; Radue, M.S.; Chinkanjanarot, S.; Bednarcyk, B.A.; Pineda, E.J.; Waters, K.; Pandey, R.; King, J.A.; Odegard, G.M. Multiscale modeling of PEEK using reactive molecular dynamics modeling and micromechanics. Polymer 2019, 163, 96–105. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of hartree-fock, moller-plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Prediction of PEKK properties related to crystallization by molecular dynamics simulations with a united-atom model. Polymer 2019, 174, 25–32. [Google Scholar] [CrossRef]
- Apra, E.; Bhattarai, A.; Baxter, E.; Wang, S.Y.; Johnson, G.E.; Govind, N.; El-Khoury, P.Z. Simplified ab initio molecular dynamics-based raman spectral simulations. Appl. Spectrosc. 2020, 74, 1350–1357. [Google Scholar] [CrossRef]
- Kaminski, S.; Gaus., M.; Phatak, P.; von Stetten, D.; Elstner, M.; Mroginski, M.A. Vibrational Raman spectra from the self-consistent charge density functional tight binding method via classical time-correlation functions. J. Chem. Theory Comput. 2020, 6, 1240–1255. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yokokura, S.; Nagahama, T.; Yamaguchi, M.; Shimada, T. Molecular Dynamics Simulation of Poly(Ether Ether Ketone) (PEEK) Polymer to Analyze Intermolecular Ordering by Low Wavenumber Raman Spectroscopy and X-ray Diffraction. Polymers 2022, 14, 5406. https://doi.org/10.3390/polym14245406
Yang X, Yokokura S, Nagahama T, Yamaguchi M, Shimada T. Molecular Dynamics Simulation of Poly(Ether Ether Ketone) (PEEK) Polymer to Analyze Intermolecular Ordering by Low Wavenumber Raman Spectroscopy and X-ray Diffraction. Polymers. 2022; 14(24):5406. https://doi.org/10.3390/polym14245406
Chicago/Turabian StyleYang, Xiaoran, Seiya Yokokura, Taro Nagahama, Makoto Yamaguchi, and Toshihiro Shimada. 2022. "Molecular Dynamics Simulation of Poly(Ether Ether Ketone) (PEEK) Polymer to Analyze Intermolecular Ordering by Low Wavenumber Raman Spectroscopy and X-ray Diffraction" Polymers 14, no. 24: 5406. https://doi.org/10.3390/polym14245406
APA StyleYang, X., Yokokura, S., Nagahama, T., Yamaguchi, M., & Shimada, T. (2022). Molecular Dynamics Simulation of Poly(Ether Ether Ketone) (PEEK) Polymer to Analyze Intermolecular Ordering by Low Wavenumber Raman Spectroscopy and X-ray Diffraction. Polymers, 14(24), 5406. https://doi.org/10.3390/polym14245406





