Muscle-like Scaffolds for Biomechanical Stimulation in a Custom-Built Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Design and Fabrication of the In-Vitro Study System
2.3. Fabrication of the Scaffold and Its Assembly on the In-Vitro Study System
2.4. Collagen Coating of the Microfilament Scaffold
2.5. Cell Seeding and Biomechanica Stimulation System Arrangement
2.6. Cell Viability Measurements
2.7. Biomechanical Stimulation of a Cell-Seeded Scaffold
2.8. Cell Morphology on the Microfilament Scaffold
3. Results
3.1. Microfilament Scaffold and Assembly of the System
3.2. Cell Viability Results on the Microfilament Scaffolds
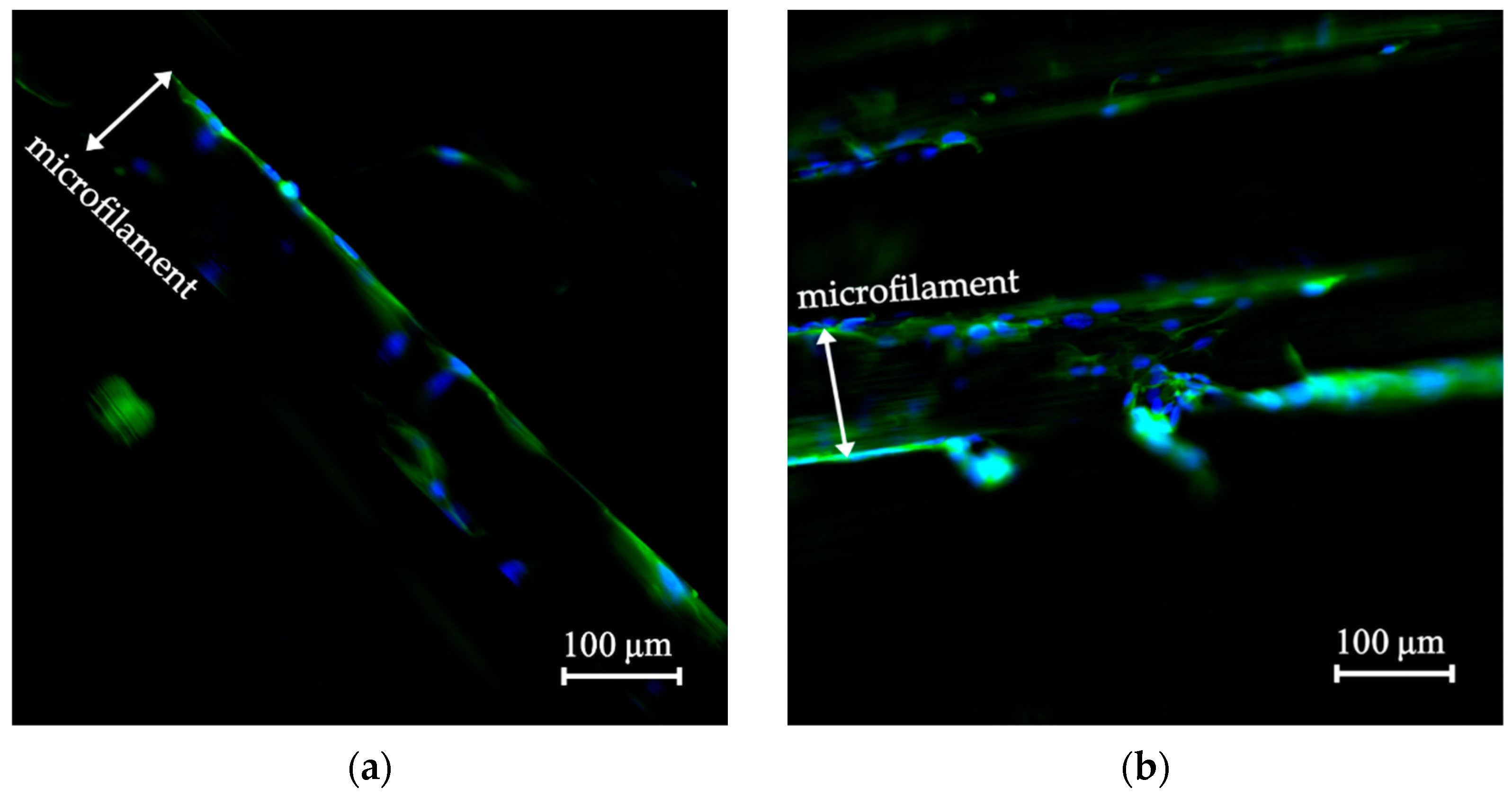
3.3. Cell Morphology on the Scaffolds
3.4. Cell Morphology of the Biomechanically Stimulated Scaffolds
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; Abedi-Dorcheh, K.; Vaziri, A.S.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Adib, F.R.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, J.; Liu, X.; Liu, J.; Zhang, Q.; Yan, X.; Yuan, X.; Ren, D. Multiple Effects of Mechanical Stretch on Myogenic Progenitor Cells. Stem Cells Dev. 2020, 29, 336–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed. Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials Based Strategies for Skeletal Muscle Tissue Engineering: Existing Technologies and Future Trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Browe, D.; Freeman, J. Optimizing C2C12 Myoblast Differentiation Using Polycaprolactone–Polypyrrole Copolymer Scaffolds. J. Biomed. Mater. Res.-Part A 2019, 107, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.S.; Fernandes, E.M.; Pina, S.; Silva-Correia, J.; Vieira, S.; Oliveira, J.M.; Reis, R.L. Polymers of Biological Origin. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 228–252. [Google Scholar]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of Polymeric Biomaterials: A Strategy for Tissue Engineering and Medical Devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef] [Green Version]
- Haycock, J.W. 3D Cell Culture: A Review of Current Approaches and Techniques. Methods Mol. Biol. 2011, 695, 1–15. [Google Scholar] [CrossRef]
- Eugenis, I.; Wu, D.; Rando, T.A. Cells, Scaffolds, and Bioactive Factors: Engineering Strategies for Improving Regeneration Following Volumetric Muscle Loss. Biomaterials 2021, 278, 121173. [Google Scholar] [CrossRef]
- Blake, C.; Massey, O.; Boyd-Moss, M.; Firipis, K.; Rifai, A.; Franks, S.; Quigley, A.; Kapsa, R.; Nisbet, D.R.; Williams, R.J. Replace and Repair: Biomimetic Bioprinting for Effective Muscle Engineering. APL Bioeng. 2021, 5, 031502. [Google Scholar] [CrossRef]
- Alexeev, D.; Goedecke, N.; Snedeker, J.; Ferguson, S. Mechanical Evaluation of Electrospun Poly(ε-Caprolactone) Single Fibers. Mater. Today Commun. 2020, 24, 101211. [Google Scholar] [CrossRef]
- Moyle, L.A.; Jacques, E.; Gilbert, P.M. Engineering the next Generation of Human Skeletal Muscle Models: From Cellular Complexity to Disease Modeling. Curr. Opin. Biomed. Eng. 2020, 16, 9–18. [Google Scholar] [CrossRef]
- Roberts, T.J. Contribution of Elastic Tissues to the Mechanics and Energetics of Muscle Function during Movement. J. Exp. Biol. 2016, 219, 266–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells, Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-Based Biomaterials for Tissue Engineering and Drug Delivery: Current Scenario and Challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532. [Google Scholar] [CrossRef]
- Coudane, J.; Nottelet, B.; Mouton, J.; Garric, X.; Van Den Berghe, H. Poly(ε-Caprolactone)-Based Graft Copolymers: Synthesis Methods and Applications in the Biomedical Field: A Review. Molecules 2022, 27, 7339. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Klingner, A. A Review on Electrospun Polymeric Nanofibers: Production Parameters and Potential Applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Górecka, Ż.; Idaszek, J.; Kołbuk, D.; Choińska, E.; Chlanda, A.; Święszkowski, W. The Effect of Diameter of Fibre on Formation of Hydrogen Bonds and Mechanical Properties of 3D-Printed PCL. Mater. Sci. Eng. C 2020, 114, 111072. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.H.; Park, W.J.; Lee, J.E.; Kim, B.; Han, D.W. Advanced Techniques for Skeletal Muscle Tissue Engineering and Regeneration. Bioengineering 2020, 7, 99. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Fleming, J.W.; Player, D.J.; Baker, L.A.; Turner, M.C.; Jones, J.M.; Martin, N.R.W.; Ferguson, R.A.; Mudera, V.C.; et al. Scalable 3D Printed Molds for Human Tissue Engineered Skeletal Muscle. Front. Bioeng. Biotechnol. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, J.; Auzmendi, O.; Amestoy, H.; Diez-Torre, A.; Sarasua, J.R. Mechanical Properties and Fatigue Analysis on Poly(ε-Caprolactone)-Polydopamine-Coated Nanofibers and Poly(ε-Caprolactone)-Carbon Nanotube Composite Scaffolds. Eur. Polym. J. 2017, 94, 208–221. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, M.; Kim, G.H. 3D-Printed Biomimetic Scaffold Simulating Microfibril Muscle Structure. Adv. Funct. Mater. 2018, 28, 1800405. [Google Scholar] [CrossRef]
- Wragg, N.M.; Player, D.J.; Martin, N.R.W.; Liu, Y.; Lewis, M.P. Development of Tissue-Engineered Skeletal Muscle Manufacturing Variables. Biotechnol. Bioeng. 2019, 116, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Ghobeira, R.; Asadian, M.; Vercruysse, C.; Declercq, H.; De Geyter, N.; Morent, R. Wide-Ranging Diameter Scale of Random and Highly Aligned PCL Fibers Electrospun Using Controlled Working Parameters. Polymer 2018, 157, 19–31. [Google Scholar] [CrossRef]
- An, J.; Chua, C.K.; Leong, K.F.; Chen, C.H.; Chen, J.P. Solvent-Free Fabrication of Three Dimensionally Aligned Polycaprolactone Microfibers for Engineering of Anisotropic Tissues. Biomed. Microdevices 2012, 14, 863–872. [Google Scholar] [CrossRef]
- Visco, A.; Scolaro, C.; Giamporcaro, A.; De Caro, S.; Tranquillo, E.; Catauro, M. Threads Made with Blended Biopolymers: Mechanical, Physical and Biological Features. Polymers 2019, 11, 901. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; An, J.; Chua, C.K.; Tan, L.P. Bioprinting of 3D in Vitro Skeletal Muscle Models: A Review. Mater. Des. 2020, 193, 108794. [Google Scholar] [CrossRef]
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M. Natural Polymers: A Source of Inspiration; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; ISBN 9780081002667. [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liu, W.; Guan, L.; Chen, J.; Duan, L.; Jia, Z.; Huang, J.; Li, W.; Liu, J.; Xiong, J.; et al. A 3D-Printed PLCL Scaffold Coated with Collagen Type i and Its Biocompatibility. BioMed Res. Int. 2018, 2018, 5147156. [Google Scholar] [CrossRef]
- Egorov, A.; Riedel, B.; Vinke, J.; Schmal, H.; Thomann, R.; Thomann, Y.; Seidenstuecker, M. The Mineralization of Various 3D-Printed PCL Composites. J. Funct. Biomater. 2022, 13, 238. [Google Scholar] [CrossRef]
- Bara, J.J.; Guilak, F. Engineering Functional Tissues: In Vitro Culture Parameters. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–177. [Google Scholar]
- Kazimierczak, P.; Przekora, A. Bioengineered Living Bone Grafts—A Concise Review on Bioreactors and Production Techniques In Vitro. Int. J. Mol. Sci. 2022, 23, 1765. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Engineering Functional Tissues: In Vitro Culture Parameters. In Principles of Tissue Engineering, 5th ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–157. [Google Scholar]
- Beldjilali-Labro, M.; Garcia, A.G.; Farhat, F.; Bedoui, F.; Grosset, J.F.; Dufresne, M.; Legallais, C. Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges. Materials 2018, 11, 1116. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhuang, X.; Niu, F. Quantitative Investigation of the Link between Actin Cytoskeleton Dynamics and Cellular Behavior. Micromachines 2022, 13, 1885. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Guo, P.; Li, X.; He, Z.; Li, Z.; Stoddart, M.J.; Grad, S.; Tian, W.; Chen, D.; et al. Effect of Cyclic Mechanical Loading on Immunoinflammatory Microenvironment in Biofabricating Hydroxyapatite Scaffold for Bone Regeneration. Bioact. Mater. 2021, 6, 3097–3108. [Google Scholar] [CrossRef]
- Maleiner, B.; Tomasch, J.; Heher, P.; Spadiut, O.; Rünzler, D.; Fuchs, C. The Importance of Biophysical and Biochemical Stimuli in Dynamic Skeletal Muscle Models. Front. Physiol. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Rojas-Rojas, L.; Guillén-Girón, T. Mechanical Properties of Polycaprolactone Microfilaments for Muscular Tissue Engineering. Tecnol. En Marcha 2023, 36. In Press. [Google Scholar]
- Rojas-Rojas, L.; Ulloa-Fernández, A.; Castro-Piedra, S.; Vargas-Segura, W.; Guillén-Girón, T. Evaluation of Biomechanical and Chemical Properties of Gamma-Irradiated Polycaprolactone Microfilaments for Musculoskeletal Tissue Engineering Applications. Int. J. Biomater. 2022, 9, 5266349. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji—An Open Platform for Biological Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Freshney, R.I. Basic Principles of Cell Culture. In Culture of Cells for Tissue Engineering; Vunjak-Novakovic, G., Freshney, R.I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 3–22. [Google Scholar]
- Zhou, Y.; Sooriyaarachchi, D.; Liu, D.; Tan, G.Z. Biomimetic Strategies for Fabricating Musculoskeletal Tissue Scaffolds: A Review. Int. J. Adv. Manuf. Technol. 2021, 112, 1211–1229. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent Progress in the Fabrication Techniques of 3D Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, J.U.; Kim, G.H. The Fabrication of Uniaxially Aligned Micro-Textured Polycaprolactone Struts and Application for Skeletal Muscle Tissue Regeneration. Biofabrication 2019, 11, 025005. [Google Scholar] [CrossRef]
- Jana, S.; Levengood, S.K.L.; Zhang, M. Anisotropic Materials for Skeletal-Muscle-Tissue Engineering. Adv. Mater. 2016, 28, 10588–10612. [Google Scholar] [CrossRef] [Green Version]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S.N. Collagen Based Biomaterials for Tissue Engineering Applications: A Review. In Processes and Phenomena on the Boundary between Biogenic and Abiogenic Nature; Frank-Kamenetskaya, O.V., Vlasov, D.Y., Panova, E.G., Lessovaia, S.N., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–22. [Google Scholar]
- Steynberg, T.; Visagie, M.; Mqoco, T.; Idicula, A.; Moolman, S.; Richter, W.; Joubert, A. Qualitative Assessment of Smooth Muscle Cells Propagated on 2d-and 3dpolycaprolactone Polymers via Scanning Electron Microscope. Biomed. Res. 2012, 23, 191–198. [Google Scholar]
- Milasincic, D.J.; Dhawan, J.; Farmer, S.R. Anchorage-Dependent Control of Muscle-Specific Gene Expression in C2C12 Mouse Myoblasts. Vitr. Cell. Dev. Biol.-Anim. 1996, 32, 90–99. [Google Scholar] [CrossRef]
- Frleta, R.; Popovi, M.; Smital, T.; Šimat, V. Comparison of Growth and Chemical Profile of Diatom Skeletonema Grevillei in Bioreactor and Incubation-Shaking Cabinet in Two Growth Phases. Mar. Durgs 2022, 20, 697. [Google Scholar] [CrossRef]
- Stampoultzis, T.; Karami, P.; Pioletti, D.P. Thoughts on Cartilage Tissue Engineering: A 21st Century Perspective. Curr. Res. Transl. Med. 2021, 69, 103299. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering 2020, 7, 85. [Google Scholar] [CrossRef]
- Castro, N.; Ribeiro, S.; Fernandes, M.M.; Ribeiro, C.; Cardoso, V.; Correia, V.; Minguez, R.; Lanceros-Mendez, S. Physically Active Bioreactors for Tissue Engineering Applications. Adv. Biosyst. 2020, 4, 2000125. [Google Scholar] [CrossRef]
- Somers, S.M.; Spector, A.A.; Digirolamo, D.J.; Grayson, W.L. Biophysical Stimulation for Engineering Functional Skeletal Muscle. Tissue Eng.-Part B Rev. 2017, 23, 362–372. [Google Scholar] [CrossRef]
- Heher, P.; Maleiner, B.; Prüller, J.; Teuschl, A.H.; Kollmitzer, J.; Monforte, X.; Wolbank, S.; Redl, H.; Rünzler, D.; Fuchs, C. A Novel Bioreactor for the Generation of Highly Aligned 3D Skeletal Muscle-like Constructs through Orientation of Fibrin via Application of Static Strain. Acta Biomater. 2015, 24, 251–265. [Google Scholar] [CrossRef]
- Rimington, R.P.; Capel, A.J.; Chaplin, K.F.; Fleming, J.W.; Bandulasena, H.C.H.; Bibb, R.J.; Christie, S.D.R.; Lewis, M.P. Differentiation of Bioengineered Skeletal Muscle within a 3D Printed Perfusion Bioreactor Reduces Atrophic and Inflammatory Gene Expression. ACS Biomater. Sci. Eng. 2019, 5, 5525–5538. [Google Scholar] [CrossRef]
- Salinas, E.Y.; Hu, J.C.; Athanasiou, K. A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng.-Part B Rev. 2018, 24, 345–358. [Google Scholar] [CrossRef]
- Behary, N.; Eap, S.; Cayla, A.; Chai, F.; Benkirane-Jessel, N.; Campagne, C. Nano-Strucutred Ridged Micro-Filaments (≥100 Μm Diameter) Produced Using a Single Step Strategy for Improved Bone Cells Adhesion and Proliferation in Textile Scaffolds. Molecules 2022, 27, 3790. [Google Scholar] [CrossRef]








| Time (h) | Methodology | Details | Test |
|---|---|---|---|
| 0 | Cell seeding C2C12 | Aluminum holder in incubator | A0 |
| 24 | Viability measurements | Mobile grip and control grip inside incubator | A1 |
| 48 | Viability measurements | A2 | |
| 72 | Viability measurements | Mobile grip located in biomechanical stimulation system and control grip inside incubator. Cell density estimation | A3 |
| 96 | Viability measurements | A4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Rojas, L.; Espinoza-Álvarez, M.L.; Castro-Piedra, S.; Ulloa-Fernández, A.; Vargas-Segura, W.; Guillén-Girón, T. Muscle-like Scaffolds for Biomechanical Stimulation in a Custom-Built Bioreactor. Polymers 2022, 14, 5427. https://doi.org/10.3390/polym14245427
Rojas-Rojas L, Espinoza-Álvarez ML, Castro-Piedra S, Ulloa-Fernández A, Vargas-Segura W, Guillén-Girón T. Muscle-like Scaffolds for Biomechanical Stimulation in a Custom-Built Bioreactor. Polymers. 2022; 14(24):5427. https://doi.org/10.3390/polym14245427
Chicago/Turabian StyleRojas-Rojas, Laura, María Laura Espinoza-Álvarez, Silvia Castro-Piedra, Andrea Ulloa-Fernández, Walter Vargas-Segura, and Teodolito Guillén-Girón. 2022. "Muscle-like Scaffolds for Biomechanical Stimulation in a Custom-Built Bioreactor" Polymers 14, no. 24: 5427. https://doi.org/10.3390/polym14245427





