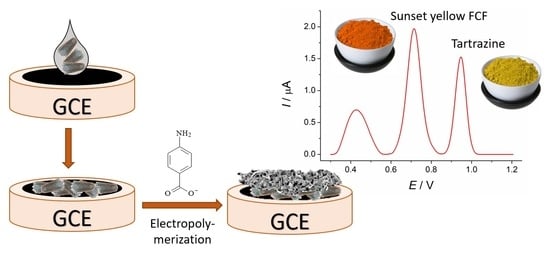
Electropolymerized 4-Aminobenzoic Acid Based Voltammetric Sensor for the Simultaneous Determination of Food Azo Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Procedures
2.3.1. Electrode Surface Modification
2.3.2. Electrochemical Measurements
2.3.3. Real Samples Analysis
2.3.4. Statistical Treatment
3. Results and Discussion
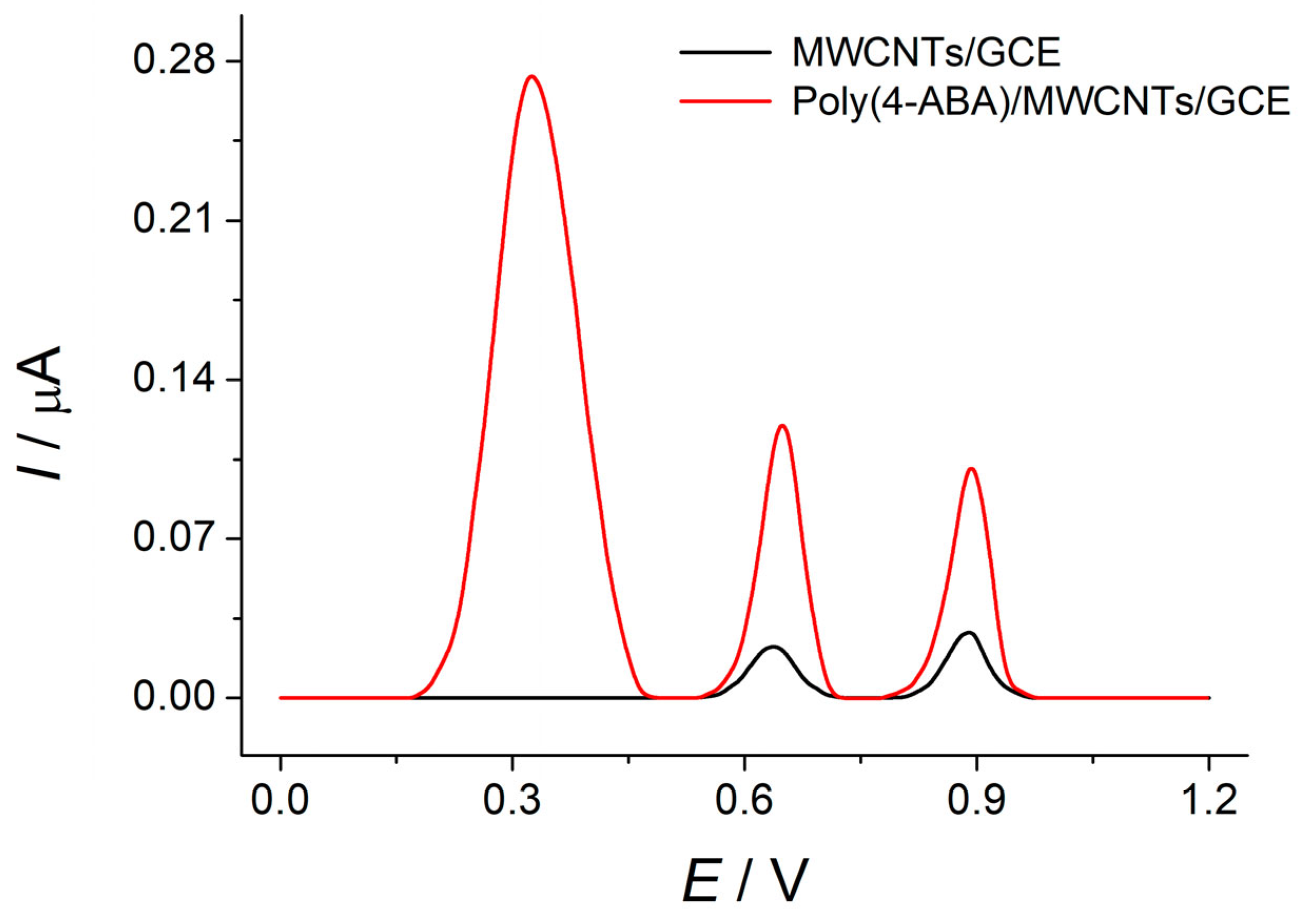
3.1. Voltammetric Behavior of Azo Dyes at Bare GCE and MWCNTs/GCE
3.2. Fabrication of 4-ABA-Modified Electrode
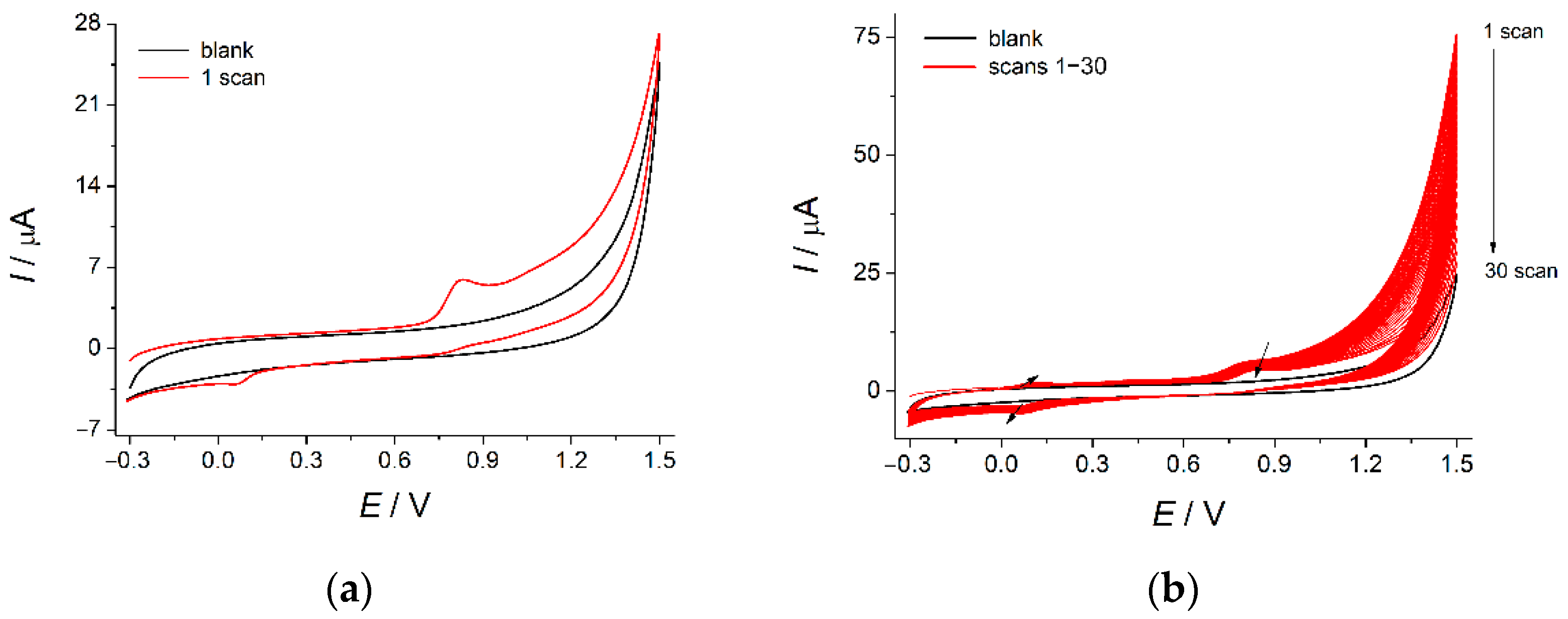
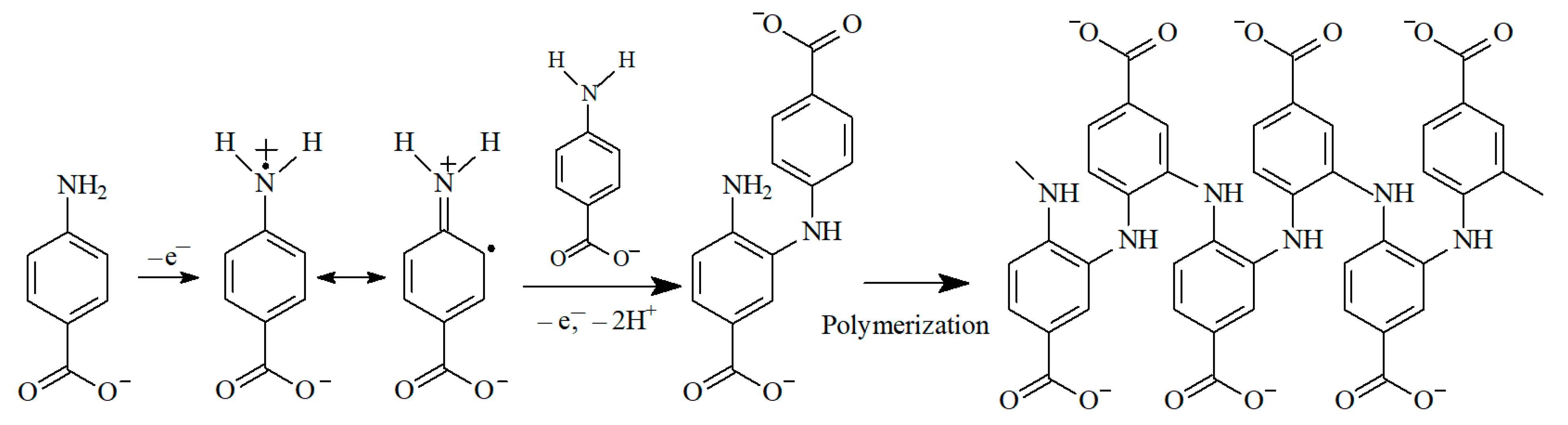
3.2.1. Electropolymerization of 4-ABA at the MWCNTs/GCE
3.2.2. Optimization of 4-ABA Electropolymerization Conditions
3.3. Bare and Modified Electrodes Characterization
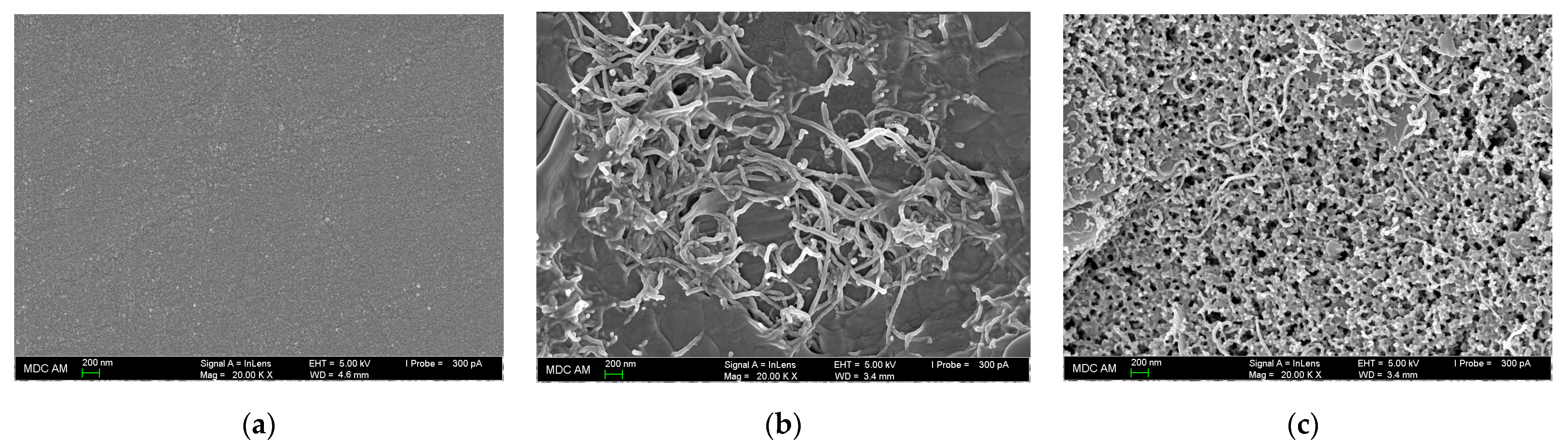
3.3.1. Surface Morphology
3.3.2. Effective Surface Area and Electron Transfer Properties
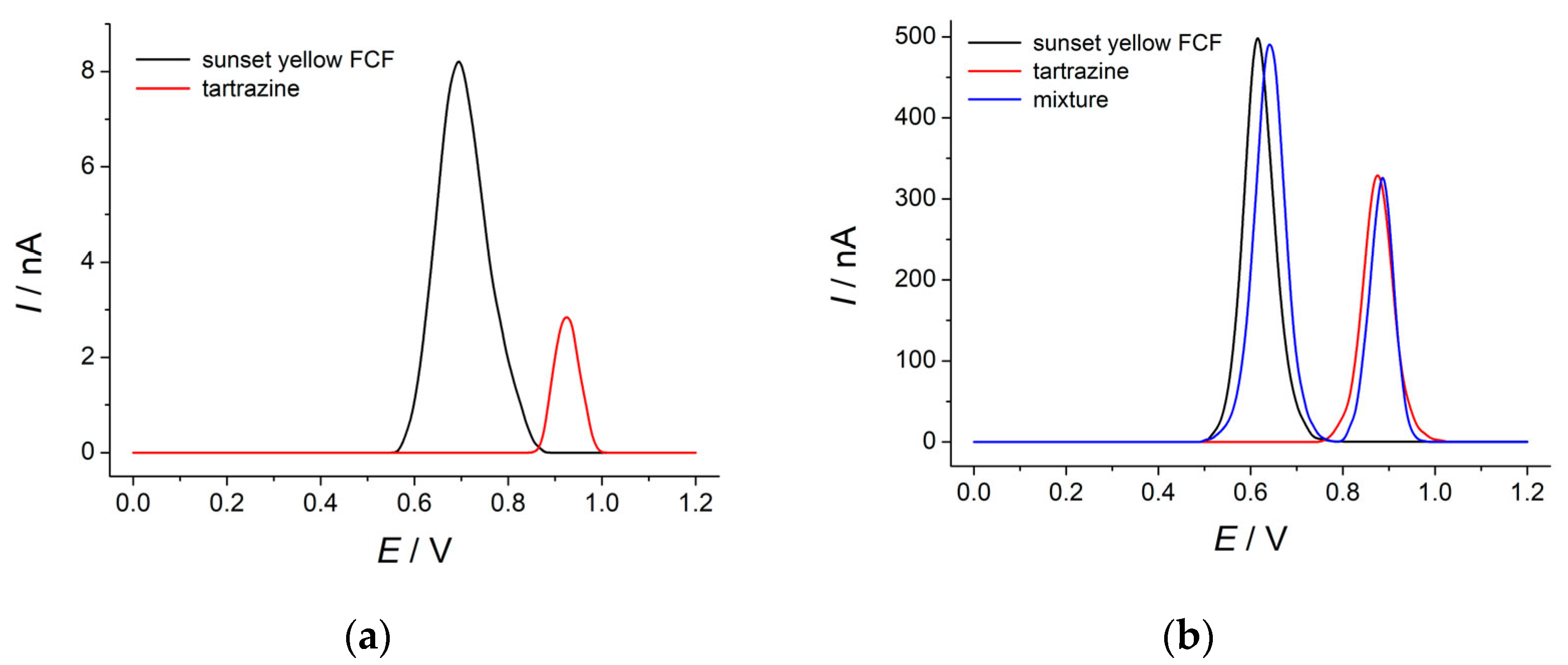
3.4. Voltammetric Response of Dyes Mixture at the Poly(4-ABA)-Modified Electrode
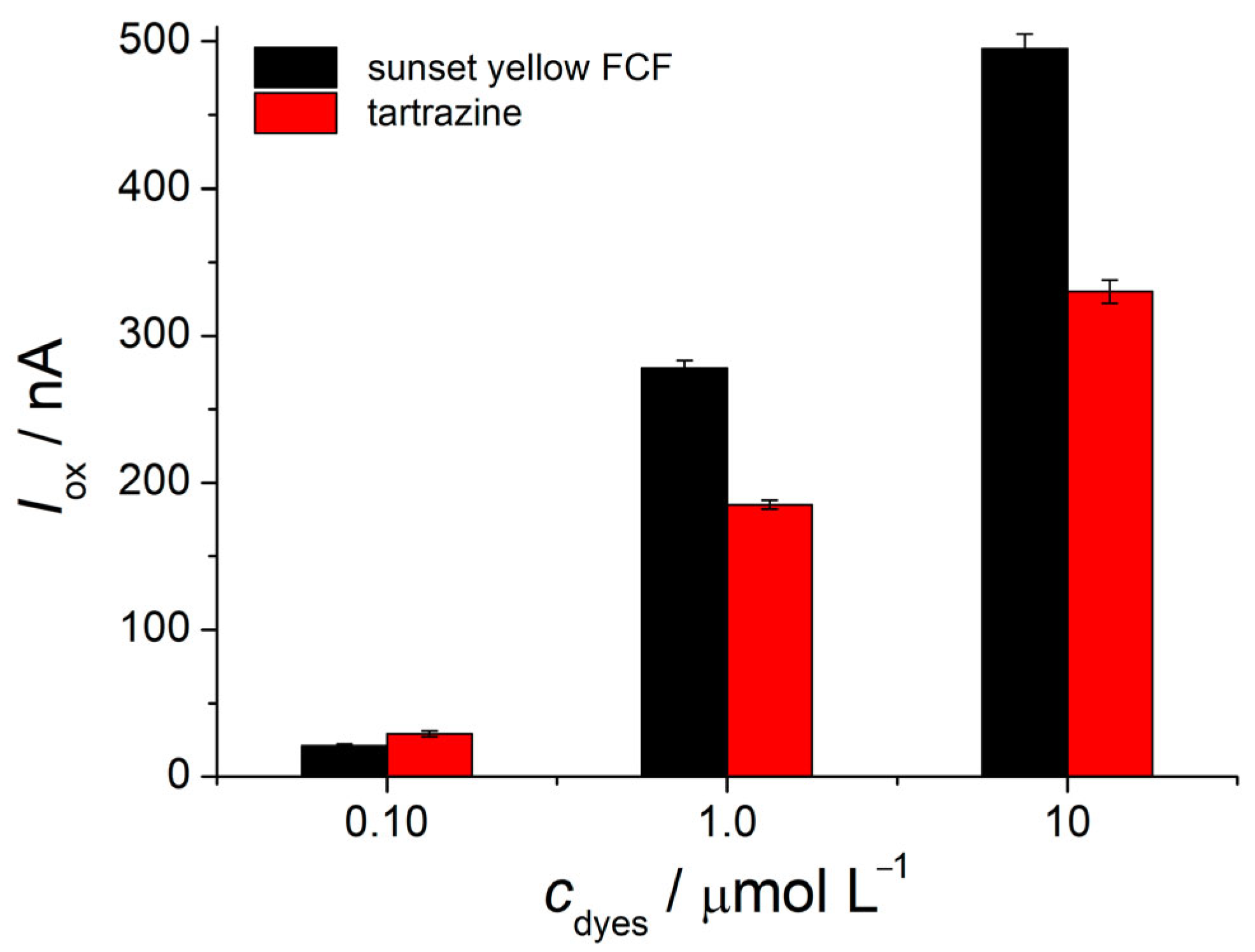
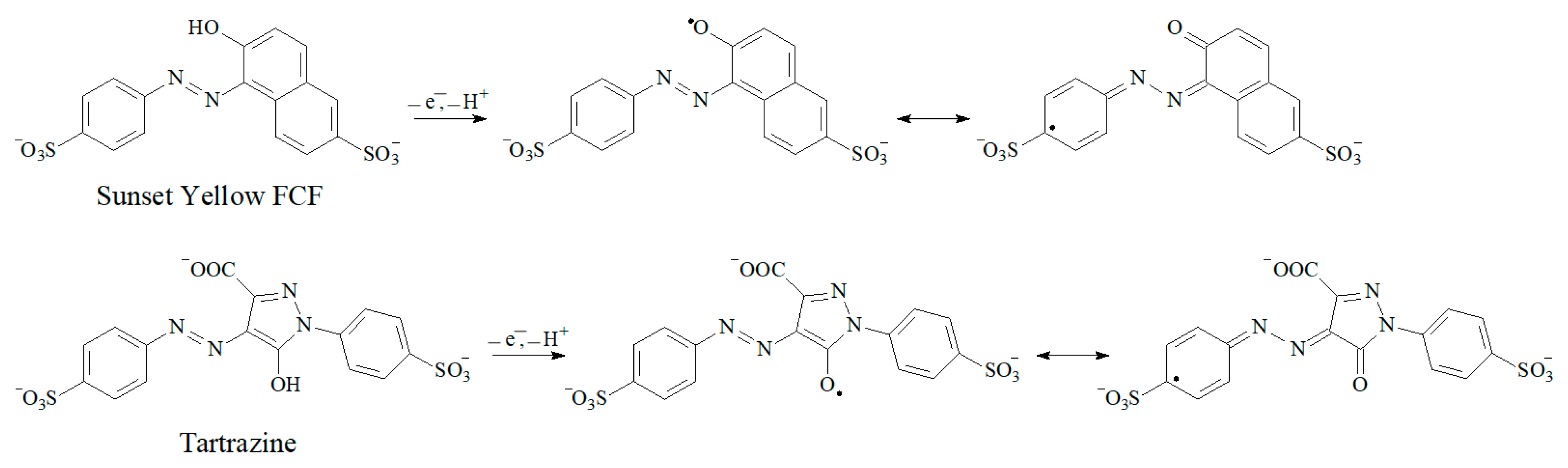
3.5. Electrooxidation of Sunset Yellow FCF and Tartrazine at the Polymer-Modified Electrode
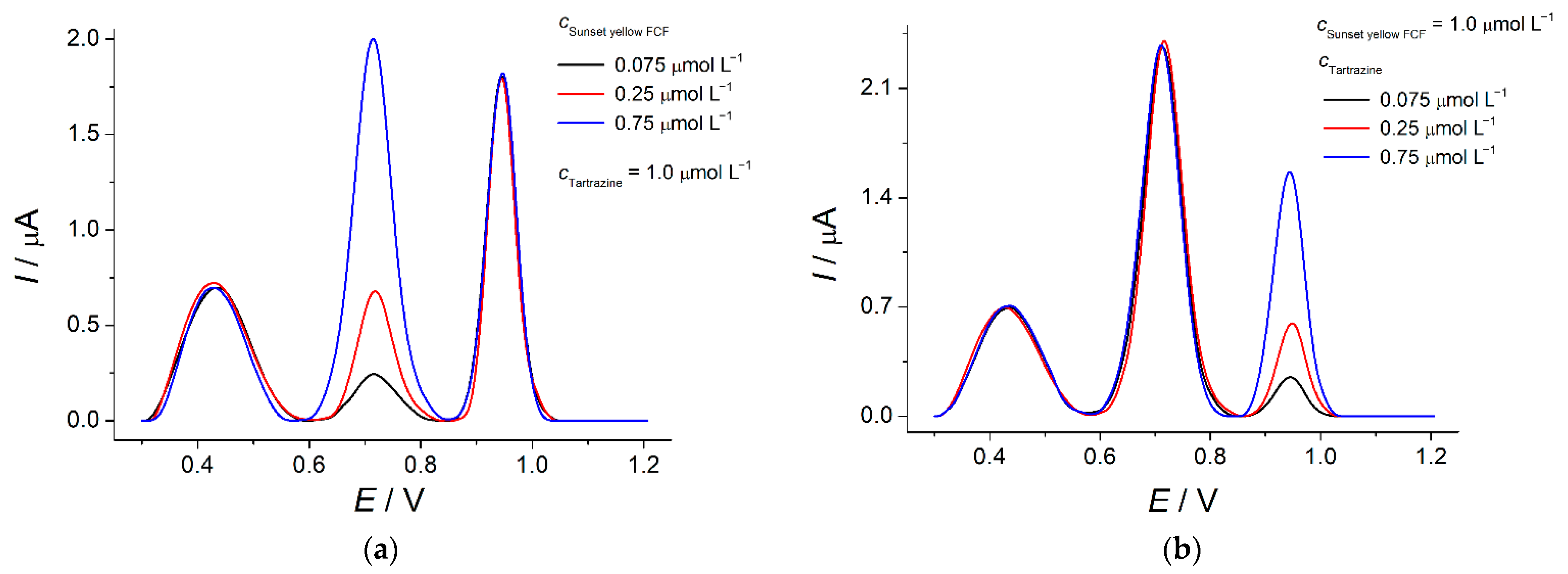
3.6. Simultaneous Determination of Azo Dyes Using Poly(4-ABA)-Based Sensor
3.6.1. Optimization of Pulse Parameters
3.6.2. Analytical Characteristics of Azo Dyes
3.6.3. Accuracy, Reproducibility, and Robustness of the Sensor Response to Azo Dyes
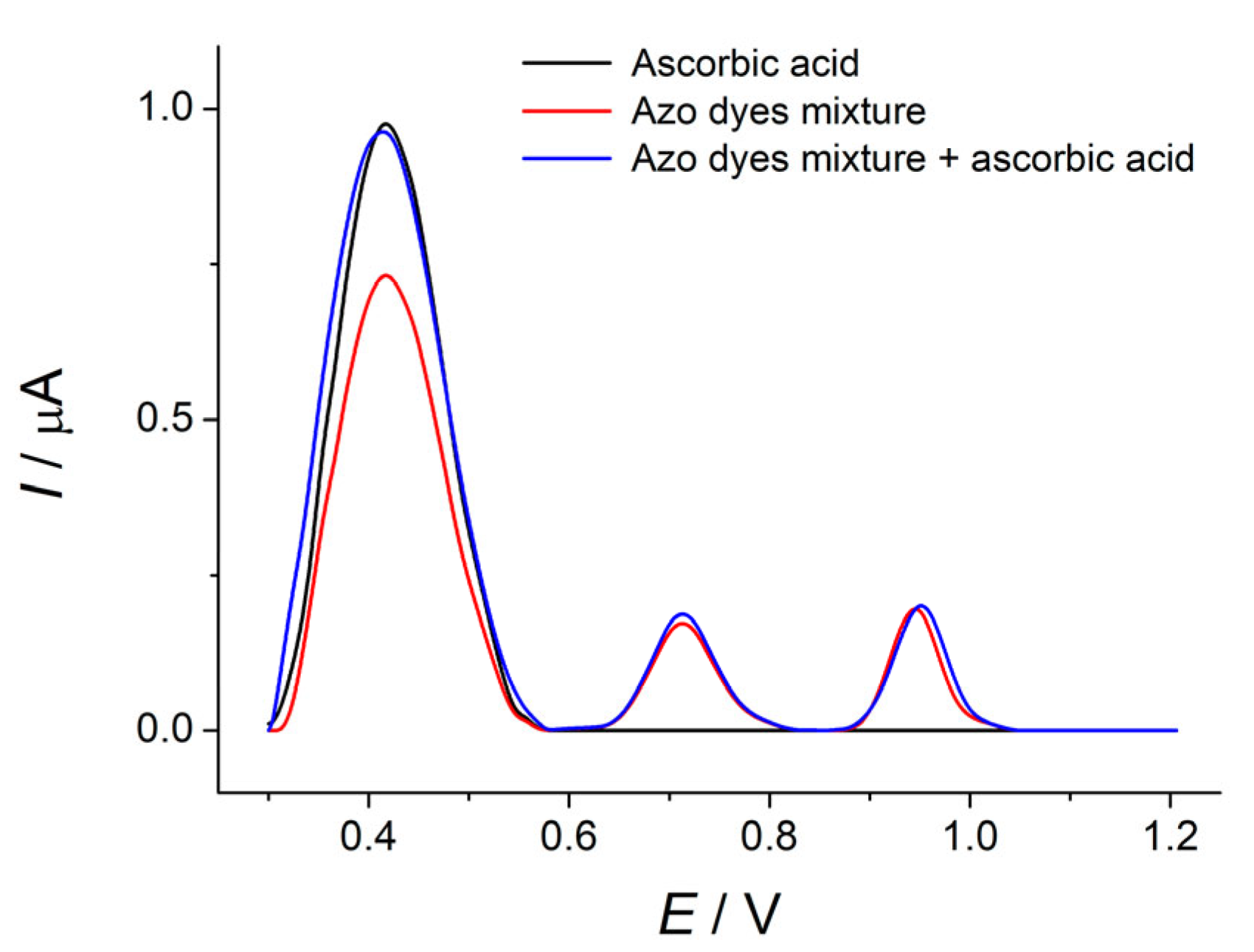
3.6.4. Interference Study
3.7. Practical Application of the Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Molina, B.G. Polymers and Plastics Modified Electrodes for Biosensors: A Review. Molecules 2020, 25, 2446. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, G.; Cheng, W.; Li, S. Poly (o-aminobenzoic acid) modified glassy carbon electrode for electrochemical detection of dopamine in the presence of ascorbic acid. Front. Biosci. 2005, 10, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Benyoucef, A.; Huerta, F.; Vázquez, J.L.; Morallon, E. Synthesis and in situ FTIRS characterization of conducting polymers obtained from aminobenzoic acid isomers at platinum electrodes. Eur. Polym. J. 2005, 41, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.; Wang, J.; Wang, X.; Wang, Z.; Xu, H.; Di, S.; Wang, Q.; Wang, X. Sensitive and selective detection of the highly toxic pesticide carbofuran in vegetable samples by a molecularly imprinted electrochemical sensor with signal enhancement by AuNPs. RSC Adv. 2018, 8, 25334–25341. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Gao, M.; Shi, G.; Wang, L.; Zhang, W.; Xue, J.; Jin, L.; Jin, J. Simultaneous detection of monoamines in rat striatal microdialysate at poly(para-aminobenzoic acid) modified electrode by high-performance liquid chromatography. Anal. Chim. Acta 2001, 439, 239–246. [Google Scholar] [CrossRef]
- Titretir Duran, S. Determination of bisphenol a with poly (p-aminobenzoic acid) modified gold electrode by using differential pulse voltammetry. Gazi Univ. J. Sci. 2019, 32, 426–438. [Google Scholar]
- Liu, Y.T.; Deng, J.; Xiao, X.L.; Ding, L.; Yuan, Y.L.; Li, H.; Li, X.T.; Yan, X.N.; Wang, L.L. Electrochemical sensor based on a poly(para-aminobenzoic acid) film modified glassy carbon electrode for the determination of melamine in milk. Electrochim. Acta 2011, 56, 4595–4602. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Q.; Chen, Y.; Wang, F. Simultaneous determination of hydroquinone and catechol using poly(p-aminobenzoic acid) modified glassy carbon electrode. J. Appl. Polym. Sci. 2009, 113, 2881–2886. [Google Scholar] [CrossRef]
- Silva, M.M.; Reboredo, F.H.; Lidon, F.C. Food colour additives: A synoptical overview on their chemical properties, applications in food products, and health side effects. Foods 2022, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T. Azo dyes and human health. J. Environ. Sci. Health. Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- EFSA. Reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- Scientific Opinion. On re-evaluation of Tartrazine (E102) as a food additive. In: EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). EFSA J. 2009, 7, 1331. [Google Scholar] [CrossRef]
- Georgescu State, R.; van Staden, J.(K.)F.; Stefan-van Staden, R.-I. Recent trends on the electrochemical sensors used for the determination of tartrazine and sunset yellow FCF from food and beverage products. J. Electrochem. Soc. 2022, 169, 017509. [Google Scholar] [CrossRef]
- Kaya, S.I.; Cetinkaya, A.; Ozkan, S.A. Latest advances on the nanomaterials-based electrochemical analysis of azo toxic dyes sunset yellow and tartrazine in food samples. Food Chem. Toxicol. 2021, 156, 112524. [Google Scholar] [CrossRef]
- Rozi, N.; Ahmad, A.; Yook Heng, L.; Shyuan, L.K.; Hanifah, S.A. Electrochemical Sunset Yellow Biosensor Based on Photocured Polyacrylamide Membrane for Food Dye Monitoring. Sensors 2018, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Sierra-Rosales, P.; Toledo-Neira, C.; Squella, J.A. Electrochemical determination of food colorants in soft drinks using MWCNT-modifed GCEs. Sens. Actuat. B 2017, 240, 1257–1264. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of sunset yellow and tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.Y.; Khamzina, E.I.; Bukharinova, M.A.; Tarasov, A.V. An electrochemical sensor based on carbon paper modified with graphite powder for sensitive determination of sunset yellow and tartrazine in drinks. Sensors 2022, 22, 4092. [Google Scholar] [CrossRef] [PubMed]
- Marahel, F.; Niknam, L. Application electrochemical sensor based on nanosheets G-C3N4/CPE by square-wave anodic stripping voltammetric for measure amounts of toxic tartrazine color residual in different drink and foodstuffs. J. Environ. Sci. Health B 2022, 57, 489–496. [Google Scholar] [CrossRef]
- Tran, Q.T.; Phung, T.T.; Nguyen, Q.T.; Le, T.G.; Lagrost, C. Highly sensitive and rapid determination of sunset yellow in drinks using a low-cost carbon material-based electrochemical sensor. Anal. Bioanal. Chem. 2019, 411, 7539–7549. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Golestaneh, M. Simultaneous determination of sunset yellow and tartrazine in soft drinks using gold nanoparticles carbon paste electrode. Food Chem. 2012, 132, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Kolozof, P.-A.; Florou, A.B.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. In-situ tailoring of the electrocatalytic properties of screen-printed graphite electrodes with sparked generated molybdenum nanoparticles for the simultaneous voltammetric determination of sunset yellow and tartrazine. Sens. Actuat. B 2020, 304, 127268. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Budnikov, H.C. Voltammetric determination of tartrazine on an electrode modified with cerium dioxide nanoparticles and cetyltriphenylphosphonium bromide. J. Anal. Chem. 2022, 77, 664–670. [Google Scholar] [CrossRef]
- Moarefdoust, M.M.; Jahani, S.; Moradalizadeh, M.; Motaghi, M.M.; Foroughi, M.M. An electrochemical sensor based on hierarchical nickel oxide nanostructures doped with indium ions for voltammetric simultaneous determination of sunset yellow and tartrazine colorants in soft drink powders. Anal. Methods 2021, 13, 2396–2404. [Google Scholar] [CrossRef]
- Nagles, E.; Ceroni, M.; Hurtado, J. Simultaneous detection of tartrazine-sunset yellow in food samples using bioxide/carbon paste microcomposite with lanthanum and titanium. J. Electrochem. Sci. Technol. 2020, 11, 421–429. [Google Scholar] [CrossRef]
- Marquez-Mariño, K.; Penagos-Llanos, J.; García-Beltrán, O.; Nagles, E.; Hurtado, J.J. Development of a novel electrochemical sensor based on a carbon paste electrodedecorated with Nd2O3 for the simultaneous detection of tartrazine and sunset yellow. Electroanalysis 2018, 30, 2760–2767. [Google Scholar] [CrossRef]
- Baytak, A.K.; Aslanoglu, M. Praseodymium doped dysprosium oxide-carbon nanofibers based voltammetric platform for the simultaneous determination of sunset yellow and tartrazine. Electroanalysis 2022. [Google Scholar] [CrossRef]
- Nguyen, Q.-T.; Le, T.-G.; Bergonzo, P.; Tran, Q.-T. One-Step Fabrication of nickel-electrochemically reduced graphene oxide nanocomposites modified electrodes and application to the detection of sunset yellow in drinks. Appl. Sci. 2022, 12, 2614. [Google Scholar] [CrossRef]
- Arvand, M.; Parhizi, Y.; Mirfathi, S.H. Simultaneous voltammetric determination of synthetic colorants in foods using a magnetic core–shell Fe3O4@SiO2/MWCNTs nanocomposite modified carbon paste electrode. Food Anal. Methods 2016, 9, 863–875. [Google Scholar] [CrossRef]
- Yu, L.; Zheng, H.; Shi, M.; Jing, S.; Qu, L. A novel electrochemical sensor based on poly (diallyldimethylammonium chloride)-dispersed graphene supported palladium nanoparticles for simultaneous determination of sunset yellow and tartrazine in soft drinks. Food Anal. Methods 2017, 10, 200–209. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Ultrasensitive detection of food colorant sunset yellow using nickel nanoparticles promoted lettuce-like spinel Co3O4 anchored GO nanosheets. Food Chem. Toxicol. 2022, 159, 112725. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Cao, S.; Gao, F.; Zhang, Y.; Yang, Y. One-step electrochemical approach for the preparation of graphene wrapped-phosphotungstic acid hybrid and its application for simultaneous determination of sunset yellow and tartrazine. Electrochim. Acta 2012, 74, 151–157. [Google Scholar] [CrossRef]
- Wu, J.-H.; Lee, H.-L. Determination of sunset yellow and tartrazine in drinks using screen-printed carbon electrodes modified with reduced graphene oxide and NiBTC frameworks. Microchem. J. 2020, 158, 105133. [Google Scholar] [CrossRef]
- Alves, G.F.; de Faria, L.V.; Lisboa, T.P.; de Souza, C.C.; Fernandes, B.L.M.; Matos, M.A.C.; Matos, R.C. A portable and affordable paper electrochemical platform for the simultaneous detection of sunset yellow and tartrazine in food beverages and desserts. Microchem. J. 2022, 181, 107799. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, P.; Wu, J.; Wang, W.; Ye, B. Highly sensitive determination of sunset yellow in drink using a poly(L-cysteine) modified glassy carbon electrode. Anal. Methods 2013, 5, 5044–5050. [Google Scholar] [CrossRef]
- Koyun, O.; Sahin, Y. Poly(L-cysteine) modified pencil graphite electrode for determination of sunset yellow in food and beverage samples by differential pulse voltammetry. Int. J. Electrochem. Sci. 2018, 13, 159–174. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A novel voltammetric method for the enhanced detection of the food additive tartrazine using an electrochemical sensor. Heliyon 2018, 4, e00986. [Google Scholar] [CrossRef] [PubMed]
- Karim-Nezhad, G.; Khorablou, Z.; Zamani, M.; Dorraji, P.S.; Alamgholiloo, M. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2017, 25, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.-C.; Hsieh, B.-C.; Chuang, M.-C. Electropolymerised-hemin-catalysed reduction and analysis of tartrazine and sunset yellow. Electrochim. Acta 2019, 319, 766–774. [Google Scholar] [CrossRef]
- Chao, M.; Ma, X. Convenient electrochemical determination of sunset yellow and tartrazine in food samples using a poly(L-phenylalanine)-modified glassy carbon electrode. Food Anal. Methods 2015, 8, 130–138. [Google Scholar] [CrossRef]
- Akkapinyo, C.; Subannajui, K.; Poo-arporn, Y.; Poo-arporn, R.P. Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode. Molecules 2021, 26, 2312. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Liu, G.; Sun, D. Determination of sunset yellow and tartrazine using silver and poly (L-cysteine) composite film modified glassy carbon electrode. Indian J. Chem. 2016, 55A, 298–303. [Google Scholar]
- Wang, M.; Gao, Y.; Sun, Q.; Zhao, J. Sensitively simultaneous determination of sunset yellow and tartrazine in foods based on polypyrrole modified oxidized single-walled carbon nanotubes. J. Electrochem. Soc. 2014, 161, B297–B304. [Google Scholar] [CrossRef]
- Baiju, J. Polymer nanocomposite-based electrochemical sensors and biosensors. In Nanorods and Nanocomposites; Ghamsari, M., Dhara, S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Ziyatdinova, G.; Budnikov, H. Carbon nanomaterials and surfactants as electrode surface modifiers in organic electroanalysis. In Nanoanalytics: Nanoobjects and Nanotechnologies in Analytical Chemistry; Shtykov, S.N., Ed.; De Gruyter: Berlin, Germany; Boston, MA, Germany, 2018; pp. 223–252. [Google Scholar] [CrossRef]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry: A review. Anal. Chim. Acta. 2008, 622, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Santos, C.; Pimenta, T.C.; Thomasini, R.L.; Verly, M.R.; Franco, D.L.; Ferreira, L.C. Electropolymerization of phenol and aniline derivatives: Synthesis, characterization and application as electrochemical transducers. J. Electroanal. Chem. 2019, 846, 113163. [Google Scholar] [CrossRef]
- Matos, M.F.F.; Soares, P.I.; Lima, T.M.; Pereira, A.C.; Franco, D.L.; Ferreira, L.C. Comparison of the modification of graphite electrodes with poly(4-aminobenzoic acid) and poly(4-hydroxyphenylacetic acid) for determination of Pb(II). Chem. Pap. 2022, 76, 5691–5704. [Google Scholar] [CrossRef]
- Lasia, A. Electrochemical Impedance Spectroscopy and Its Applications; Springer: New York, NY, USA, 2014; 367p. [Google Scholar] [CrossRef]
- Saleh, M.M.S.; Hashem, E.Y.; Al- Salahi, N.O.A. Oxidation and complexation-based spectrophotometric methods for sensitive determination of tartrazine E102 in some commercial food samples. Comput. Chem. 2016, 4, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, M.; Sajjadi, S.M.; Mousavi, H.Z. Kinetic analysis of azo dye decolorization during their acid-base equilibria: Photocatalytic degradation of tartrazine and sunset yellow. Reac. Kinet. Mech. Cat. 2019, 128, 555–570. [Google Scholar] [CrossRef]
- Pérez-Urquiza, M.; Beltrán, J.L. Determination of the dissociation constants of sulfonated azo dyes by capillary zone electrophoresis and spectrophotometry methods. J. Cromatogr. A 2001, 917, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001; 864p. [Google Scholar]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 4, 3731–3737. [Google Scholar] [CrossRef]
- Majidi, M.R.; Pournaghi-Azar, M.H.; Bajeh Baj, R.F.; Naseri, A. Formation of graphene nanoplatelet-like structures on carbon-ceramic electrode surface: Application for simultaneous determination of sunset yellow and tartrazine in some food samples. Ionics 2015, 21, 863–875. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Electrochemical fabrication of a novel ZnO/cysteic acid nanocomposite modified electrode and its application to simultaneous determination of sunset yellow and tartrazine. Food Chem. 2017, 227, 73–77. [Google Scholar] [CrossRef]
- Bonan, S.; Fedrizzi, G.; Menotta, S.; Elisabetta, C. Simultaneous determination of synthetic dyes in foodstuffs and beverages by high-performance liquid chromatography coupled with diode-array detector. Dyes Pigm. 2013, 99, 36–40. [Google Scholar] [CrossRef]






| Sensor | Dye | Method | Redox Process | tacc (s) | Detection Limit (µmol L−1) | Linear Dynamic Range (µmol L−1) | Ref |
|---|---|---|---|---|---|---|---|
| Poly(L-cysteine)/GCE 1 | Sunset yellow FCF | DPV 2 | Oxidation | 300 | 0.0040 | 0.0080–0.70 | [39] |
| Poly(L-cysteine)/PGE 3 | — | 0.125 | 1.0–1000 | [40] | |||
| Polyglycine/CPE 4 | Tartrazine | CV 5 | Oxidation | — | 0.283 | 1–27 and 35–87 | [41] |
| Poly (p-aminobenzenesulfonic acid)/ZnO nanoparticles-CPE | DPV | — | 0.080 | 0.0349–1.246 and 1.246–5.44 | [42] | ||
| Electropolymerized hemin/GCE | Sunset yellow FCF | SWV6 | Reduction | — | 0.384 | 0.5–10 | [43] |
| Tartrazine | — | 0.360 | 0.5–10 | ||||
| Poly(L-phenylalanine)/GCE | Sunset yellow FCF | DPV | Reduction | 60 | 0.040 | 0.40–14 | [44] |
| Tartrazine | Oxidation | 80 | 0.020 | 0.40–20 and 20–165 | |||
| Poly(L-methionine)/Reduced graphene oxide/SPCE 7 | Sunset yellow FCF | DPV | Oxidation | 300 | 0.048 | 1–10 and 10–50 | [45] |
| Tartrazine | 420 | 0.041 | 1–10 and 10–85 | ||||
| Poly(L-cysteine)/Ag nanoparticles/GCE | Sunset yellow FCF | DPV | Oxidation | — | 0.075 | 0.50–10 and 10–300 | [46] |
| Tartrazine | — | 0.25 | 0.75–75 and 75–750 | ||||
| Polypyrrole-SWCNTs-COOH 8/GCE | Sunset yellow FCF | SWV | Oxidation | 300 | 0.00070 | 0.0050–1.0 | [47] |
| Tartrazine | 300 | 0.0010 | 0.0070–1.0 |
| Electrode | Rs (Ω) | Ret (kΩ) | Q (µΩ−1) | n | W (µΩ−1) | χ2 |
|---|---|---|---|---|---|---|
| GCE | 245 ± 5 | 72 ± 3 | 3.7 ± 0.2 | 0.789 | – | 0.028 |
| MWCNTs/GCE | 199 ± 3 | 12.1 ± 0.9 | 3.6 ± 0.1 | 0.775 | 230 ± 5 | 0.015 |
| Poly(4-ABA)/MWCNTs/GCE | 113 ± 2 | 4.18 ± 0.05 | 389 ± 4 | 0.913 | 246 ± 3 | 0.020 |
| Dye | Linear Regression Parameters | ||
|---|---|---|---|
| a ± SD | b ± SD | R2 | |
| I (µA) = a + bυ1/2 (mV s−1) | |||
| Sunset yellow FCF | 0.28 ± 0.05 | 0.301 ± 0.007 | 0.9970 |
| Tartrazine | 0.18 ± 0.04 | 0.332 ± 0.005 | 0.9989 |
| lnI (µA) = a + blnυ (V s−1) | |||
| Sunset yellow FCF | 2.17 ± 0.04 | 0.43 ± 0.01 | 0.9967 |
| Tartrazine | 2.29 ± 0.02 | 0.45 ± 0.01 | 0.9990 |
| E (V) = a + blnυ (V s−1) | |||
| Sunset yellow FCF | 0.842 ± 0.003 | 0.023 ± 0.001 | 0.9960 |
| Tartrazine | 1.109 ± 0.005 | 0.022 ± 0.002 | 0.9834 |
| Parameter | Equation | Sunset Yellow FCF | Tartrazine |
|---|---|---|---|
| D (cm2 s−1) | (1.06 ± 0.07) × 10−6 | (1.91 ± 0.06) × 10−7 | |
| k0 (cm s−1) | 6.4 × 10−4 | 2.1 × 10−4 |
| Dye | Limit of Detection (µmol−1 L) | Linear Dynamic Range (µmol−1 L) | I = a + bc | ||
|---|---|---|---|---|---|
| a ± SD (µA) | (b ± SD) × 10−5 (µA mol−1 L) | R2 | |||
| Sunset yellow FCF | 0.0023 | 0.010–0.75 | 0.039 ± 0.002 | 26.14 ± 0.07 | 0.9999 |
| 0.75–5.0 | 0.99 ± 0.06 | 14.1 ± 0.2 | 0.9993 | ||
| Tartrazine | 0.0030 | 0.010–0.75 | 0.096 ± 0.002 | 20.08 ± 0.06 | 0.9999 |
| 0.75–5.0 | 0.93 ± 0.04 | 9.2 ± 0.1 | 0.9994 | ||
| Dye | Added (µg) | Found (µg) | RSD (%) | R (%) |
|---|---|---|---|---|
| Sunset yellow FCF | 0.0226 | 0.0227 ± 0.0004 | 1.6 | 100 ± 2 |
| 0.113 | 0.113 ± 0.006 | 5.1 | 100 ± 5 | |
| 0.226 | 0.227 ± 0.004 | 1.5 | 100 ± 2 | |
| 1.13 | 1.13 ± 0.02 | 1.5 | 100 ± 2 | |
| 2.26 | 2.27 ± 0.02 | 0.91 | 100.4 ± 0.9 | |
| 11.3 | 11.32 ±0.08 | 0.54 | 100.2 ± 0.7 | |
| Tartrazine | 11.3 | 11.32 ± 0.08 | 2.9 | 100.2 ± 0.7 |
| 0.0267 | 0.0263 ± 0.0009 | 4.9 | 99 ± 3 | |
| 0.134 | 0.134 ± 0.007 | 1.9 | 100 ± 5 | |
| 0.267 | 0.265 ± 0.006 | 2.0 | 99 ± 2 | |
| 1.34 | 1.34 ± 0.03 | 2.2 | 100 ± 2 | |
| 13.4 | 13.4 ± 0.1 | 0.64 | 100.0 ± 0.7 |
| Sample | Dye | Spiked (µmol L−1) | Found (µmol L−1) | RSD (%) | R (%) |
|---|---|---|---|---|---|
| Sample 1 | Sunset yellow FCF | 0 | 0.144 ± 0.005 | 1.4 | |
| 0.072 | 0.21 ± 0.01 | 2.9 | 97 ± 3 | ||
| 0.144 | 0.289 ± 0.005 | 0.69 | 100 ± 2 | ||
| Tartrazine | 0 | 0.37 ± 0.01 | 1.9 | ||
| 0.186 | 0.55 ± 0.01 | 0.92 | 98 ± 2 | ||
| 0.37 | 0.74 ± 0.02 | 1.0 | 100 ± 3 | ||
| Sample 2 | Sunset yellow FCF | 0 | 0.60 ± 0.01 | 0.92 | |
| 0.30 | 0.89 ± 0.03 | 1.2 | 99 ± 3 | ||
| 0.60 | 0.120 ± 0.004 | 1.3 | 100 ± 3 | ||
| Tartrazine | 0 | 0.17 ± 0.01 | 2.9 | ||
| 0.085 | 0.255 ± 0.009 | 2.0 | 100 ± 3 | ||
| 0.17 | 0.343 ± 0.006 | 0.73 | 101 ± 2 |
| Sample | Dye | Dye Contents (mg L−1) | t-Test * | F-Test ** | |||
|---|---|---|---|---|---|---|---|
| Voltammetry | RSD (%) | Chromatography | RSD (%) | ||||
| 1 | Sunset yellow FCF | 6.5 ± 0.2 | 1.3 | 6.7 ± 0.5 | 5.0 | 0.634 | 15.5 |
| Tartrazine | 19.6 ± 0.5 | 1.7 | 20.0 ± 0.9 | 1.8 | 1.38 | 1.14 | |
| 2 | Sunset yellow FCF | 26.9 ± 0.6 | 0.97 | 27 ± 1 | 2.1 | 0.575 | 4.56 |
| Tartrazine | 9.2 ± 0.7 | 2.9 | 9 ± 1 | 8.3 | 0.279 | 7.86 | |
| 3 | Sunset yellow FCF | 27 ± 1 | 1.6 | 28 ± 3 | 4.9 | 0.153 | 9.44 |
| Tartrazine | 7.8 ± 0.7 | 3.4 | 7.9 ± 0.5 | 2.7 | 0.609 | 1.52 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziyatdinova, G.; Titova, M.; Davletshin, R. Electropolymerized 4-Aminobenzoic Acid Based Voltammetric Sensor for the Simultaneous Determination of Food Azo Dyes. Polymers 2022, 14, 5429. https://doi.org/10.3390/polym14245429
Ziyatdinova G, Titova M, Davletshin R. Electropolymerized 4-Aminobenzoic Acid Based Voltammetric Sensor for the Simultaneous Determination of Food Azo Dyes. Polymers. 2022; 14(24):5429. https://doi.org/10.3390/polym14245429
Chicago/Turabian StyleZiyatdinova, Guzel, Maria Titova, and Rustam Davletshin. 2022. "Electropolymerized 4-Aminobenzoic Acid Based Voltammetric Sensor for the Simultaneous Determination of Food Azo Dyes" Polymers 14, no. 24: 5429. https://doi.org/10.3390/polym14245429