Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation
Multifunctional Polymers Used in Agricultural Application and Environmental Treatment
)
Abstract
:1. Introduction
2. Materials and Methods
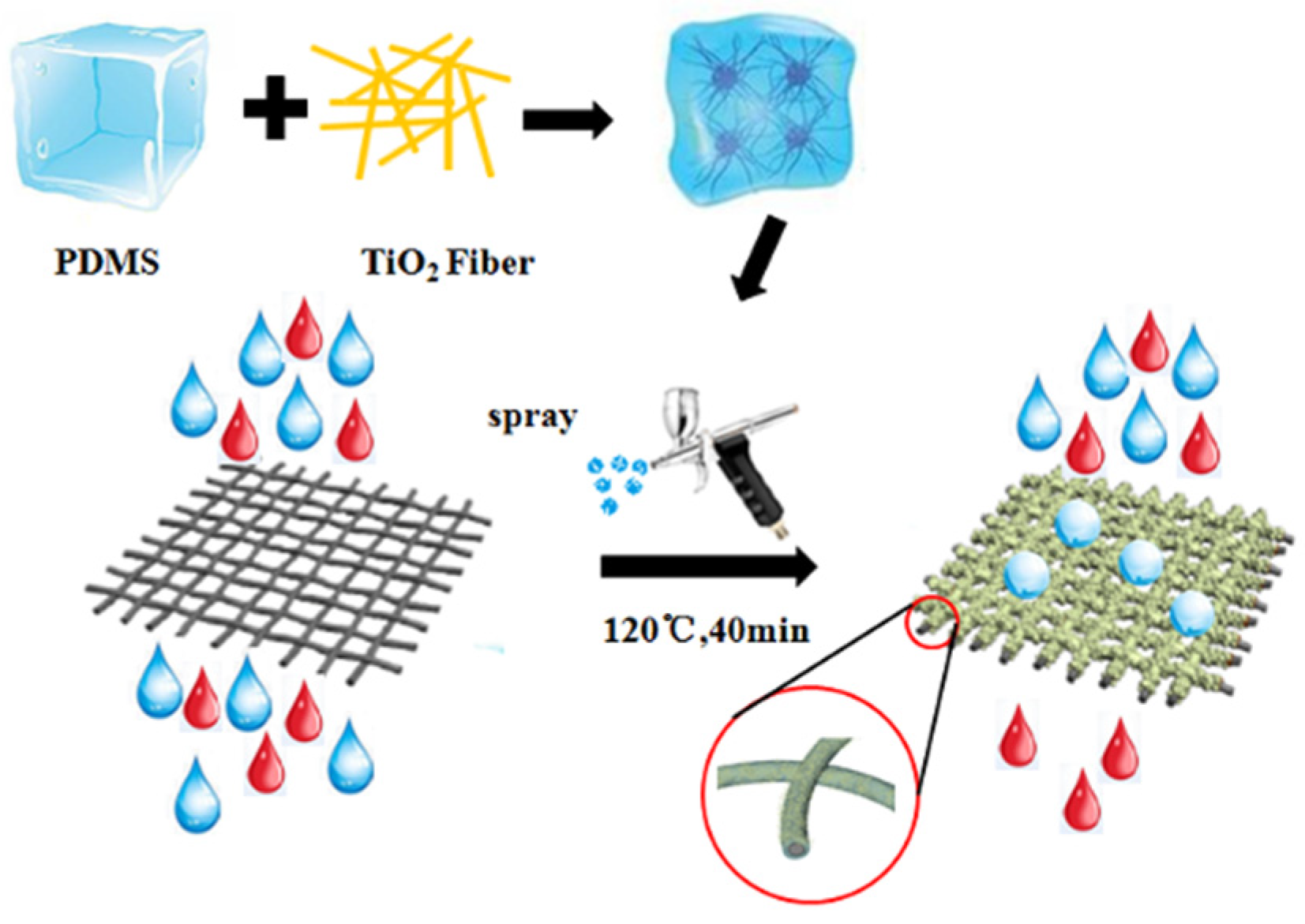
2.1. Preparation of TiO2 Nanofibers
2.2. Preparation of PDMS/TiO2 Composite Coating
2.3. Sample Testing and Characterization
2.4. Anti-Corrosion Property Test
2.5. Oil–Water Separation Performance Test
2.6. Durability Test
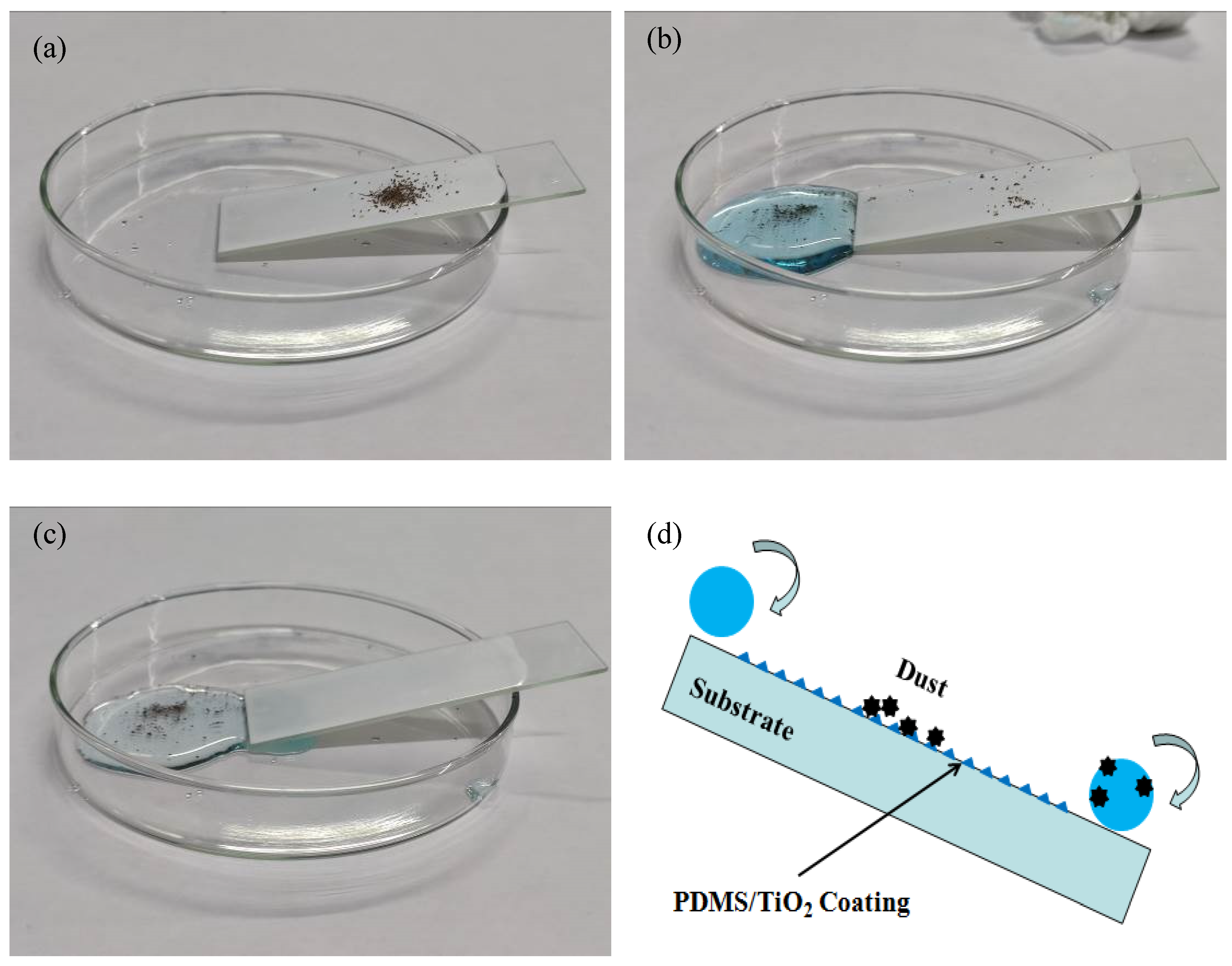
2.7. Self-Cleaning Test
2.8. Water Jet Stability
3. Results and Discussion
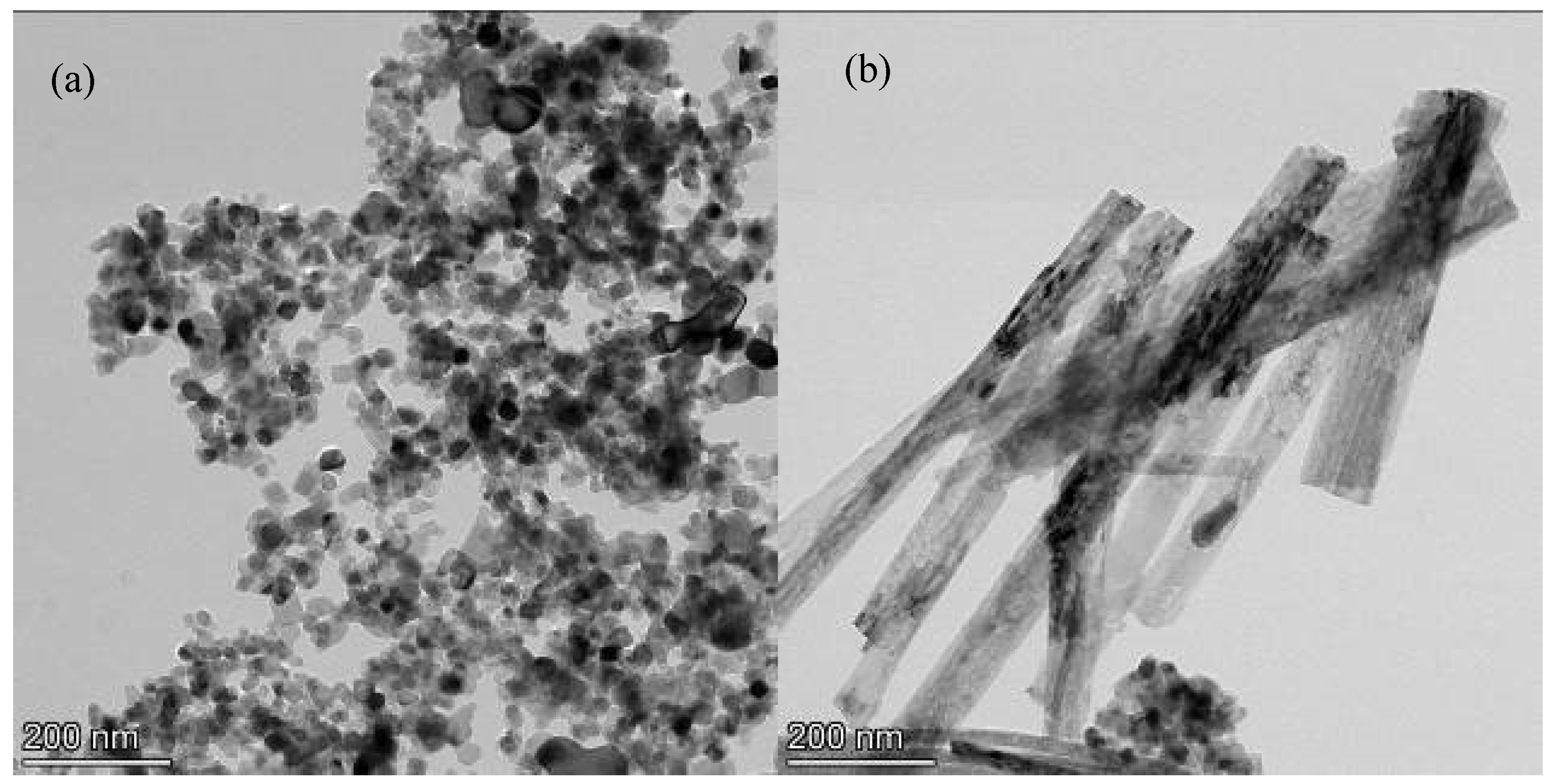
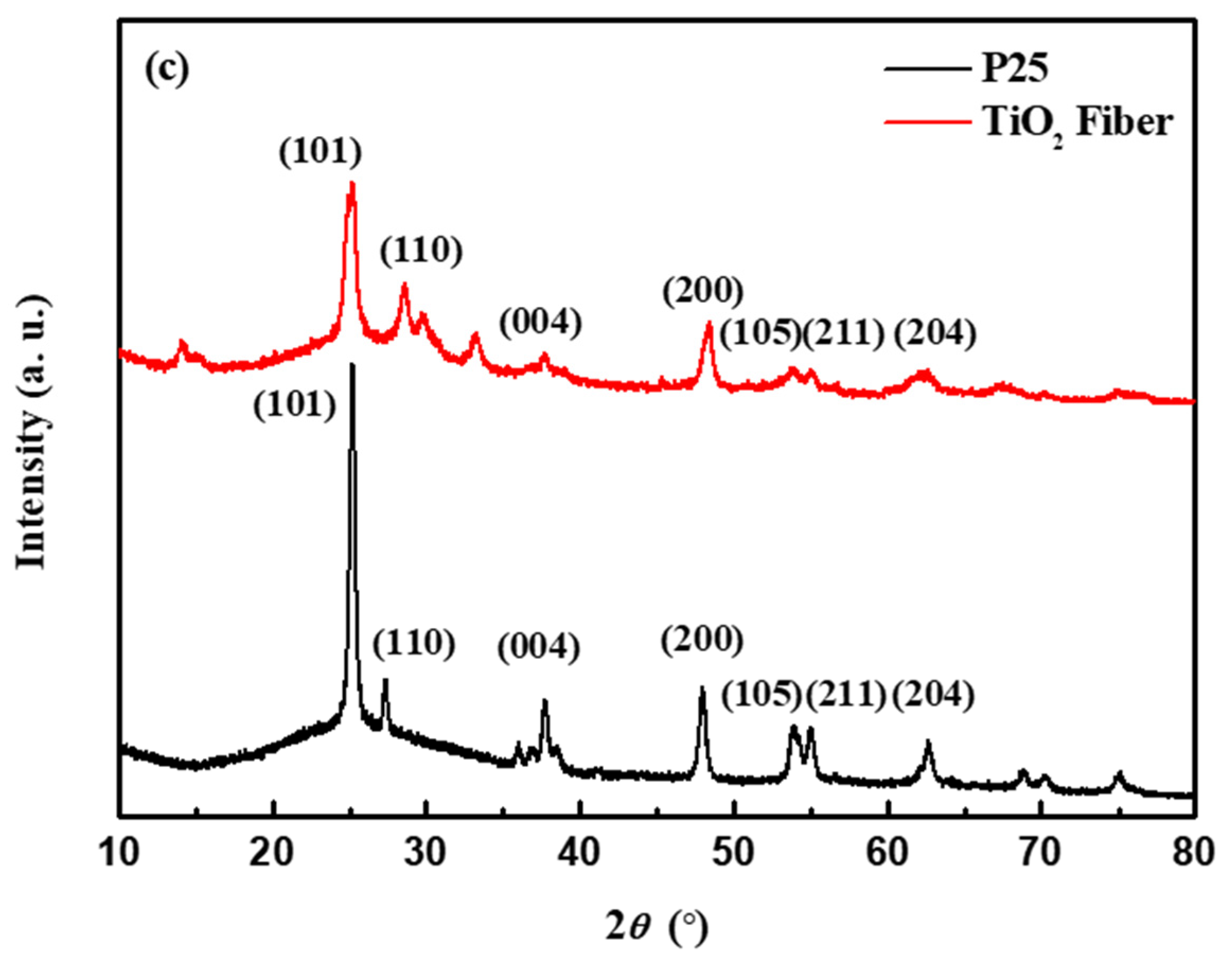
3.1. Morphology and Composition of TiO2 Nanofibers
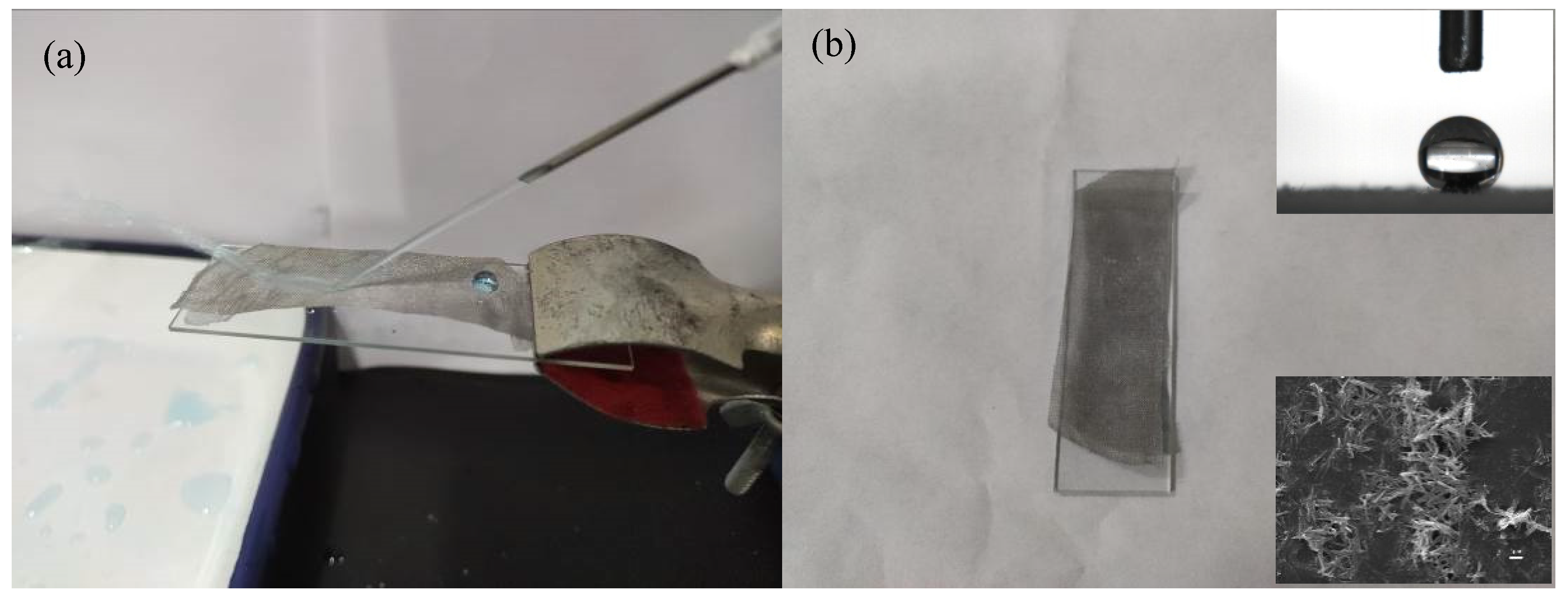
3.2. PDMS/TiO2-Coated Stainless Steel Mesh
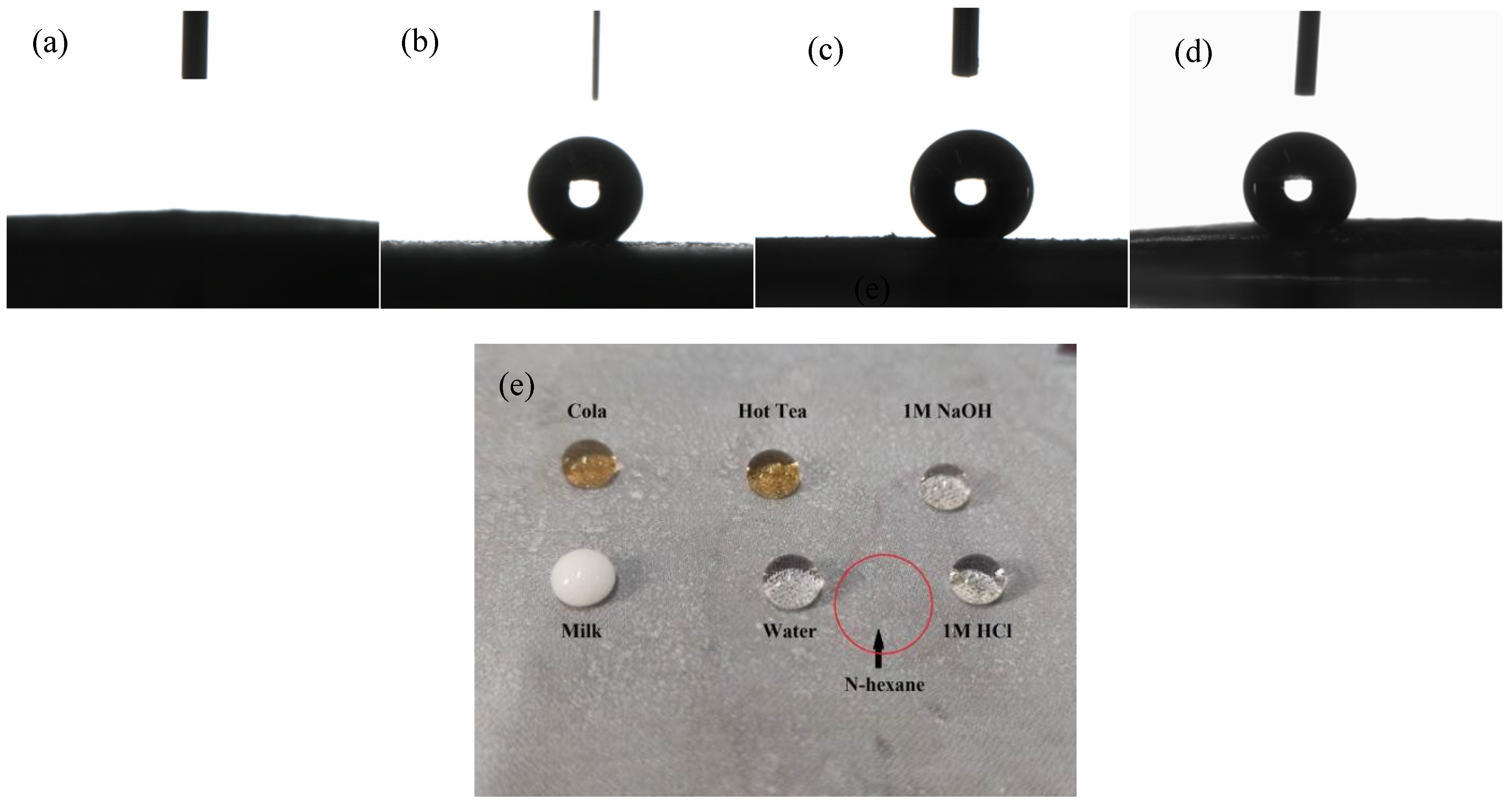
3.3. Contact Angle Measurement
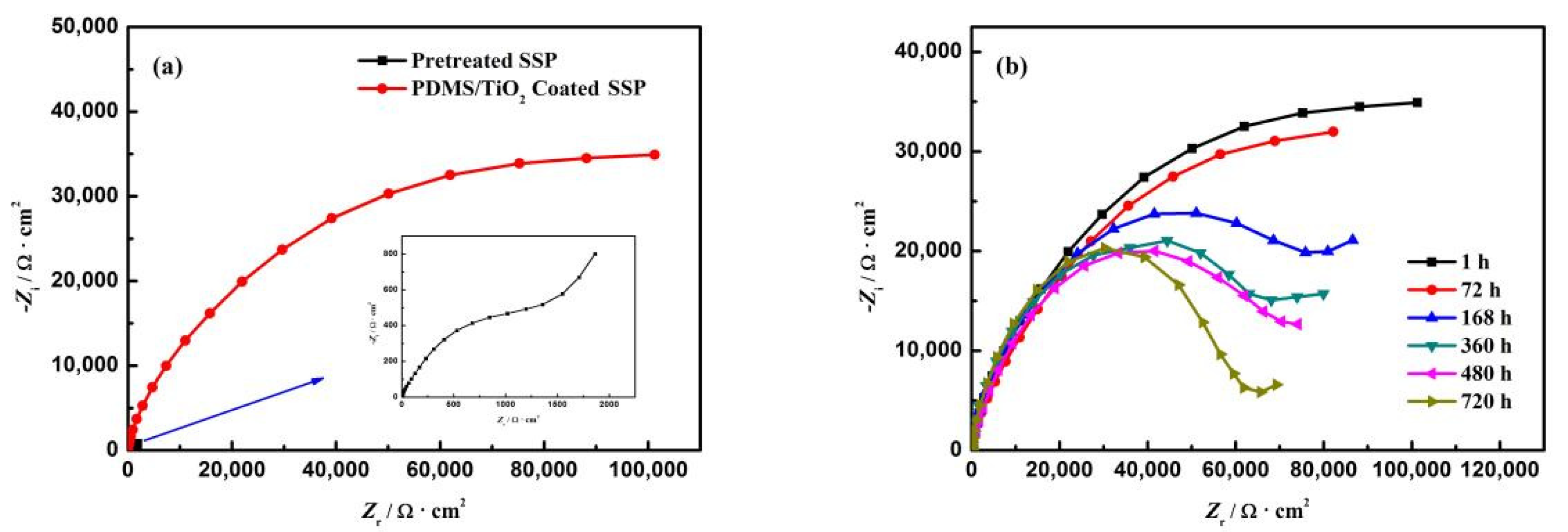
3.4. Corrosion Resistance Test
3.5. Oil–Water Separation Performance
3.6. Durability Measurement
3.7. Self-Cleaning Performance
4. Conclusions
- (1)
- The TiO2 nanofibers prepared by the hydrothermal method are uniform in size. The TiO2 nanofibers are added to the PDMS to form a superhydrophobic coating, which is sprayed on the stainless steel mesh to obtain good superhydrophobic performance. At the same time, the superhydrophobic coating has excellent corrosion resistance and can still protect the stainless steel mesh after 720 h of immersion;
- (2)
- When the superhydrophobic SSM is used as oil–water separation material, the separation efficiency of different oil–water mixtures is above 94%, and the separation efficiency is basically unchanged in acid, alkali, and salt environments;
- (3)
- After 50 times of continuous separation, the separation efficiency is still above 92%. After 40 cycles of polishing, the coating still has a high water contact angle, indicating that the superhydrophobic coating has good wear durability;
- (4)
- The PDMS/TiO2 composite coating has excellent self-cleaning performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, Z.L.; Feng, Y.J.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.X.; Cao, Y.Z.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.X.; Guo, Z.G.; Liu, W.M. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef]
- Ma, Q.L.; Cheng, H.F.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef]
- Li, J.; Kang, R.M.; Tang, X.H.; She, H.D.; Yang, Y.X.; Zha, F. Superhydrophobic meshes that can repel hot water and strong corrosive liquids used for efficient gravity-driven oil/water separation. Nanoscale 2016, 8, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhu, L.T.; Luo, Z.H. Electrospun fibrous membrane with enhanced swithchable oil/water wettability for oily water separation. Chem. Eng. J. 2016, 287, 474–481. [Google Scholar] [CrossRef]
- Yu, Y.L.; Chen, H.; Liu, Y.; Craig, V.; Li, L.H.; Chen, Y. Superhydrophobic and superoleophilic boron nitride nanotube-coated stainless steel meshes for oil and water separation. Adv. Mater. Interfaces 2014, 1, 1300002. [Google Scholar] [CrossRef]
- Kwon, G.; Kota, A.K.; Li, Y.X.; Sohani, A.; Mabry, J.M.; Tuteja, A. On-demand separation of oil–water mixtures. Adv. Mater. 2012, 24, 3666–3671. [Google Scholar] [CrossRef]
- Salehabadi, S.; Seyfi, J.; Hehazi, I.; Davachi, S.M.; Naeini, A.H.; Khakbaz, M. Nanosilica-decorated sponges for efficient oil/water separation: Role of nanoparticle’s type and concentration. J. Mater. Sci. 2017, 52, 7017–7027. [Google Scholar] [CrossRef]
- Zhang, C.L.; Li, P.; Cao, B. Fabrication of superhydrophobic–superoleophilic fabrics by an etching and dip-coating two-step method for oil–water separation. Ind. Eng. Chem. Res. 2016, 55, 5030–5035. [Google Scholar] [CrossRef]
- Ge, B.; Zhang, Z.Z.; Zhu, X.T.; Men, X.H.; Zhou, X.Y.; Xue, Q.J. A graphene coated cotton for oil/water separation. Compos. Sci. Technol. 2014, 102, 100–105. [Google Scholar] [CrossRef]
- Piltan, S.; Seyfi, J.; Hejazi, I.; Davachi, S.M.; Khonakdar, H.A. Superhydrophobic filter paper via an improved phase separation process for oil/water separation: Study on surface morphology, composition and wettability. Cellulose 2016, 23, 3913–3924. [Google Scholar] [CrossRef]
- Wang, F.J.; Lei, S.; Xu, Y.; Ou, J.F. Green approach to the fabrication of superhydrophobic mesh surface for oil/water separation. ChemPhysChem 2015, 16, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, Y.N.; Liu, N.; Cao, Y.Z.; Xu, L.X.; Zhang, W.F.; Feng, L. In situ ultrafast separation and purification of oil/water emulsions by superwetting TiO2 nanocluster-based mesh. Nanoscale 2016, 8, 8525–8529. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Li, H.Y.; Li, J.P.; Zha, F.; Lei, Z.Q. A facile one-step spray-coating process for the fabrication of superhydrophobic attapulgite coated mesh used in oil/water separation. RSC Adv. 2015, 5, 53802–53808. [Google Scholar] [CrossRef]
- Cao, M.; Luo, X.M.; Ren, H.J.; Feng, J.Y. Hot water-repellent and mechanically durable superhydrophobic mesh for oil/water separation. J. Colloid Interf. Sci. 2018, 512, 567–574. [Google Scholar] [CrossRef]
- Lu, Z.X.; Huang, X.; Wang, L.S. Superhydrophobic hierarchical structure carbon mesh films for oil/water separation application. Appl. Phys. A-Mater. 2017, 123, 538. [Google Scholar] [CrossRef]
- Lee, S.; Kim, W.; Yong, K. Overcoming the water vulnerability of electronic devices: A highly water-resistant ZnO nanodevice with multifunctionality. Adv. Mater. 2011, 23, 4398–4402. [Google Scholar] [CrossRef]
- Chang, F.M.; Cheng, S.L.; Hong, S.J.; Sheng, Y.J.; Tsao, H.K. Superhydrophilicity to superhydrophobicity transition of CuO nanowire films. Appl. Phys. Lett. 2010, 96, 114101. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Nishimoto, S.; Saito, H.; Murakami, T.; Fujishima, A. Preparation and photocatalytic wettability conversion of TiO2-based superhydrophobic surfaces. Langmuir 2006, 22, 9477–9479. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube-TiO2 composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar]
- Dawidczyk, T.J.; Walton, M.D.; Jang, W.S.; Grunlan, J.C. Layer-by-layer assembly of UV-resistant poly (3,4-ethylenedioxythiophene) thin films. Langmuir 2008, 24, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhou, B.; Bai, J.; Li, L.; Jin, Z.; Zhang, J.; Li, J. Self-organized TiO2 nanotube array sensor for the determination of chemical oxygen demand. Adv. Mater. 2008, 20, 1044–1049. [Google Scholar]
- Bolvardia, B.; Seyfib, J.; Hejazic, I.; Otadia, M.; Khonakdard, H.A.; Davachi, S.M. Towards an efficient and durable superhydrophobic mesh coated by PDMS/TiO2 nanocomposites for oil/water separation. Appl. Surf. Sci. 2019, 492, 862–870. [Google Scholar] [CrossRef]
- Qing, Y.Q.; Yang, C.N.; Sun, Y.Z.; Zheng, Y.S.; Wang, X.D.; Shang, Y.; Wang, L.S.; Liu, C.S. Facile fabrication of superhydrophobic surfaces with corrosion resistance by nanocomposite coating of TiO2 and polydimethylsiloxane. Colloid. Surf. A Physicochem. Eng. Asp. 2015, 484, 471–477. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Gao, L.; Yu, X.Q.; Liang, C.H.; Zhang, Y.F. Durability evaluation of superhydrophobic copper foams for long-term oil-water separation. Appl. Surf. Sci. 2017, 407, 145–155. [Google Scholar] [CrossRef]
- Latthe, S.S.; Terashima, C.; Nakata, K.; Sakai, M.; Fujishima, A. Development of sol-gel processed semi-transparent and self-cleaning superhydrophobic coatings. J. Mater. Chem. A 2014, 2, 5548–5553. [Google Scholar] [CrossRef]
- Yong, C.J.; Bhushan, B. Mechanically durable carbon nanotube-composite hierarchical structures with superhydrophobicity, self-cleaning, and low-drag. ACS Nano 2009, 3, 4155–4163. [Google Scholar]
- Gurav, A.B.; Xu, Q.F.; Latthe, S.S.; Vhatkar, R.S.; Liu, S.H.; Yoon, H.; Yoon, S.S. Superhydrophobic coatings prepared from methyl-modified silica particles using simple ip-coating method. Ceram. Int. 2015, 41, 3017–3023. [Google Scholar] [CrossRef]
- Gao, J.F.; Huang, X.W.; Xue, H.G.; Tang, L.C.; Li, R.K. Facile preparation of hybrid microspheres for super-hydrophobic coating and oil-water separation. Chem. Eng. J. 2017, 326, 443–453. [Google Scholar] [CrossRef]
- Cao, W.T.; Liu, Y.J.; Ma, M.G.; Zhu, J.F. Facile preparation of robust and superhydrophobic materials for self-cleaning and oil/water separation. Colloid. Surf. A Physicochem. Eng. Asp. 2017, 529, 18–25. [Google Scholar] [CrossRef]
- Xu, P.; Li, X.X. Fabrication of TiO2/SiO2 superhydrophobic coating for efficient oil/water separation. J. Environ. Chem. Eng. 2021, 9, 105538. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wei, Y.Q.; Tao, F.R.; Zhang, X.; Gai, L.G.; Liu, L.B. All-water-based superhydrophobic coating with reversible wettability for oil-water separation and wastewater purification. Prog. Org. Coat. 2022, 165, 106726. [Google Scholar] [CrossRef]
- Chen, F.J.; Hao, S.M.; Huang, S.; Lu, Y. Nanoscale SiO2-coated superhydrophobic meshes via electro-spray deposition for oil-water separation. Powder Technol. 2020, 373, 82–92. [Google Scholar] [CrossRef]
- Liu, G.Y.; Wang, J.J.; Wang, W.; Yu, D. A novel PET fabric with durable anti-fouling performance for reusable and efficient oil-water separation. Colloid. Surf. A Physicochem. Eng. Asp. 2019, 583, 123941. [Google Scholar] [CrossRef]
- Cai, P.L.; Di, K.Y.; Lv, J.S.N.; Li, S.; Chen, X.T. Environmentally benign and durable superhydrophobic coatings based on short fluorocarbon chain siloxane modified halloysite nanotubes for oil/water separation. Colloid. Surf. A Physicochem. Eng. Asp. 2021, 630, 127540. [Google Scholar] [CrossRef]
- Zeng, Z.W.; Wu, X.Z.; Liu, Y.; Chen, C.; Tian, D.; He, Y.; Wang, L.L.; Yang, G.; Zhang, X.H.; Zhang, Y.Z. Fabrication of a durable coral-like superhydrophilic MgO coating on stainless steel mesh for efficient oil/water separation. Chem. Eng. Sci. 2022, 248, 117144. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Li, H.; Liu, P.Z.; Liu, X.L.; Gan, S.P.; Chang, X.; Zhu, L.; Wei, B.J.; Xue, Q.Z. Recyclable superhydrophilic meshes with scalable and robust coating for separating oily wastewater. Appl. Surf. Sci. 2022, 602, 154396. [Google Scholar] [CrossRef]




| Materials | Base Materials | Oil | Separation Efficiency (%) | References |
|---|---|---|---|---|
| TiO2 nanoparticles + PDMS | Iron–chromium–nickel alloy meshes | Hexane | 95 | [24] |
| SiO2 + PVDF | Filter paper | Dichloromethane | 97.5 | [30] |
| Magnesium stearate + phenol formaldehyde resin | SSM | Hexane | 95 | [31] |
| TiO2/SiO2 + epoxy resin | SSM | Soybean oil | 95 | [32] |
| TiO2 + propyl methacrylate | Cotton fabrics | Dichloromethane | 96 | [33] |
| Nanoscale SiO2 + dodecyl trimethoxy silane | SSM | Kerosene | 95 | [34] |
| Docosanethiol + octavinylsilsesquioxane | PET fabrics | Toluene | 97 | [35] |
| F-HNTs + epoxy resin | SSM | Dichloromethane | 97.8 | [36] |
| TiO2 nanofibers + PDMS | SSM | Hexane | 95 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, K.; Huang, X.; Pan, J. Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation. Polymers 2022, 14, 5431. https://doi.org/10.3390/polym14245431
Cao K, Huang X, Pan J. Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation. Polymers. 2022; 14(24):5431. https://doi.org/10.3390/polym14245431
Chicago/Turabian StyleCao, Kun, Xi Huang, and Jie Pan. 2022. "Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation" Polymers 14, no. 24: 5431. https://doi.org/10.3390/polym14245431
APA StyleCao, K., Huang, X., & Pan, J. (2022). Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation. Polymers, 14(24), 5431. https://doi.org/10.3390/polym14245431






