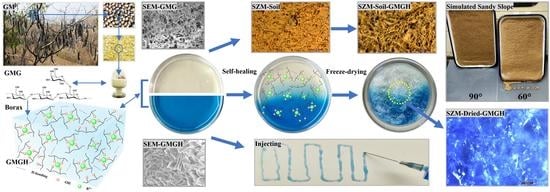
Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
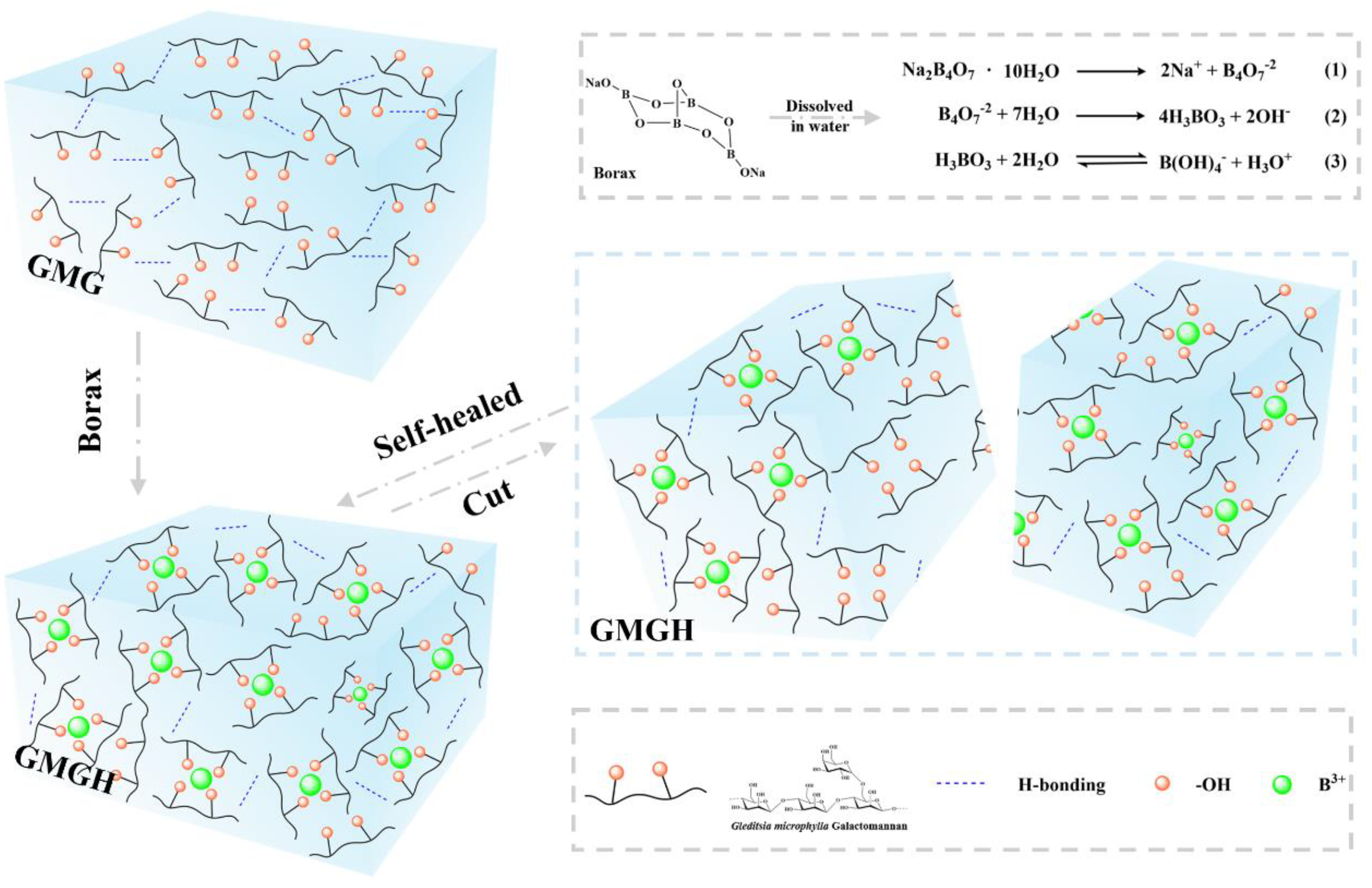
2.2. Synthesis of Hydrogels
2.3. Characterization of Hydrogels
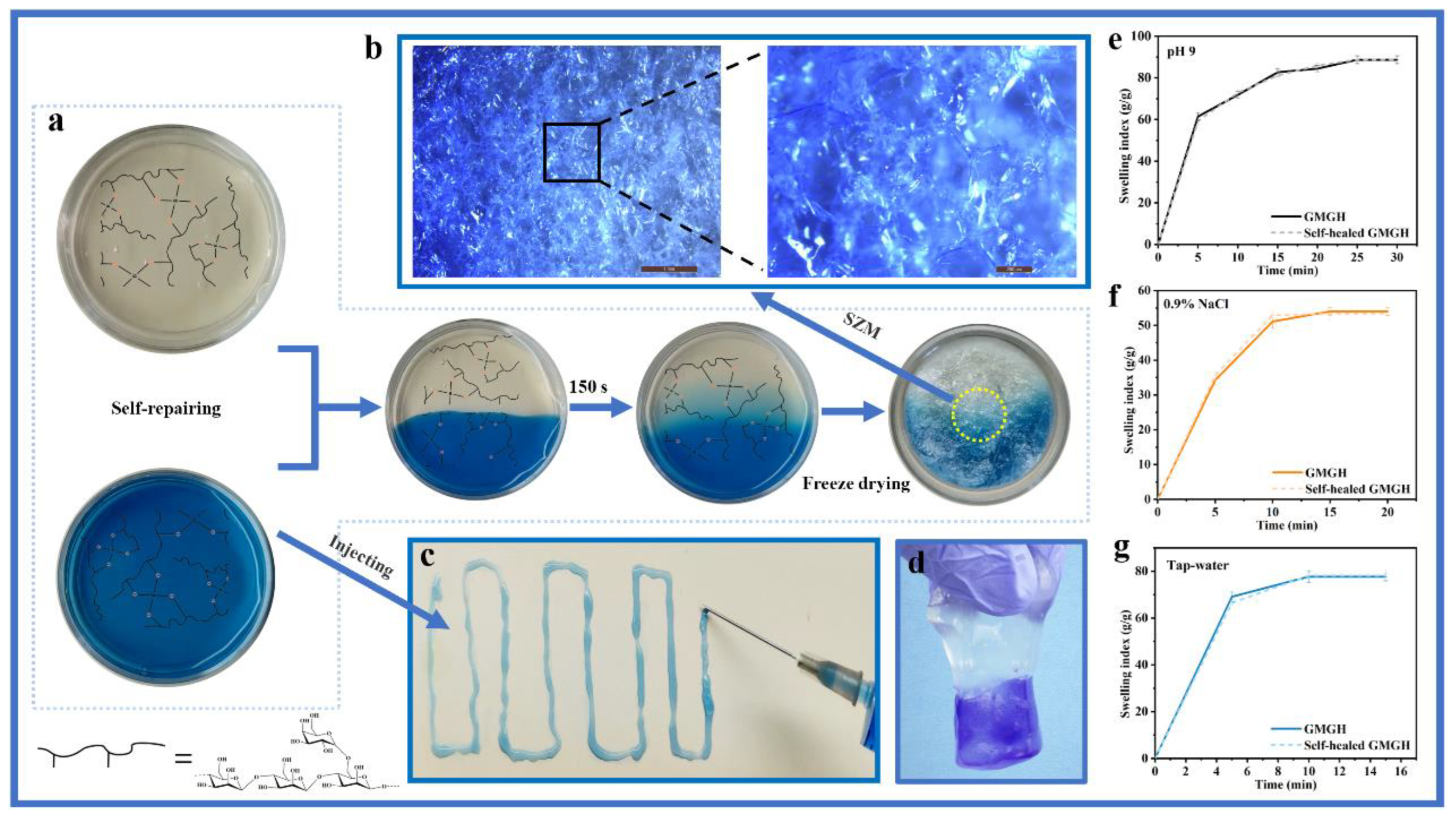
2.4. Self-Repair Performance
2.5. Swelling Property of GMGH
2.6. WHC of the Soil–GMGH
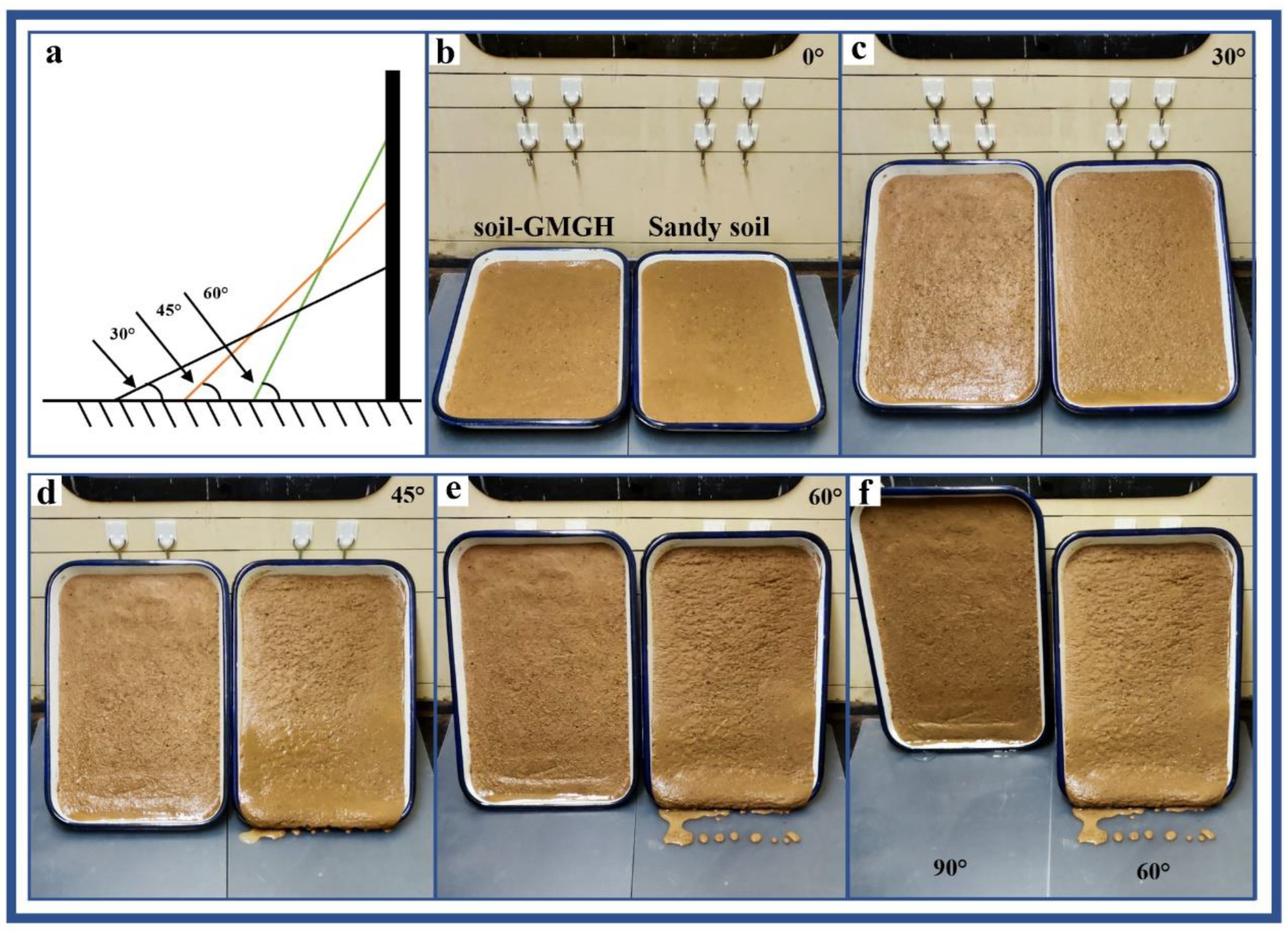
2.7. Sandy Slope Simulation Experiments
3. Results and Discussions
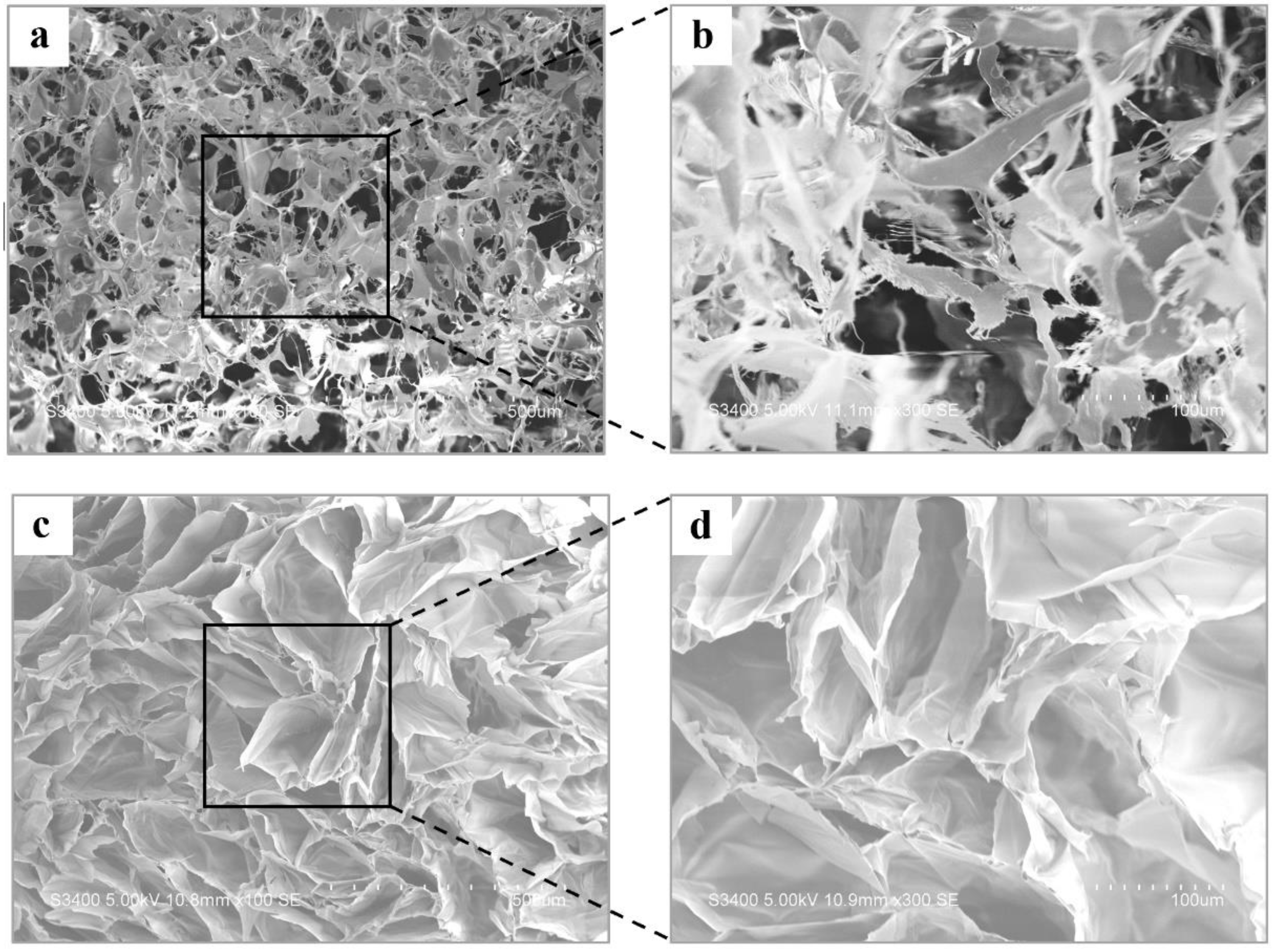
3.1. Characterization of GMGH
3.2. Self-Healing and Injection Ability
3.3. Water Absorption and Retention of GMGH
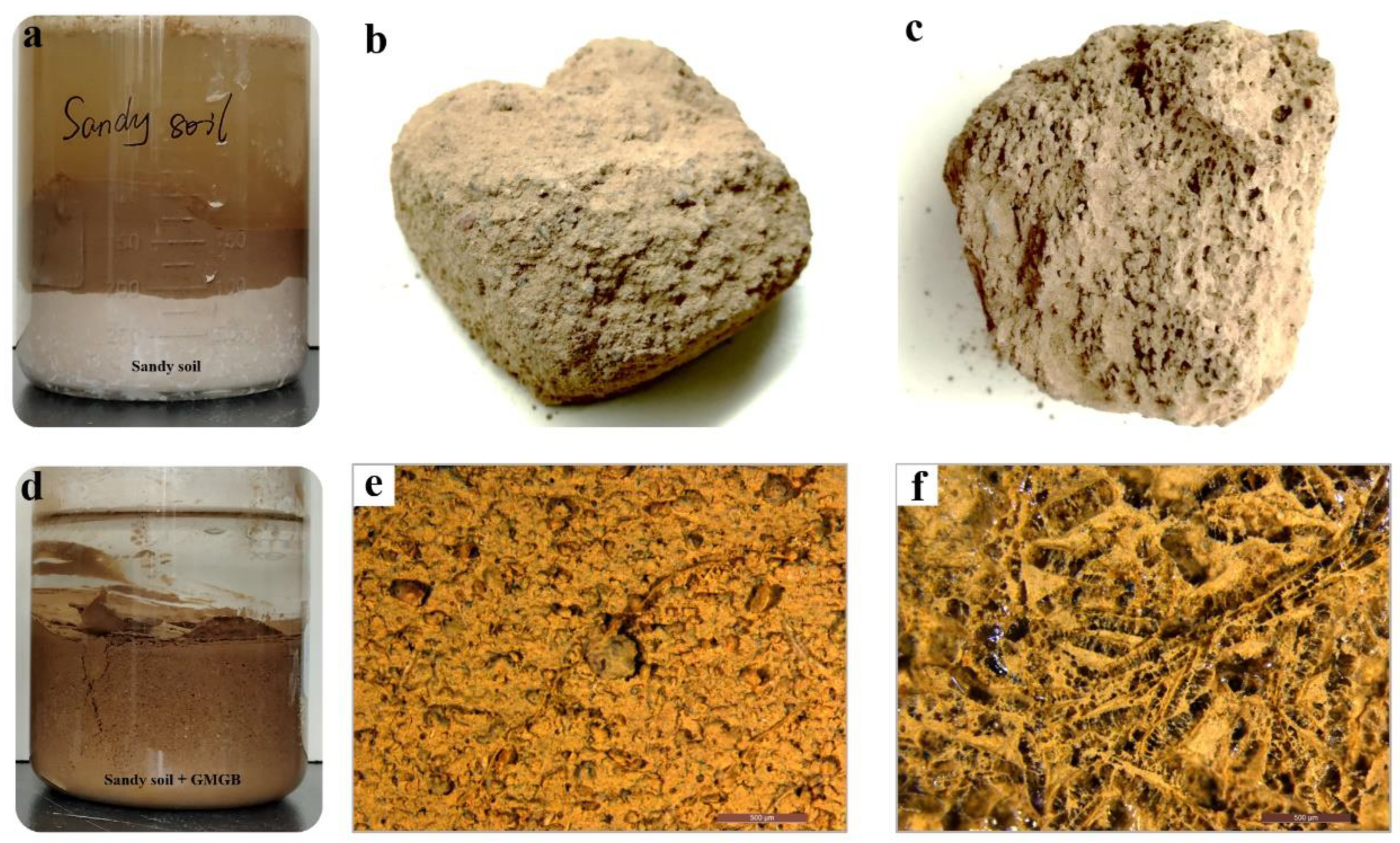
3.4. Determination of WHC of Sandy Soil
3.5. Cyclic Water Absorption and Water Retention Capacity of the Soil-GMGH
3.6. Results of the Sandy Slope Simulation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Oztas, T.; Koc, A.; Comakli, B. Changes in vegetation and soil properties along a slope on overgrazed and eroded rangelands. J. Arid. Environ. 2003, 55, 93–100. [Google Scholar] [CrossRef]
- Zou, C.; Wang, K.; Wang, T.; Xu, W. Overgrazing and soil carbon dynamics in eastern inner mongolia of china. Ecol. Res. 2007, 22, 135–142. [Google Scholar] [CrossRef]
- Zhu, Y.; Drake, R.; Lue, R.; Xia, R. Analysis of temporal and spatial differences in eco-environmental carrying capacity related to water in the haihe river basins, china. Water Resour. Manag. 2010, 24, 1089–1105. [Google Scholar] [CrossRef]
- Chen, I.S.; Weng, C.J.; Chen, Y.R.; Huang, S.P.; Wen, Z.H.; Jang-Liaw, N.H.; Tsai, T.H. The checklist of inland-water and mangrove fish fauna of kinmen island, fujian province, taiwan with comments on ecological conservation of native fishes. J. Mar. Sci. Technol. 2013, 21, 316–319. [Google Scholar]
- Athukorala, W.; Wilson, C. Groundwater overuse and farm-level technical inefficiency: Evidence from sri lanka. Hydrogeol. J. 2012, 20, 893–905. [Google Scholar] [CrossRef]
- Forkutsa, I.; Sommer, R.; Shirokova, Y.I.; Lamers, J.P.A.; Kienzler, K.; Tischbein, B.; Martius, C.; Vlek, P.L.G. Modeling irrigated cotton with shallow groundwater in the Aral Sea Basin of Uzbekistan: I. Water dynamics. Irrigation Sci. 2009, 27, 319–330. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Sidhu, G.S. Soil degradation in India: Causes, major threats, and management options. In Proceedings of the MARCO Symposium, Tsukuba, Japan, 26–28 August 2015; pp. 151–156. [Google Scholar]
- Arnaud-Fassetta, G. River channel changes in the rhone delta (france) since the end of the little ice age: Geomorphological adjustment to hydroclimatic change and natural resource management. Catena 2003, 51, 141–172. [Google Scholar] [CrossRef]
- Zheng, H.; Guan, D. Major effects of road construction on eco-environment. Chin. J. Ecol. 2005, 24, 1520–1524. [Google Scholar]
- Langergraber, G. The role of plant uptake on the removal of organic matter and nutrients in subsurface flow constructed wetlands: A simulation study. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2005, 51, 213–223. [Google Scholar] [CrossRef]
- Elena, S.; Gladys, L.; Claudia, A.; Xavier, S.; Salvador, N. Photosynthesis, resource acquisition and growth responses of two biomass crops subjected to water stress. J. Plant Sci. 2018, 6, 68–86. [Google Scholar]
- Savage, J.A.; Beecher, S.D.; Clerx, L.; Gersony, J.T.; Knoblauch, J.; Losada, J.M.; Jensen, K.H.; Knoblauch, M.; Holbrook, N.M. Maintenance of carbohydrate transport in tall trees. Nat. Plants 2017, 3, 965–972. [Google Scholar] [CrossRef]
- Baiamonte, G.; De Pasquale, C.; Marsala, V.; Cimò, G.; Alonzo, G.; Crescimanno, G.; Conte, P. Structure alteration of a sandy-clay soil by biochar amendments. J. Soil. Sediments 2015, 15, 816–824. [Google Scholar] [CrossRef]
- Nadaf, S.A.; Chidanandappa, H.M. Effect of zinc and boron application on distribution and contribution of zinc fractions to the total uptake of zinc by groundnut (Arachis hypogaea L.) in sandy loam soils of Karnataka, India. Legume Res. 2015, 38, 598–602. [Google Scholar] [CrossRef][Green Version]
- Nadaf, S.A.; Chidanandappa, H.M. Content and uptake of macronutrients by groundnut (Arachis hypogaea L.) as influenced by soil application of zinc and boron in sandy loam soils of Karnataka, India. Legume Res. 2015, 38, 363–366. [Google Scholar] [CrossRef]
- Mandal, K.G.; Sinha, A.C. Nutrient management effects on light interception, photosynthesis, growth, dry-matter production and yield of Indian mustard (Brassica juncea). J. Agron. Crop Sci. 2004, 190, 119–129. [Google Scholar] [CrossRef]
- Liu, C.; Lei, F.; Li, P.; Jiang, J.; Wang, K. Borax crosslinked fenugreek galactomannan hydrogel as potential water-retaining agent in agriculture. Carbohyd. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superab-sorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax cross-linked guar gum hydrogels as potential adsorbents for water purification. Carbohyd. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Mishra, S.; Siddiqui, M.Z.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohyd. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Jnanesha, A.C.; Kumar, A.; Lal, R.K. Hydrogel application improved growth and yield in Senna (Cassia angustifolia Vahl.). Ind. Crop. Prod. 2021, 174, 114175. [Google Scholar] [CrossRef]
- Čechmánková, J.; Skála, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability 2021, 13, 10701. [Google Scholar] [CrossRef]
- Agaba, H.; Orikiriza, L.J.B.; Esegu, J.F.O.; Obua, J.; Kabasa, J.D.; Huttermann, A. Effects of Hydrogel Amendment to Different Soils on Plant Available Water and Survival of Trees under Drought Conditions. Clean-Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Kapur, G.S. Enzyme-based green approach for the synthesis of gum tragacanth and acrylic acid cross-linked hydrogel: Its utilization in controlled fertilizer release and enhancement of water-holding capacity of soil. Iran. Polym. J. 2013, 22, 561–570. [Google Scholar]
- Costa, M.C.G.; Freire, A.G.; Lourenco, D.V.; De Sousa, R.R.; Feitosa, J.P.D.; Mota, J.C.A. Hydrogel composed of potassium acrylate, acrylamide, and mineral as soil conditioner under saline conditions. Sci. Agr. 2022, 79, e20200235. [Google Scholar] [CrossRef]
- Kareem, S.A.; Dere, I.; Gungula, D.T.; Andrew, F.P.; Saddiq, A.M.; Adebayo, E.F.; Tame, V.T.; Kefas, H.M.; Joseph, J.; Patrick, D.O. Synthesis and Characterization of Slow-Release Fertilizer Hydrogel Based on Hydroxy Propyl Methyl Cellulose, Polyvinyl Alcohol, Glycerol and Blended Paper. Gels 2021, 7, 262. [Google Scholar] [CrossRef]
- Womack, N.C.; Piccoli, I.; Camarotto, C.; Squartini, A.; Guerrini, G.; Gross, S.; Maggini, M.; Cabrera, M.L.; Morari, F. Hydrogel application for improving soil pore network in agroecosystems. Preliminary results on three different soils. Catena 2022, 208, 105759. [Google Scholar] [CrossRef]
- Rinaudo, M. New way to crosslink chitosan in aqueous solution. Eur. Polym. J. 2010, 46, 1537–1544. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohyd. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Rodrigues Noleto, G.; Oliveira Petkowicz, C.L. Potential for Biomedical Applications of Galactomannans and Xyloglucans from Seeds: An Overview. Med. Chem. 2016, 6, 658–661. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Fahmideh-Rad, E. Guar seed gum: Biological properties, chemical modifications, and structural analysis- A review. Int. J. Biol. Macromol. 2019, 138, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, W.; Lei, F.; Li, P.; Jiang, J. Comparison and characterization of galactomannan at different developmental stages of Gleditsia sinensis Lam. Carbohyd. Polym. 2019, 223, 115127. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, P.J. Design and development of guar gum and borax crosslinked guar gum matrix tablets of theophylline for colon specific drug. J. Chem. Pharm. Res. 2012, 4, 1052–1060. [Google Scholar]
- Li, N.; Liu, C.; Chen, W. Facile access to guar gum based supramolecular hydrogels with rapid self-healing ability and multistimuli responsive gel-sol transitions. J. Agr. Food Chem. 2019, 67, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, F.; Li, P.; Wang, K.; Jiang, J. A review on preparations, properties, and applications of cis-ortho-hydroxyl polysaccharides hydrogels crosslinked with borax. Int. J. Biol. Macromol. 2021, 182, 1179–1191. [Google Scholar] [CrossRef]
- Rani, G.U.; Dey, K.P.; Bharti, S.; Mishra, S. Controlled drug release of 5-amino salicylic acid by poly (2-hydroxyethylmethacrylate) grafted agar. Front. Chem. Sci. Eng. 2014, 8, 465–470. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Tan, T. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly (aspartic acid) and poly (acrylic acid). Polymer 2006, 47, 7702–7710. [Google Scholar] [CrossRef]
- Guo, L.; Ning, T.; Nie, L.; Li, Z.; Lal, R. Interaction of deep placed controlled-release urea and water retention agent on nitrogen and water use and maize yield. Eur. J. Agron. 2016, 75, 118–129. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Dunlop, J.M. Hydrophilic gel amendments to sand soil can increase growth and nitrogen uptake efficiency of citrus seedlings. Hort Sci. 2004, 39, 267–271. [Google Scholar] [CrossRef]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gómez-Cadenas, A. Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Tang, M.; Zhang, F.; Lei, F.; Li, P.; Wang, K.; Zeng, H.; Jiang, J. Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil. Polymers 2022, 14, 5430. https://doi.org/10.3390/polym14245430
Liu C, Tang M, Zhang F, Lei F, Li P, Wang K, Zeng H, Jiang J. Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil. Polymers. 2022; 14(24):5430. https://doi.org/10.3390/polym14245430
Chicago/Turabian StyleLiu, Chuanjie, Meng Tang, Fenglun Zhang, Fuhou Lei, Pengfei Li, Kun Wang, Hongbo Zeng, and Jianxin Jiang. 2022. "Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil" Polymers 14, no. 24: 5430. https://doi.org/10.3390/polym14245430
APA StyleLiu, C., Tang, M., Zhang, F., Lei, F., Li, P., Wang, K., Zeng, H., & Jiang, J. (2022). Facile Access to Gleditsia microphylla Galactomannan Hydrogel with Rapid Self-Repair Capacity and Multicyclic Water-Retaining Performance of Sandy Soil. Polymers, 14(24), 5430. https://doi.org/10.3390/polym14245430