Towards a Circular Economy: Study of the Mechanical, Thermal, and Electrical Properties of Recycled Polypropylene and Their Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of vPP/rPP Blends
2.3. Tensile Test
2.4. FTIR-ATR Analysis
2.5. Surface Resistivity Test
2.6. Differential Scanning Calorimetry Test
3. Results and Discussion
3.1. Feasibility of the Obtained vPP/rPP Blends
3.2. Tensile Test
3.3. FTIR–ATR Analysis
3.4. Surface Resistivity Test
3.5. Differential Scanning Calorimetry Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amrullah, A.; Farobie, O.; Septarini, S.; Satrio, J.A. Synergetic biofuel production from co-pyrolysis of food and plastic waste: Reaction kinetics and product behavior. Heliyon 2022, 8, e10278. [Google Scholar] [CrossRef]
- Kameel, N.I.A.; Daud, W.M.A.W.; Patah, M.F.A.; Zulkifli, N.W.M. Influence of reaction parameters on thermal liquefaction of plastic wastes into oil: A review. Energy Convers. Manag. X 2022, 14, 100196. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.-X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef] [Green Version]
- Râpă, M.; Spurcaciu, B.N.; Ion, R.-M.; Grigorescu, R.M.; Darie-Niță, R.N.; Iancu, L.; Nicolae, C.-A.; Gabor, A.R.; Matei, E.; Predescu, C. Valorization of Polypropylene Waste in the Production of New Materials with Adequate Mechanical and Thermal Properties for Environmental Protection. Materials 2022, 15, 5978. [Google Scholar] [CrossRef]
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 2021, 428, 131928. [Google Scholar] [CrossRef]
- Takarada, W.; Barique, M.A.; Kunimitsu, T.; Kameda, T.; Kikutani, T. Verification of the Influence of Processing History through Comparing High-Speed Melt Spinning Behavior of Virgin and Recycled Polypropylene. Polymers 2022, 14, 3238. [Google Scholar] [CrossRef]
- Cabernard, L.; Pfister, S.; Oberschelp, C.; Hellweg, S. Growing environmental footprint of plastics driven by coal combustion. Nat. Sustain. 2021, 5, 139–148. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, G.; Wang, Y.; Chen, S.; Xu, L.; Wang, R. Modelling the global impact of China’s ban on plastic waste imports. Resour. Conserv. Recycl. 2019, 154, 104607. [Google Scholar] [CrossRef]
- Morales, M.A.; Maranon, A.; Hernandez, C.; Porras, A. Development and Characterization of a 3D Printed Cocoa Bean Shell Filled Recycled Polypropylene for Sustainable Composites. Polymers 2021, 13, 3162. [Google Scholar] [CrossRef]
- Alhazmi, H.; Almansour, F.; Aldhafeeri, Z. Plastic Waste Management: A Review of Existing Life Cycle Assessment Studies. Sustainability 2021, 13, 5340. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Fletes, R.C.V.; López, E.O.C.; Sánchez, F.J.M.; Mendizábal, E.; Núñez, R.G.; Rodrigue, D.; Gudiño, P.O. Morphological and Mechanical Properties of Bilayers Wood-Plastic Composites and Foams Obtained by Rotational Molding. Polymers 2020, 12, 503. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Stache, E.E. Chemical Upcycling of Commercial Polystyrene via Catalyst-Controlled Photooxidation. J. Am. Chem. Soc. 2022, 144, 5745–5749. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K. A Brief Review of Poly(Vinyl Chloride) (PVC) Recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef]
- Assaad, J.J.; Khalil, M.; Khatib, J. Alternatives to Enhance the Structural Performance of PET-Modified Reinforced Concrete Beams. Environments 2022, 9, 37. [Google Scholar] [CrossRef]
- Assaad, J.J.; Khatib, J.M.; Ghanem, R. Bond to Bar Reinforcement of PET-Modified Concrete Containing Natural or Recycled Coarse Aggregates. Environments 2022, 9, 8. [Google Scholar] [CrossRef]
- Dziuba, R.; Kucharska, M.; Madej-Kiełbik, L.; Sulak, K.; Wiśniewska-Wrona, M. Biopolymers and Biomaterials for Special Applications within the Context of the Circular Economy. Materials 2021, 14, 7704. [Google Scholar] [CrossRef]
- Soares, C.T.D.M.; Ek, M.; Östmark, E.; Gällstedt, M.; Karlsson, S. Recycling of multi-material multilayer plastic packaging: Current trends and future scenarios. Resour. Conserv. Recycl. 2021, 176, 105905. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Gharde, S.; Kandasubramanian, B. Mechanothermal and chemical recycling methodologies for the Fibre Reinforced Plastic (FRP). Environ. Technol. Innov. 2019, 14, 100311. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.; Cho, H.; Kim, J. Novel mechanical vapor recompression-assisted evaporation process for improving energy efficiency in pulp and paper industry. Int. J. Energy Res. 2021, 46, 3409–3427. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, Y.; Cho, H.; Kim, J. Economic performance assessment of elemental sulfur recovery with carbonate melt desulfurization process. Process Saf. Environ. Prot. 2022, 158, 123–133. [Google Scholar] [CrossRef]
- Fico, D.; Rizzo, D.; De Carolis, V.; Montagna, F.; Corcione, C.E. Sustainable Polymer Composites Manufacturing through 3D Printing Technologies by Using Recycled Polymer and Filler. Polymers 2022, 14, 3756. [Google Scholar] [CrossRef]
- Canevarolo, S.V. Chain scission distribution function for polypropylene degradation during multiple extrusions. Polym. Degrad. Stab. 2000, 70, 71–76. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupancic, B.; Emri, I. The effect of extensive mechanical recycling on the properties of ow density polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Tominaga, A.; Sekiguchi, H.; Nakano, R.; Yao, S.; Takatori, E. Thermal process-dependence of the mechanical properties and inner structures of pre-consumer recycled polypropylene. AIP Conf. Proc. 2015, 1664, 150011. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Velidakis, E.; Mountakis, N.; Fischer-Griffiths, P.E.; Grammatikos, S.A.; Tzounis, L. Fused Filament Fabrication 3D printed polypropylene/alumina nanocomposites: Effect of filler loading on the mechanical reinforcement. Polym. Test. 2022, 109, 107545. [Google Scholar] [CrossRef]
- Kolek, Z. Recycled polymers from food packaging in relation to environmental protection. Pol. J. Environ. Stud. 2001, 10, 73–76. [Google Scholar]
- Jiun, Y.L.; Tze, C.T.; Moosa, U.; Tawawneh, M.A. Effects of Recycling Cycle on Used Thermoplastic Polymer and Thermoplastic Elastomer Polymer. Polym. Polym. Compos. 2016, 24, 735–740. [Google Scholar] [CrossRef]
- Reis, J.; Pacheco, L.; Mattos, H.D.C. Tensile behavior of post-consumer recycled high-density polyethylene at different strain rates. Polym. Test. 2013, 32, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Żenkiewicz, M.; Dzwonkowski, J. Effects of electron radiation and compatibilizers on impact strength of composites of recycled polymers. Polym. Test. 2007, 26, 903–907. [Google Scholar] [CrossRef]
- Yam, K.L.; Gogoi, B.K.; Lai, C.C.; Selke, S.E. Composites from compounding wood fibers with recycled high density polyethylene. Polym. Eng. Sci. 1990, 30, 693–699. [Google Scholar] [CrossRef]
- Poulakis, J.; Papaspyrides, C. Recycling of polypropylene by the dissolution/reprecipitation technique: I. A model study. Resour. Conserv. Recycl. 1997, 20, 31–41. [Google Scholar] [CrossRef]
- Achilias, D.S.; Roupakias, C.; Megalokonomos, P.; Lappas, A.A.; Antonakou, E.V. Chemical recycling of plastic wastes made from polyethylene (LDPE and HDPE) and polypropylene (PP). J. Hazard. Mater. 2007, 149, 536–542. [Google Scholar] [CrossRef]
- Deka, B.; Maji, T. Study on the properties of nanocomposite based on high density polyethylene, polypropylene, polyvinyl chloride and wood. Compos. Part A Appl. Sci. Manuf. 2011, 42, 686–693. [Google Scholar] [CrossRef]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Technol. Biotechnol. 2018, 94, 8–21. [Google Scholar] [CrossRef] [Green Version]
- Cardona, S.C.; Corma, A. Tertiary recycling of polypropylene by catalytic cracking in a semibatch stirred reactor: Use of spent equilibrium FCC commercial catalyst. Appl. Catal. B Environ. 2000, 25, 151–162. [Google Scholar] [CrossRef]
- Ragab, H.; Algethami, N.; Elamin, N.Y.; Asnag, G.; Rajeh, A.; Alzahrani, H.S. An insight into the influence of Ag/Se nanoparticles on the structural, optical, and electrical properties of Cs/PAM nanocomposites films as application in electrochemical devices. J. Mol. Struct. 2022, 1267, 133619. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, N.; Li, N.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Liquid sensing behaviors of conductive polypropylene composites containing hybrid fillers of carbon fiber and carbon black. Compos. Part B Eng. 2016, 94, 45–51. [Google Scholar] [CrossRef]
- Orozco, F.; Salvatore, A.; Sakulmankongsuk, A.; Gomes, D.R.; Pei, Y.; Araya-Hermosilla, E.; Pucci, A.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Electroactive performance and cost evaluation of carbon nanotubes and carbon black as conductive fillers in self-healing shape memory polymers and other composites. Polymer 2022, 260, 125365. [Google Scholar] [CrossRef]
- Yang, H.; Guan, Y.; Ye, L.; Wang, S.; Li, S.; Wen, X.; Chen, X.; Mijowska, E.; Tang, T. Synergistic effect of nanoscale carbon black and ammonium polyphosphate on improving thermal stability and flame retardancy of polypropylene: A reactive network for strengthening carbon layer. Compos. Part B Eng. 2019, 174, 107038. [Google Scholar] [CrossRef]
- Ali, A.; Azeem, M.; Noman, M.T.; Amor, N.; Militky, J.; Petru, M.; Wang, Y.; Masin, I. Development of silver plated electrically conductive elastomers embedded with carbon black particles obtained from Kevlar waste source. Polym. Test. 2022, 116, 107793. [Google Scholar] [CrossRef]
- ASTM D638; Standard Test Methods for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D257; Standard Test Method for DC Resistance of Conductance of Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2010.
- Abadchi, M.R.; Jalali-Arani, A. Crystallization and melting behavior of polypropylene (PP) in (vulcanized nanoscale polybutadiene rubber powder/PP) polymer-nanocomposites. Thermochim. Acta 2015, 617, 120–128. [Google Scholar] [CrossRef]
- Xu, J.; Hao, X.; Tang, W.; Zhou, H.; Chen, L.; Guo, C.; Wang, Q.; Ou, R. Mechanical properties, morphology, and creep resistance of ultra-highly filled bamboo fiber/polypropylene composites: Effects of filler content and melt flow index of polypropylene. Constr. Build. Mater. 2021, 310, 125289. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Jaroensuk, O.; Khankhuean, A.; Laobuthee, A.; Srisawat, N.; Pangon, A.; Mongkholrattanasit, R.; Phuengphai, P.; Wattanakornsiri, A.; Huang, C.-F. A Comprehensive Evaluation of Mechanical, Thermal, and Antibacterial Properties of PLA/ZnO Nanoflower Biocomposite Filaments for 3D Printing Application. Polymers 2022, 14, 600. [Google Scholar] [CrossRef]
- Anosike-Francis, E.N.; Obianyo, I.I.; Salami, O.W.; Ihekweme, G.O.; Ofem, M.I.; Olorunnisola, A.O.; Onwualu, A.P. Physical-Mechanical properties of wood based composite reinforced with recycled polypropylene and cowpea (Vigna unguiculata Walp.) husk. Clean. Mater. 2022, 5, 100101. [Google Scholar] [CrossRef]
- Faraj, R.H.; Sherwani, A.F.H.; Daraei, A. Mechanical, fracture and durability properties of self-compacting high strength concrete containing recycled polypropylene plastic particles. J. Build. Eng. 2019, 25, 100808. [Google Scholar] [CrossRef]
- Lin, T.A.; Lin, J.-H.; Bao, L. Polypropylene/thermoplastic polyurethane blends: Mechanical characterizations, recyclability and sustainable development of thermoplastic materials. J. Mater. Res. Technol. 2020, 9, 5304–5312. [Google Scholar] [CrossRef]
- Xiao, H.; Lu, W.; Yeh, J.-T. Effect of plasticizer on the crystallization behavior of poly(lactic acid). J. Appl. Polym. Sci. 2009, 113, 112–121. [Google Scholar] [CrossRef]
- Yang, K.; Yang, Q.; Li, G.; Zhang, Y.; Zhang, P. Mechanical properties and morphologies of polypropylene/single-filler or hybrid-filler calcium carbonate composites. Polym. Eng. Sci. 2007, 47, 95–102. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Youssef, H.; Gepreel, M.A.H.; Abbas, R.; Kandil, S. Surface Morphology and Mechanical Properties of Polyether Ether Ketone (PEEK) Nanocomposites Reinforced by Nano-Sized Silica (SiO2) for Prosthodontics and Restorative Dentistry. Polymers 2021, 13, 3006. [Google Scholar] [CrossRef]
- Caban, R. FTIR-ATR spectroscopic, thermal and microstructural studies on polypropylene-glass fiber composites. J. Mol. Struct. 2022, 1264, 133181. [Google Scholar] [CrossRef]
- Shao, Y.; Guizani, C.; Grosseau, P.; Chaussy, D.; Beneventi, D. Biocarbons from microfibrillated cellulose/lignosulfonate precursors: A study of electrical conductivity development during slow pyrolysis. Carbon 2018, 129, 357–366. [Google Scholar] [CrossRef]
- Lanyi, F.J.; Wenzke, N.; Kaschta, J.; Schubert, D.W. A method to reveal bulk and surface crystallinity of Polypropylene by FTIR spectroscopy—Suitable for fibers and nonwovens. Polym. Test. 2018, 71, 49–55. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Intaramongkol, N.; Kanjanaphong, N.; Ponjaroen, K.; Sriwiset, W.; Mongkholrattanasit, R.; Wongwachirakorn, P.; Lin, K.-Y.A.; Huang, C.-F. Study of the Enhancements of Porous Structures of Activated Carbons Produced from Durian Husk Wastes. Sustainability 2022, 14, 5896. [Google Scholar] [CrossRef]
- Gómez-Hernández, R.; Panecatl-Bernal, Y.; Méndez-Rojas, M. High yield and simple one-step production of carbon black nanoparticles from waste tires. Heliyon 2019, 5, e02139. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Lopez-Ramon, M.V.; Carrasco-Marín, F. Changes in surface chemistry of activated carbons by wet oxidation. Carbon 2000, 38, 1995–2001. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Li, S. Review of electrical properties for polypropylene based nanocomposite. Compos. Commun. 2018, 10, 221–225. [Google Scholar] [CrossRef]
- Zhang, R.-Y.; Zhao, Q.; Ge, M.-L. The effect of electrostatic shielding using invisibility cloak. AIP Adv. 2011, 1, 042126. [Google Scholar] [CrossRef] [Green Version]
- Srihata, W.; Jamnongkan, T.; Rattanasak, U.; Boonsang, S.; Kaewpirom, S. Enhanced electrostatic dissipative properties of chitosan/gelatin composite films filled with reduced graphene oxide. J. Mater. Sci. Mater. Electron. 2016, 28, 999–1010. [Google Scholar] [CrossRef]
- Su, Z.; Dong, M.; Guo, Z.; Yu, J. Study of Polystyrene and Acrylonitrile–Styrene Copolymer as Special β-Nucleating Agents To Induce the Crystallization of Isotactic Polypropylene. Macromolecules 2007, 40, 4217–4224. [Google Scholar] [CrossRef]
- Beate, K.; Regine, B.; Liane, H.; Petra, P. Ultralow percolation threshold in polyamide 6.6/MWCNT composites. Compos. Sci. Technol. 2015, 114, 119–125. [Google Scholar]
- Yetgin, S.H. Effect of multi walled carbon nanotube on mechanical, thermal and rheological properties of polypropylene. J. Mater. Res. Technol. 2019, 8, 4725–4735. [Google Scholar] [CrossRef]
- Alghyamah, A.A.; Elnour, A.Y.; Shaikh, H.; Haider, S.; Poulose, A.M.; Al-Zahrani, S.; Almasry, W.A.; Park, S.Y. Biochar/polypropylene composites: A study on the effect of pyrolysis temperature on crystallization kinetics, crystalline structure, and thermal stability. J. King Saud Univ. Sci. 2021, 33, 101409. [Google Scholar] [CrossRef]




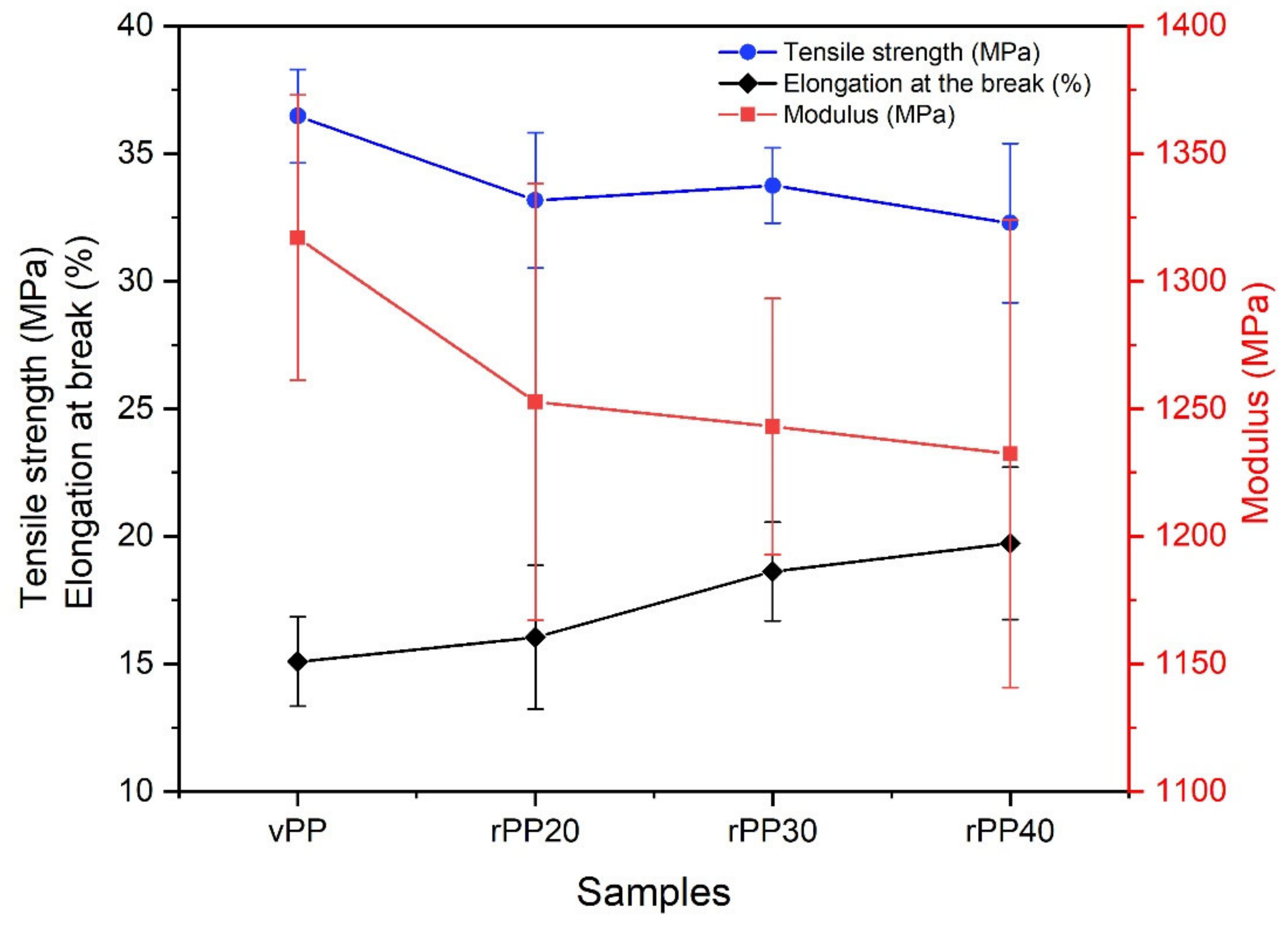

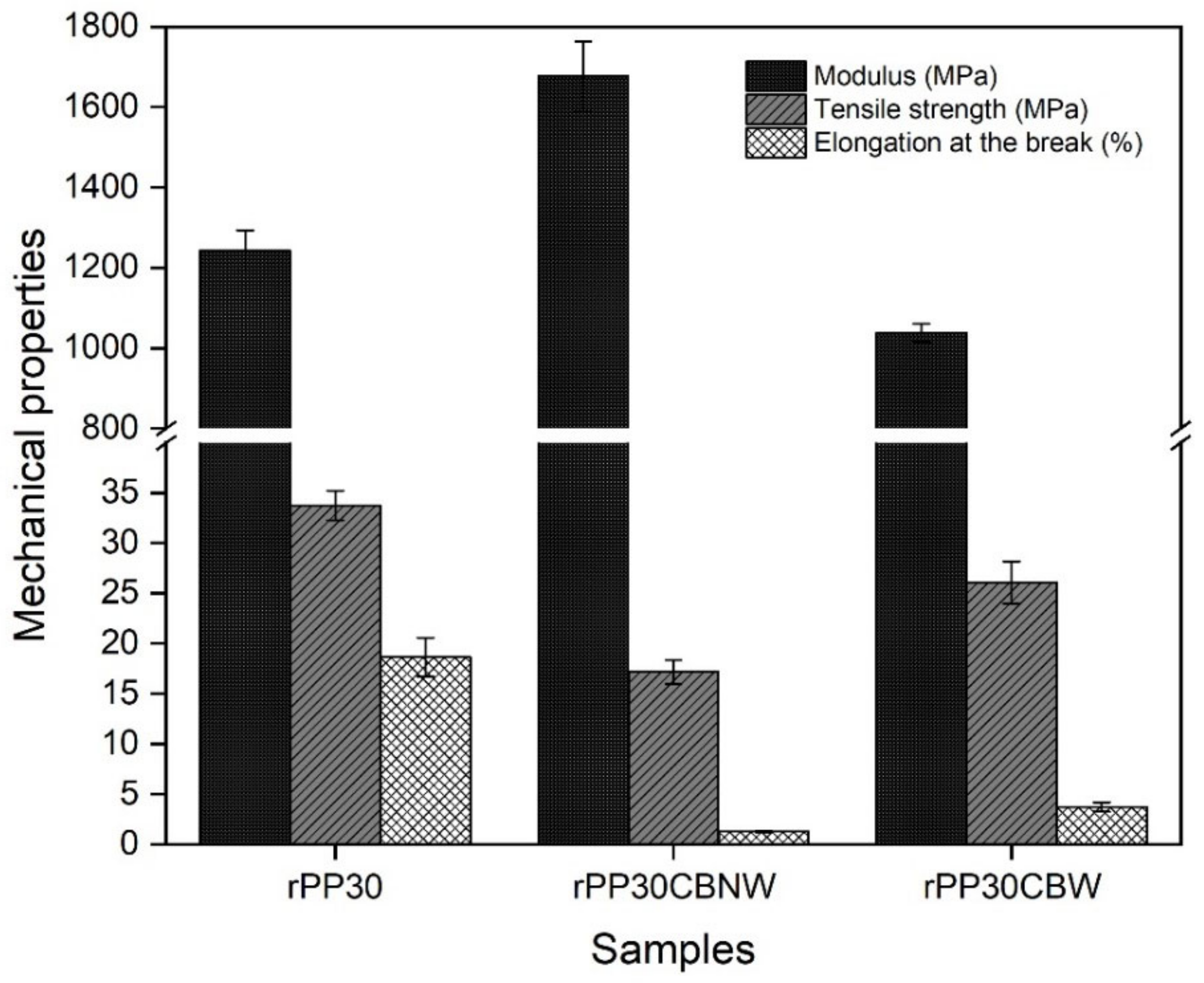



| Sample Code | Modulus (MPa) | Tensile Strength (MPa) | Elongation at the Break (%) |
|---|---|---|---|
| vPP | 1317 ± 55.85 | 36.47 ± 1.83 | 15.09 ± 1.75 |
| rPP20 | 1252 ± 85.63 | 33.17 ± 2.65 | 16.04 ± 2.82 |
| rPP30 | 1243 ± 50.15 | 33.75 ± 1.48 | 18.62 ± 1.93 |
| rPP40 | 1232 ± 91.62 | 32.28 ± 3.12 | 19.72 ± 2.99 |
| Sample Codes | Modulus (MPa) | Tensile Strength (MPa) | Elongation at the Break (%) |
|---|---|---|---|
| rPP30 | 1243 ± 50.15 | 33.75 ± 1.48 | 18.62 ± 1.93 |
| rPP30CBNW | 1676 ± 87.06 | 17.16 ± 1.18 | 1.27 ± 0.08 |
| rPP30CBW | 1038 ± 22.80 | 26.08 ± 2.11 | 3.73 ± 0.47 |
| Sample Codes | Surface Resistivity (Ohm/sq) |
|---|---|
| vPP | 1012 |
| rPP30CBNW | 104 |
| rPP30CBW | 104 |
| Sample Codes | Tc (°C) | Tm (°C) | χc (%) | |
|---|---|---|---|---|
| vPP | 113.6 | 163.2 | 93.60 | 45.22 |
| rPP30 | 121.0 | 164.6 | 82.74 | 57.10 |
| rPP30CBNW | 113.8 | 167.4 | 58.48 | 40.36 |
| rPP30CBW | 113.1 | 166.6 | 56.59 | 39.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamnongkan, T.; Intraramongkol, N.; Samoechip, W.; Potiyaraj, P.; Mongkholrattanasit, R.; Jamnongkan, P.; Wongwachirakorn, P.; Sugimoto, M.; Ito, H.; Huang, C.-F. Towards a Circular Economy: Study of the Mechanical, Thermal, and Electrical Properties of Recycled Polypropylene and Their Composite Materials. Polymers 2022, 14, 5482. https://doi.org/10.3390/polym14245482
Jamnongkan T, Intraramongkol N, Samoechip W, Potiyaraj P, Mongkholrattanasit R, Jamnongkan P, Wongwachirakorn P, Sugimoto M, Ito H, Huang C-F. Towards a Circular Economy: Study of the Mechanical, Thermal, and Electrical Properties of Recycled Polypropylene and Their Composite Materials. Polymers. 2022; 14(24):5482. https://doi.org/10.3390/polym14245482
Chicago/Turabian StyleJamnongkan, Tongsai, Nitchanan Intraramongkol, Wesarach Samoechip, Pranut Potiyaraj, Rattanaphol Mongkholrattanasit, Porntip Jamnongkan, Piyada Wongwachirakorn, Masataka Sugimoto, Hiroshi Ito, and Chih-Feng Huang. 2022. "Towards a Circular Economy: Study of the Mechanical, Thermal, and Electrical Properties of Recycled Polypropylene and Their Composite Materials" Polymers 14, no. 24: 5482. https://doi.org/10.3390/polym14245482













