Adhesive-Free Adhesion between Plasma-Treated Glass-Cloth-Containing Polytetrafluoroethylene (GC–PTFE) and Stainless Steel: Comparison between GC–PTFE and Pure PTFE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment
2.3. Water Contact Angle (WCA) Measurements
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.5. Confocal Laser Scanning Microscope (CLSM) Test
2.6. Scanning Electron Microscope (SEM) Analysis
2.7. Adhesion Strength Measurements Using a 90° Peel Test
3. Results and Discussion
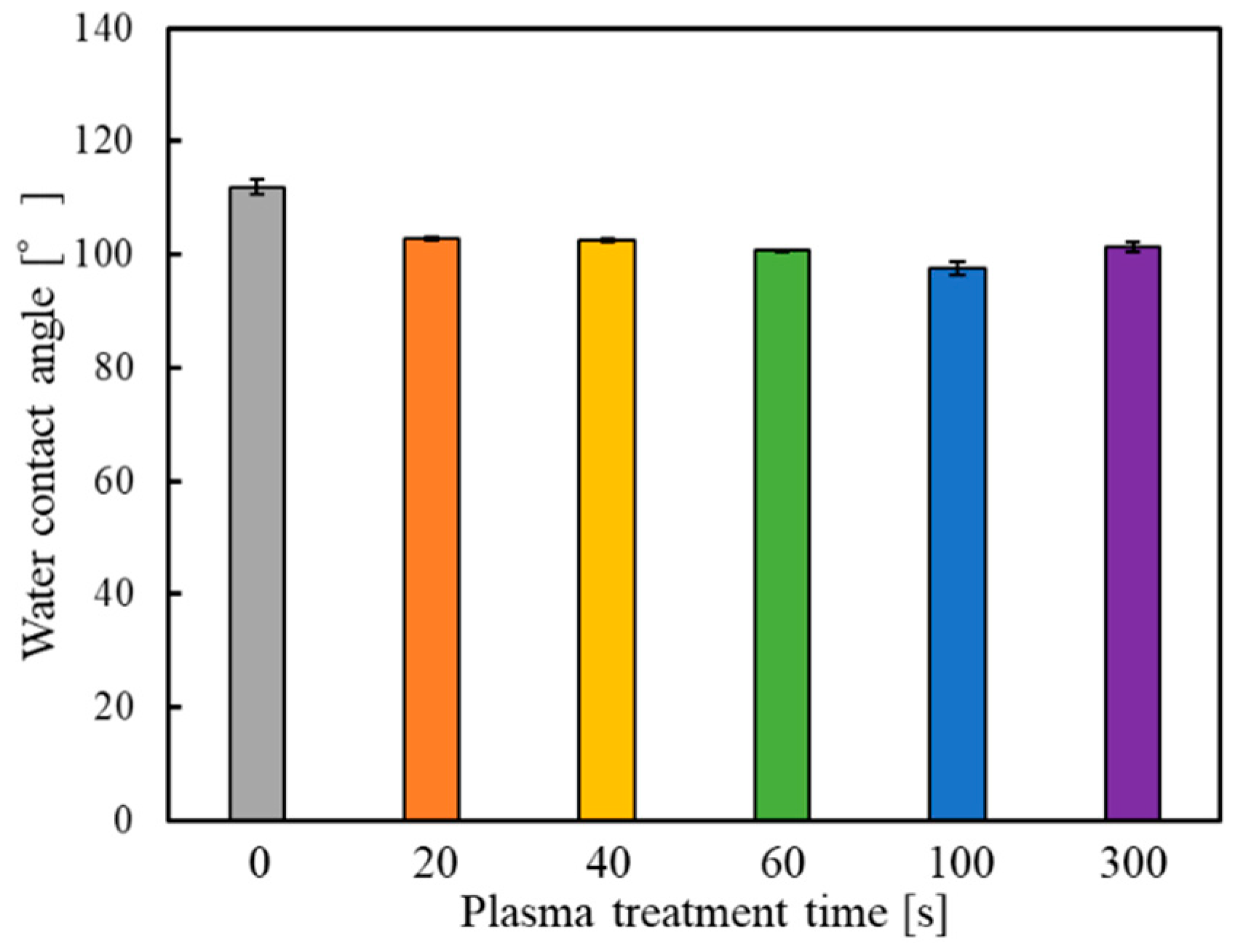
3.1. Assessment of the Wettability of GC–PTFE Using the WCA Measurement
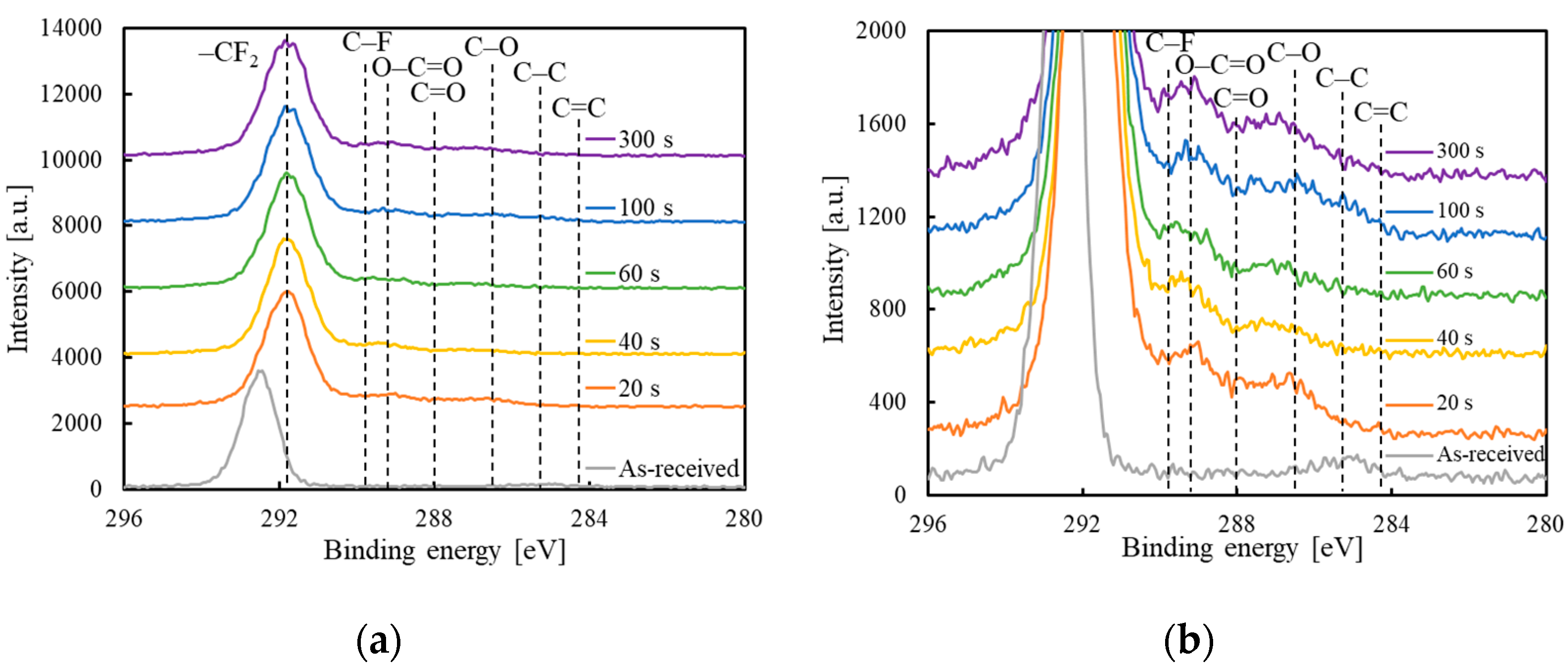
3.2. Assessment of the Chemical-Bonding State of GC–PTFE Using XPS
3.3. Surface Roughness Measurement of GC–PTFE Using CLSM
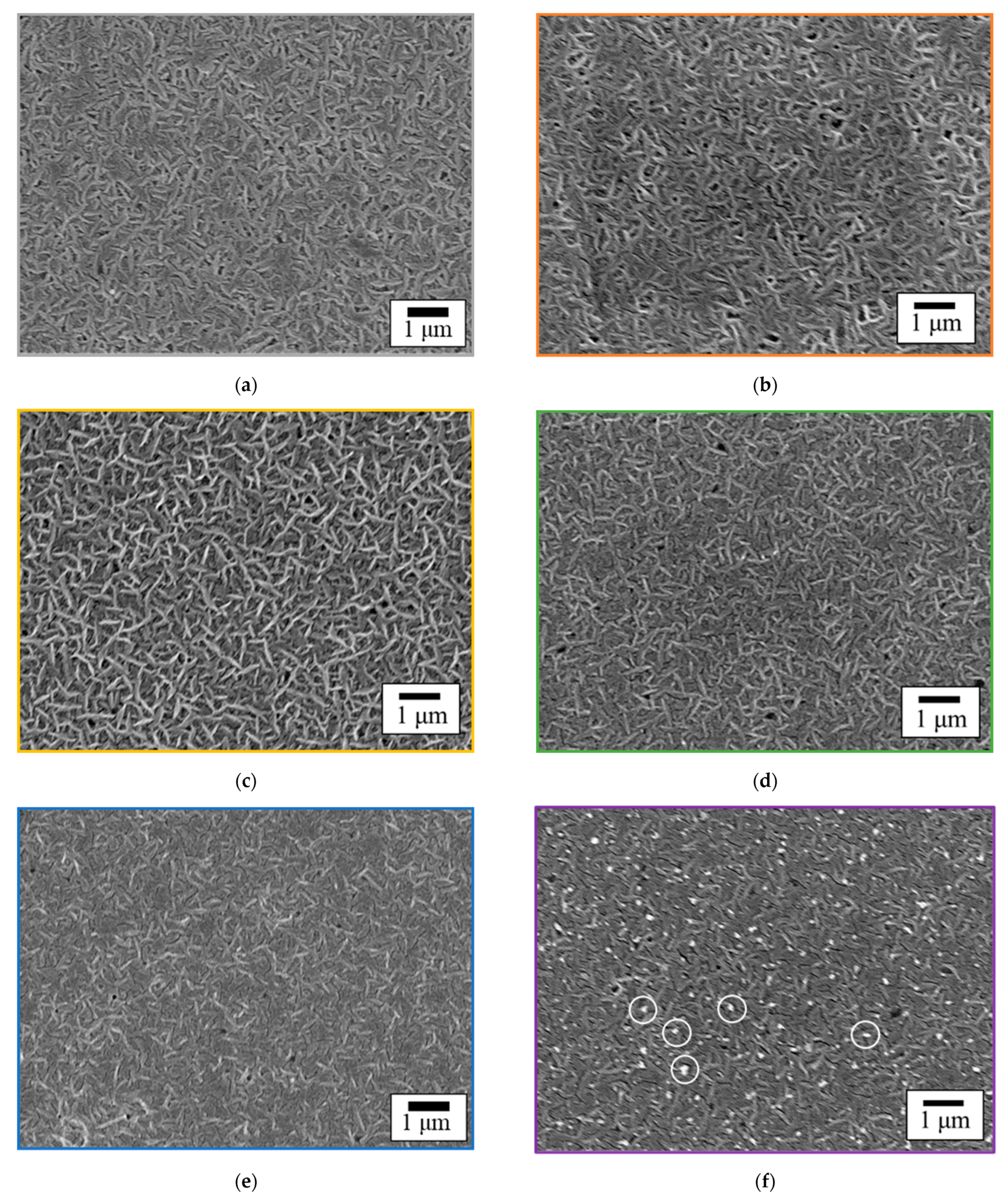
3.4. Surface Morphology of GC–PTFE
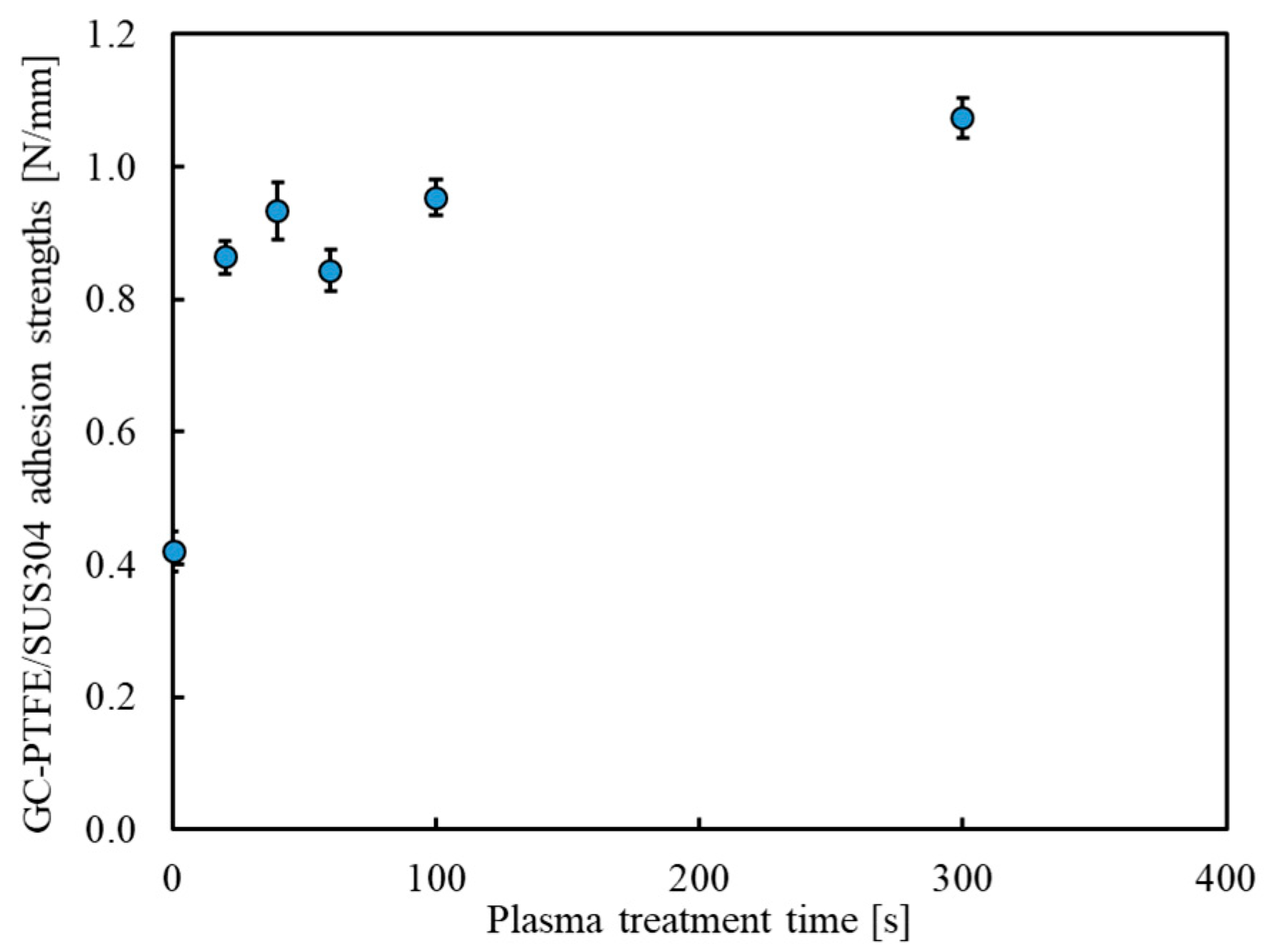
3.5. Adhesion Strength Estimated by a 90° Peel Test (Plasma-Treatment Time Dependence)
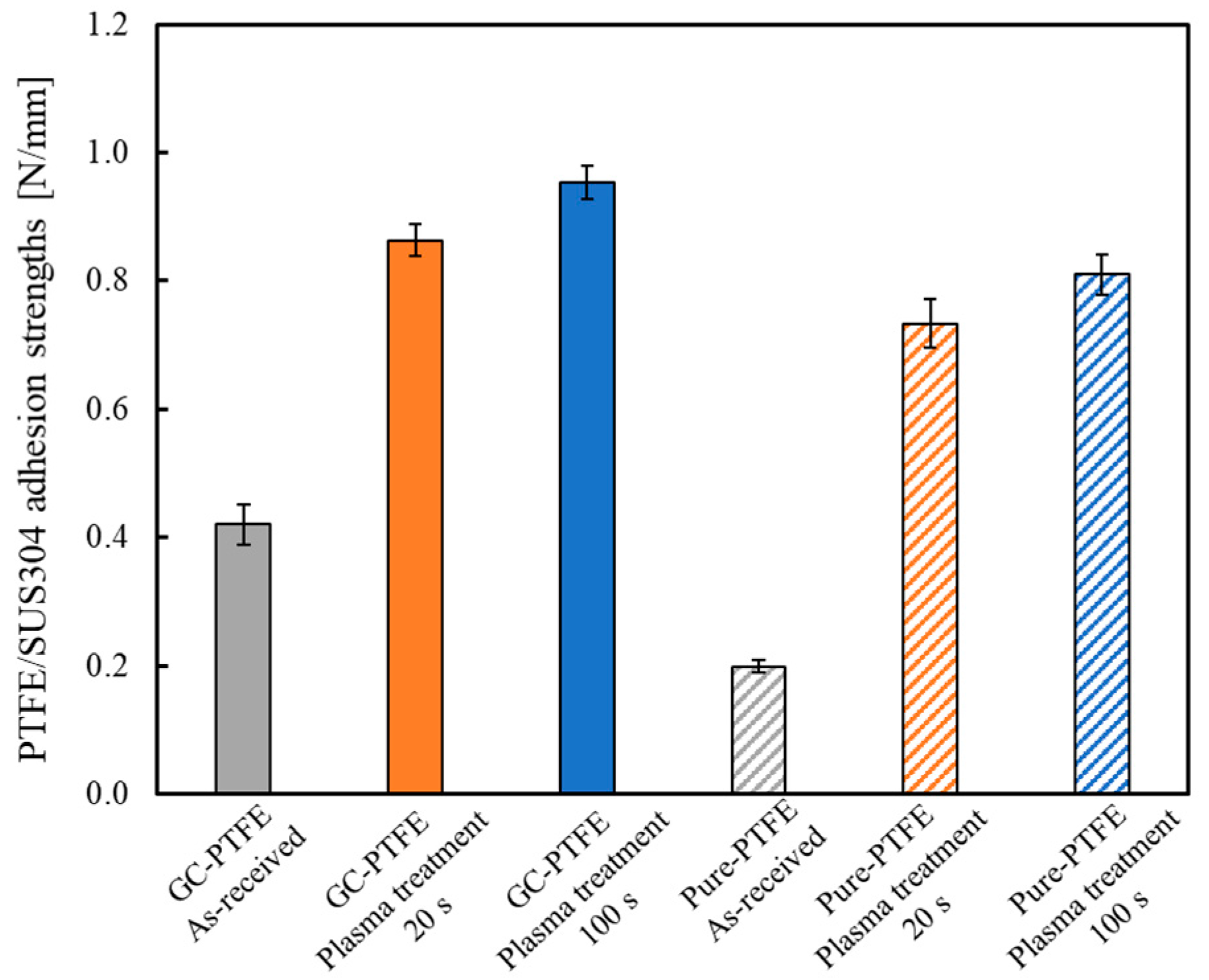
3.6. Comparison of the Adhesion Properties of GC–PTFE and Pure PTFE
3.7. Comparison of the Wettabilities of GC–PTFE and Pure PTFE Using WCA Measurement
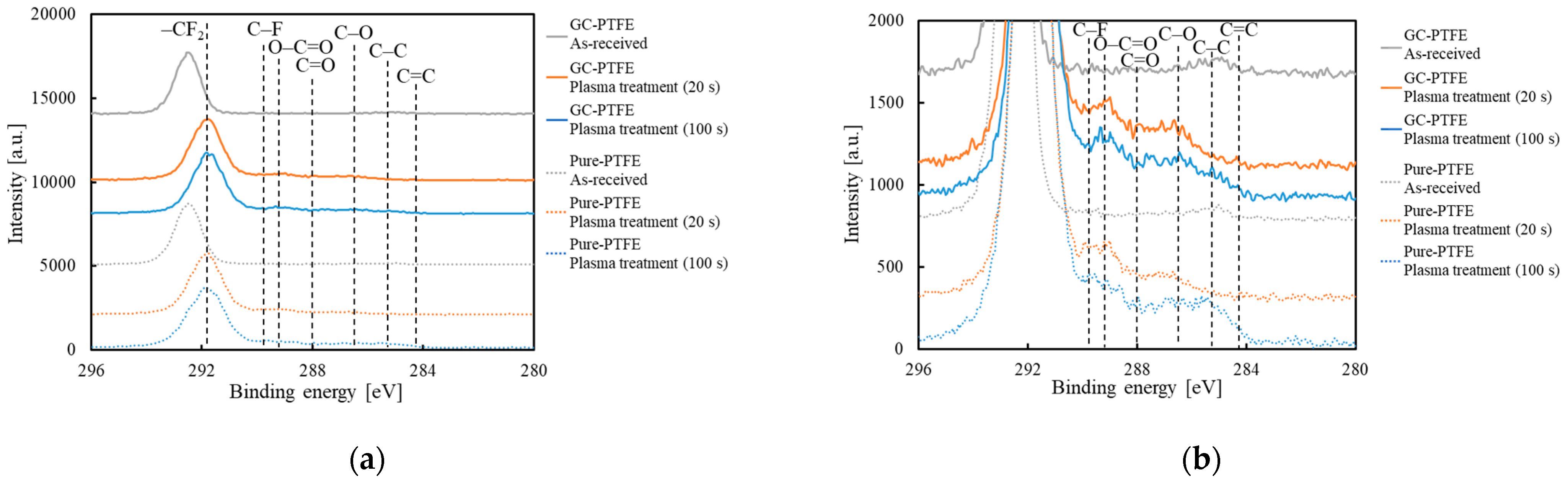
3.8. Comparison of the Chemical-Bonding States of GC–PTFE and Pure PTFE
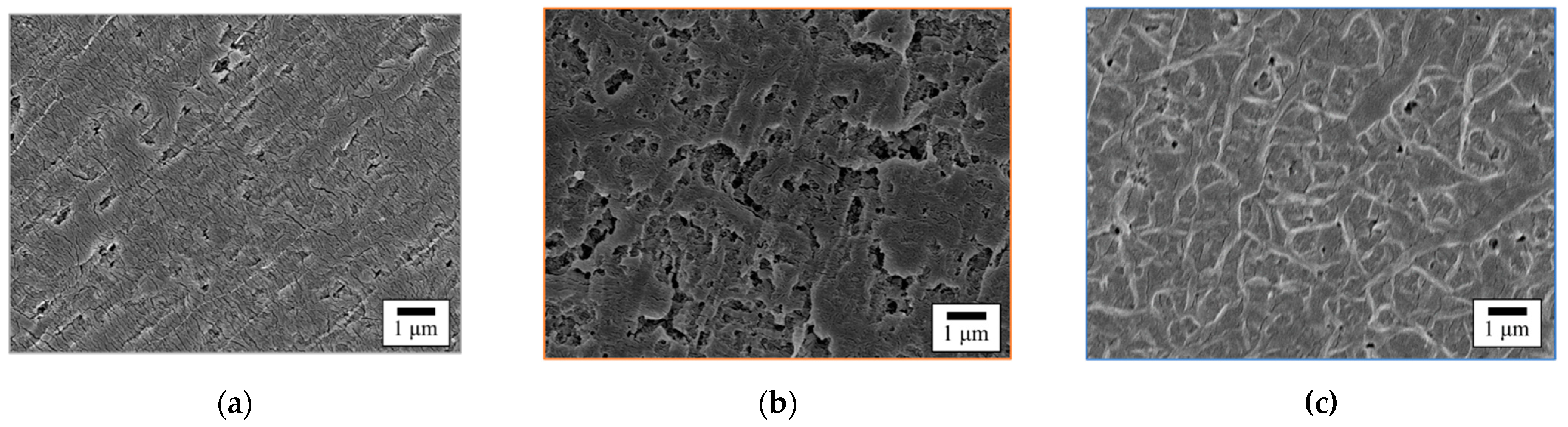
3.9. Comparison of the Surface Morphology of GC–PTFE and Pure PTFE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, J.D.; Veeramasuneni, S.; Drelich, J.; Yalamanchili, M.R.; Yamauchi, G. Effect of roughness as determined by atomic force microscopy on the wetting properties of PTFE thin films. Polym. Eng. Sci. 1996, 36, 1849–1885. [Google Scholar] [CrossRef]
- Araki, Y. Progress in processing Technique of Fluorocarbon Polymers. Polymer 1974, 23, 827–831. [Google Scholar]
- Schonhorn, H.; Hansen, R.H. Surface treatment of polymers for adhesive bonding. J. Appl. Polym. Sci. 1967, 11, 1461–1474. [Google Scholar] [CrossRef]
- Seto, Y.; Nishino, M.; Okazaki, Y.; Endo, K.; Yamamura, K.; Ohkubo, Y. Cross-sectional observation of a weak boundary layer in polytetrafluoroethylene (PTFE) using scanning electron microscope. Polym. J. 2022, 54, 79–81. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kumikov, V.K.; Khokonov, K.B. On the measurement of surface free energy and surface tension of solid metals. J. Appl. Polym. Sci. 1983, 54, 1346–1350. [Google Scholar] [CrossRef]
- Marchesi, J.T.; Keith, H.D.; Garton, A. Adhesion to sodium Naphthalenide treated fluoropolymers. Part III. Mechanism of adhesion. J. Adhes. 1992, 39, 185–205. [Google Scholar] [CrossRef]
- Lee, S.W.; Hong, J.W.; Wye, M.Y.; Kim, J.H.; Kang, H.J.; Lee, Y.S. Surface modification and adhesion improvement of PTFE film by ion beam irradiation. Nucl. Instrum. Methods. Phys. Res. B 2004, 219–220, 963–967. [Google Scholar] [CrossRef]
- Nakayama, A.; Iwao, T.; Yumoto, M. Dependence of Ion Energy on PTFE Surface Modification Effect by Nitrogen Ion Irradiation. IEEJ Trans. Fundam. Mater. 2010, 130, 331–336. [Google Scholar] [CrossRef]
- Takata, R.; Iwao, T.; Yumoto, M. Surface Modification of PTFE Using Low-Energy Nitrogen Ion Irradiation: Improvement in Adhesive Strength on Modification of Deep Modifyimg Layer. Electron. Commun. Jpn. 2016, 99, 93–99. [Google Scholar] [CrossRef]
- Girardeaux, C.; Idrissi, Y.; Pireaux, J.J.; Caudano, R. Etching and functionalization of a fluorocarbon polymer by UV laser treatment. Appl. Surf. Sci. 1996, 96, 586–590. [Google Scholar] [CrossRef]
- Hopp, B.; Geretovszky, Z.; Bertóti, I.; Boyd, I.W. Comparative tensile strength study of the adhesion improvement or PTFE by UV photon assisted surface processing. Appl. Surf. Sci. 2002, 186, 80–84. [Google Scholar] [CrossRef]
- Hopp, B.; Kresz, N.; Kokavecz, J.; Smausz, T.; Schieferdecker, H.; Döring, A.; Marti, O.; Bor, Z. Adhesive and morphological characteristics of surface chemically modified polytetrafluoroethylene films. Appl. Surf. Sci. 2004, 221, 437–443. [Google Scholar] [CrossRef]
- Jinglong, G.; Zaochun, N.; Yanhui, L. The investigation of the structural change and the wetting behavior of electron beam irradiated PTFE film. E-Polymers 2016, 16, 111–115. [Google Scholar] [CrossRef]
- Oshima, A.; Ikeda, S.; Katoh, E.; Tabata, Y. Chemical structure and physical properties of radiation-induced crosslinking of polytetrafluoroethylene. Radiat. Phys. Chem. 2001, 62, 39–45. [Google Scholar] [CrossRef]
- Katoh, E.; Sugisawa, H.; Oshima, A.; Tabata, Y.; Seguchi, T.; Yamazaki, T. Evidence for radiation induced crosslinking in polytetrafluoroethylene by means of high-resolution solid-state 19F high-speed MAS NMR. Radiat. Phys. Chem. 1999, 54, 165–171. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Zhu, B.K. Modification of polytetrafluoroethylene porous membranes by electron beam initiated surface grafting of binary monomers. J. Membr. Sci. 2009, 339, 33–38. [Google Scholar] [CrossRef]
- Oshima, A. Surface modification and improvement of adhesion of fluorinated polymers using quantum beam induced chemical reaction. J. Adhes. Sci. Jpn. 2013, 49, 433–438. [Google Scholar]
- Mishra, R.; Tripathy, S.P.; Dwivedi, K.K.; Khathing, D.T.; Ghosh, S.; Müller, M.; Fink, D. Effect of electron irradiation on polytetrafluoro ethylene. Radiat. Meas. 2003, 37, 247–251. [Google Scholar] [CrossRef]
- Lee, E.J.; Jung, C.H.; Hwang, I.T.; Choi, J.H.; Cho, S.O.; Nho, Y.C. Surface morphology control of polymer films by electron irradiation and its application to superhydrophobic surfaces. ACS Appl. Mater. Interfaces 2011, 3, 2988–2993. [Google Scholar] [CrossRef]
- Nishi, Y.; Uyama, M.; Kawazu, H.; Takei, H.; Iwata, K.; Kudoh, H.; Mitsubayashi, K. Effects of electron beam irradiation on adhesive force of laminated sheet of high strength polytetrafluoroethylene (PTFE) and bio-adaptable polydimethylsiloxane (PDMS). Mater. Trans. 2012, 53, 1657–1664. [Google Scholar] [CrossRef] [Green Version]
- Kubo, C.; Kanda, M.; Nishi, Y. Effects of homogeneous low energy electron beam irradiation (HLEBI) on adhesive force of peeling of carbon fiber reinforced epoxy polymer (CFRP) and polytetrafluoroethylene (PTFE). Mater. Trans. 2015, 56, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
- Carbone, E.A.D.; Boucher, N.; Sferrazza, M.; Reniers, F. How to increase the hydrophobicity of PTFE surfaces using an r.f. atmospheric-pressure plasma torch. Surf. Interface Anal. 2010, 42, 1014–1018. [Google Scholar] [CrossRef]
- Bournet, C.S.; Ayotte, G.; Turgeon, S.; Massines, F.; Laroche, G. Effects chemical composition and the addition of H2 in a N2 atmospheric pressure dielectric barrier discharge on polymer surface functionalization. Langmuir 2009, 25, 9432–9440. [Google Scholar] [CrossRef]
- Santiago, V.R.; Bujanda, A.A.; Stein, E.; Pappas, D.D. Atmospheric plasma processing of polymers in helium-water vapor dielectric barrier discharges. Plasma Process Polym. 2011, 8, 631–639. [Google Scholar] [CrossRef]
- Hai, W.; Hi, T.; Shimizu, K.; Yajima, T. Preparation of a super hydrophilic polytetrafluoroethylene surface using a gaseous ammonia-water low-temperature plasma. J. Photopolym. Sci. Technol. 2015, 28, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Lei, J.; Zheng, Z.; Zhu, Z.; Liu, S. The hydrophilicity improvement of polytetrafluorothylene by Ar plasma jet: The relationship of hydrophilicity, ambient and plasma parameters. Appl. Surf. Sci. 2018, 458, 183–190. [Google Scholar] [CrossRef]
- Xu, H.; Hu, Z.; Wu, S.; Chen, Y. Surface modification of polytetrafluoroethylene by microwave plasma treatment of H2O/Ar mixture at low pressure. Mater. Chem. Phys. 2003, 80, 278–282. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Kawai, H. Improved adhesion of poly(tetrafluoroethylene) by NH3-plasma treatment. J. Adhes. Sci. Technol. 1989, 3, 637–649. [Google Scholar] [CrossRef]
- Okubo, M.; Tahara, M.; Aburatani, Y.; Kuroki, T.; Hibino, T. Preparation of PTFE film with adhesive surface treated by atmospheric-pressure nonthermal plasma graft polymerization. IEEE Trans. Ind. Appl. 2010, 46, 1715–1721. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Okazaki, Y.; Shibahara, M.; Nishino, M.; Seto, Y.; Endo, K.; Yamamura, Y. Effects of He and Ar heat-assisted plasma treatments on the adhesion properties of polytetrafluoroethylene (PTFE). Polymers 2021, 13, 4266. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.P.; Kang, E.T.; Neoh, K.G.; Cui, C.Q.; Lim, T.B. Surface modification of poly(tetrafluoroethylene) films by plasma polymerization of glycidyl methacrylate for adhesion enhancement with evaporated copper. Polymer 2001, 42, 6409–6418. [Google Scholar] [CrossRef]
- Wu, S.; Kang, E.T.; Neoh, K.G.; Han, H.S.; Tan, K.L. Surface modification of poly(tetrafluoroethylene) films by graft copolymerization for adhesion improvement with evaporated copper. Macromolecules 1999, 32, 186–193. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ishihara, K.; Sato, H.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, Y. Adhesive-free adhesion between polytetrafluoroethylene (PTFE) and isobutylene–isoprene rubber (IIR) via heat-assisted plasma treatment. RSC Adv. 2017, 7, 6432–6438. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Kodama, Y.; Nishino, M.; Oshita, T.; Uehara, T.; Endo, K.; Yamamura, K. Open-air-type Ar +H2O plasma treatment of polytetrafluoroethylene for improving Ag/PTFE adhesion strength: Application to highly adhesive Ag direct wiring patterns. Jpn. J. Appl. Phys. 2020, 59, 077004. [Google Scholar] [CrossRef]
- Tetens, O. Über einige meteorologische begriffe. Z. Geophys. 1930, 6, 297–309. [Google Scholar]
- Vandencasteele, N.; Reniers, F. Plasma-modified poltmer surfaces: Characterization using XPS. J. Electron Spectros. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Hubert, J.; Dufour, T.; Vandencasteele, N.; Desbief, S.; Lazzaroni, R.; Reniers, F. Etching processes of polytetrafluoroethylene surfaces exposed to he and he–O2 atmospheric post-discharges. Langmuir 2012, 28, 9466–9474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, E.A.D.; Verhoeven, M.W.G.M.; Keuning, W.; Mullen, J.J.A.M. PTFE treatment by remote atmospheric Ar/O2 plasmas: A simple reaction scheme model proposal. J. Phys. Conf. Ser. 2016, 715, 012011. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Surface characterization of plasma-treated PTFE surface: An OES, XPS and contact angle study. Surf. Interface Anal. 2004, 36, 1027–1031. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Merche, D.; Reniers, F. XPS and contact angle study of N2 and O2 plasma-modified PTFE, PVDF and PVF surfaces. Surf. Interface Anal. 2006, 38, 526–530. [Google Scholar] [CrossRef]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Brox, B.; Olefjord, I. ESCA Studies of MoO2 and MoO3. Surf. Interface Anal. 1988, 13, 3–6. [Google Scholar] [CrossRef]
- Kuivila, C.S.; Butt, J.B.; Stair, P.C. Characterization of surface specoes on iron synthesis catalysts by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1988, 32, 99–121. [Google Scholar] [CrossRef]
- Menthe, E.; Rie, K.T.; Schltze, J.W.; Simson, S. Structure and properties of plasma-nitrided stainless steel. Surf. Coat. Technol. 1995, 74–75, 412–416. [Google Scholar] [CrossRef]
- Sagara, M.; Katada, Y.; Kodama, T.; Tsuru, T. Surface analysis of high nitrogen-bearing austenitic stainless steel using XPS. J. Jpn. Inst. Met. 2003, 67, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, J.P.; McCarthy, T.J. Ultrahydrophobic polymer surfaces prepared by simultaneous ablation of polypropylene and sputtering of poly(tetrafluoroethylene) using radio frequency plasma. Macromolecules 1999, 32, 6800–6806. [Google Scholar] [CrossRef]



| Material | Adherend | Surface Modification Treatment | Adhesion Strength | Adhesive | References |
|---|---|---|---|---|---|
| Pure PTFE | Cu | Ar+ ion irradiation | No data | No | [9] |
| Pure PTFE | Glass-cloth | Nitrogen ion irradiation | 0.02 to 0.9 N/mm | An epoxy adhesive | [10,11] |
| Pure PTFE | PMMA | UV irradiation using 1,2-diaminoethame | 0.03 to 10 MPa | An epoxy adhesive | [13] |
| Pure PTFE | Plexiglass | UV irradiation using triethylene tetramine | 0.03 to 9 MPa | An epoxy adhesive | [14] |
| Pure PTFE | CFRP | EB irradiation | 0.1 N/mm | No | [22] |
| Pure PTFE | PDMS | EB irradiation | 0.11 N/mm | No | [23] |
| Pure PTFE | Fe | Ar + H2O plasma treatment | 0.3 to 5.8 MPa | An epoxy adhesive | [29] |
| Pure PTFE | NBR | NH3 plasma treatment | 8.1 N/mm | A phenolic adhesive | [30] |
| Pure PTFE | Al | Ar + acrylic acid plasma treatment | 1.4 N/mm | An epoxy adhesive | [31] |
| Pure PTFE | Stainless steel | Heat-assisted Ar plasma treatment | 0.00 to 1.17 N/mm | An epoxy adhesive | [32] |
| Pure PTFE | Cu | Plasma polymerization using GMA | 0.03 to 0.48 N/mm | No | [33] |
| Pure PTFE | Cu | Plasma polymerization using VIDz and GMA | 1.9 N/mm | No | [34] |
| Pure PTFE | IIR | Heat-assisted He plasma treatment | 0.1 to > 2.0 N/mm | No | [35] |
| Pure PTFE | Ag | Open-air type Ar + H2O plasma treatment | 0.04 to > 1.0 N/mm | No | [36] |
| GC–PTFE | Stainless steel | Open-air type Ar + H2O plasma treatment | 0.42 to > 1.07 N/mm | No | This study |
| CF2 [%] | C–F [%] | O–C=O [%] | C=O [%] | C–O [%] | C–C [%] | C=C [%] | |
|---|---|---|---|---|---|---|---|
| 0 s | 96.7 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 |
| 20 s | 81.6 | 4.5 | 3.0 | 5.2 | 4.5 | 1.2 | 0.0 |
| 40 s | 87.2 | 3.3 | 4.0 | 2.5 | 3.0 | 0.2 | 0.0 |
| 60 s | 88.0 | 5.1 | 2.5 | 2.5 | 1.4 | 0.5 | 0.0 |
| 100 s | 80.3 | 4.2 | 3.8 | 4.5 | 4.5 | 2.3 | 0.5 |
| 300 s | 81.3 | 4.1 | 4.7 | 4.5 | 4.6 | 0.8 | 0.2 |
| Treatment Time [s] | 0 | 20 | 40 | 60 | 100 | 300 |
|---|---|---|---|---|---|---|
| Sq [μm] | 7.92 ± 0.25 | 8.82 ± 0.24 | 8.49 ± 0.19 | 8.79 ± 0.17 | 8.12 ± 0.13 | 7.29 ± 0.23 |
| Sa [μm] | 5.85 ± 0.27 | 6.08 ± 0.24 | 6.31 ± 0.15 | 6.60 ± 0.14 | 6.06 ± 0.13 | 5.30 ± 0.16 |
| Sample | CF2 [%] | C–F [%] | O–C=O [%] | C=O [%] | C–O [%] | C–C [%] | C=C [%] | |
|---|---|---|---|---|---|---|---|---|
| GC–PTFE | 0 s | 96.7 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 |
| 20 s | 81.6 | 4.5 | 3.0 | 5.2 | 4.5 | 1.2 | 0.0 | |
| 100 s | 80.3 | 4.2 | 3.8 | 4.5 | 4.5 | 2.3 | 0.5 | |
| Pure PTFE | 0 s | 98.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 | 0.0 |
| 20 s | 89.1 | 1.4 | 4.3 | 2.8 | 2.5 | 0.0 | 0.0 | |
| 100 s | 81.7 | 1.9 | 4.9 | 2.9 | 4.3 | 4.4 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishino, M.; Okazaki, Y.; Seto, Y.; Uehara, T.; Endo, K.; Yamamura, K.; Ohkubo, Y. Adhesive-Free Adhesion between Plasma-Treated Glass-Cloth-Containing Polytetrafluoroethylene (GC–PTFE) and Stainless Steel: Comparison between GC–PTFE and Pure PTFE. Polymers 2022, 14, 394. https://doi.org/10.3390/polym14030394
Nishino M, Okazaki Y, Seto Y, Uehara T, Endo K, Yamamura K, Ohkubo Y. Adhesive-Free Adhesion between Plasma-Treated Glass-Cloth-Containing Polytetrafluoroethylene (GC–PTFE) and Stainless Steel: Comparison between GC–PTFE and Pure PTFE. Polymers. 2022; 14(3):394. https://doi.org/10.3390/polym14030394
Chicago/Turabian StyleNishino, Misa, Yuki Okazaki, Yosuke Seto, Tsuyoshi Uehara, Katsuyoshi Endo, Kazuya Yamamura, and Yuji Ohkubo. 2022. "Adhesive-Free Adhesion between Plasma-Treated Glass-Cloth-Containing Polytetrafluoroethylene (GC–PTFE) and Stainless Steel: Comparison between GC–PTFE and Pure PTFE" Polymers 14, no. 3: 394. https://doi.org/10.3390/polym14030394






