Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Statistical Modelling for Optimizing the Silymarin-Loaded Transfersomes Formulation
2.3. Preparation of Silymarin Loaded Transferosomes
2.4. Characterization of Silymarin Loaded Transfersomes (SmTFs)
2.4.1. Determination of Vesicle Size
2.4.2. Encapsulation Efficiency Determination (EE%)
2.4.3. In Vitro Drug Release from Different Transfersomal Preparations
2.5. Stability Studies of the Optimized Transfersomal Formulation (SmTFs)
2.6. Prepatation of Silymarin Loaded Gel
2.6.1. Formulation of Silymarin Gel
2.6.2. Formulation of Silymarin Transfersomal Gel
2.7. Evaluation of the Prepared Silymarin Loaded Transfersomal Gel
2.7.1. Physical Inspection
2.7.2. Estimation of pH Value
2.7.3. Spreadability Test
2.7.4. Rheological Studies and Viscosity
2.7.5. Drug Content Determination
2.8. In Vitro Drug Release from Transfersomal Gel
2.9. Ex Vivo Drug Permeation Study
ER = Jss from test/Jss from control.
2.10. In Vivo Experimental Studies
2.10.1. Selection of Animals and Experimental Induction of Diabetes
2.10.2. Determination of Blood Glucose Concentration
2.11. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Studies for Preparation of Silymarin-Loaded Transfersomes (SmTFs)
3.2. Expermintal Design
3.2.1. Analysis of Box–Behenken Design (BBD)
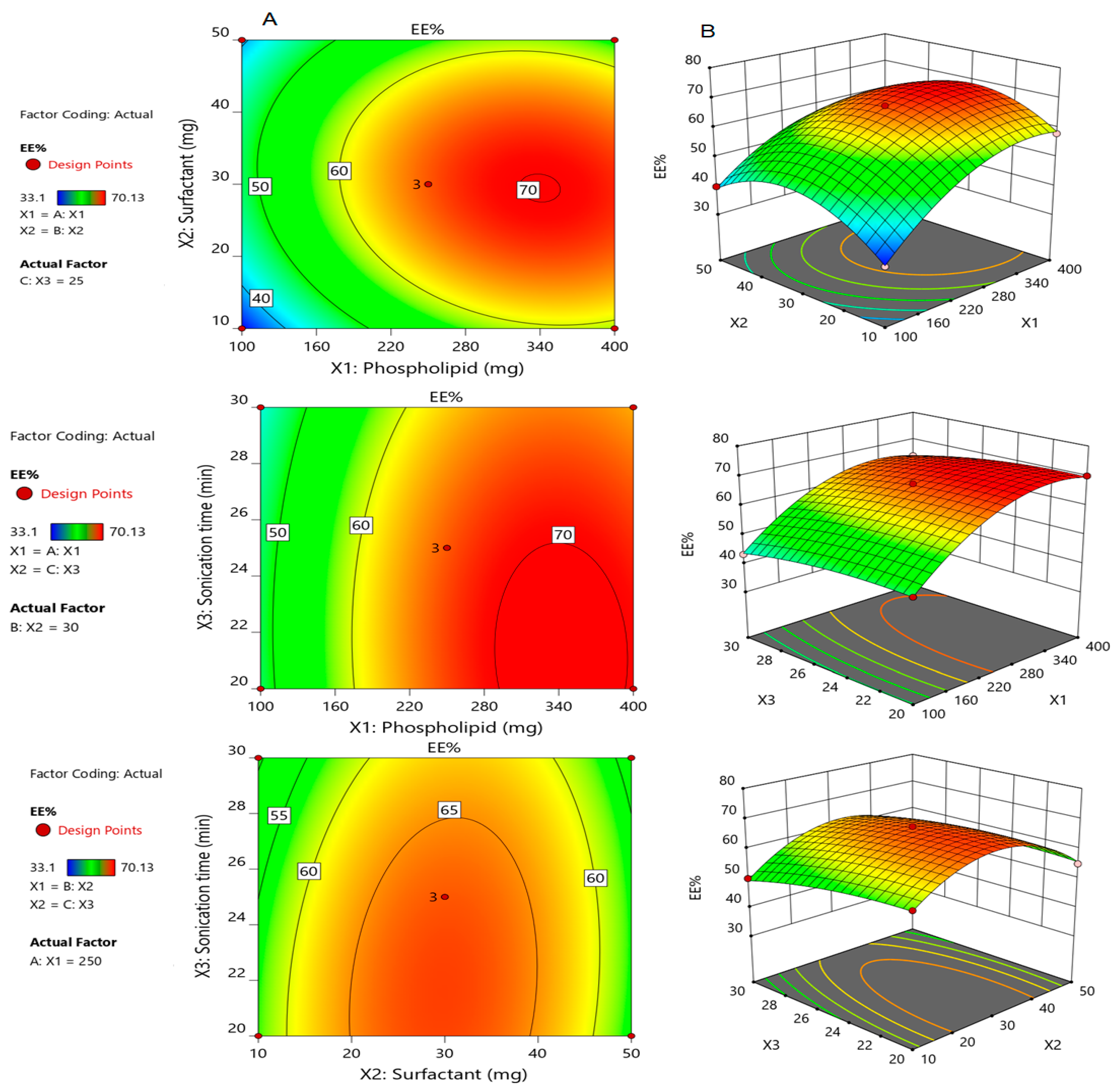
3.2.2. Effect of Formulation Parameters on the Encapsulation Efficiency (Y1)
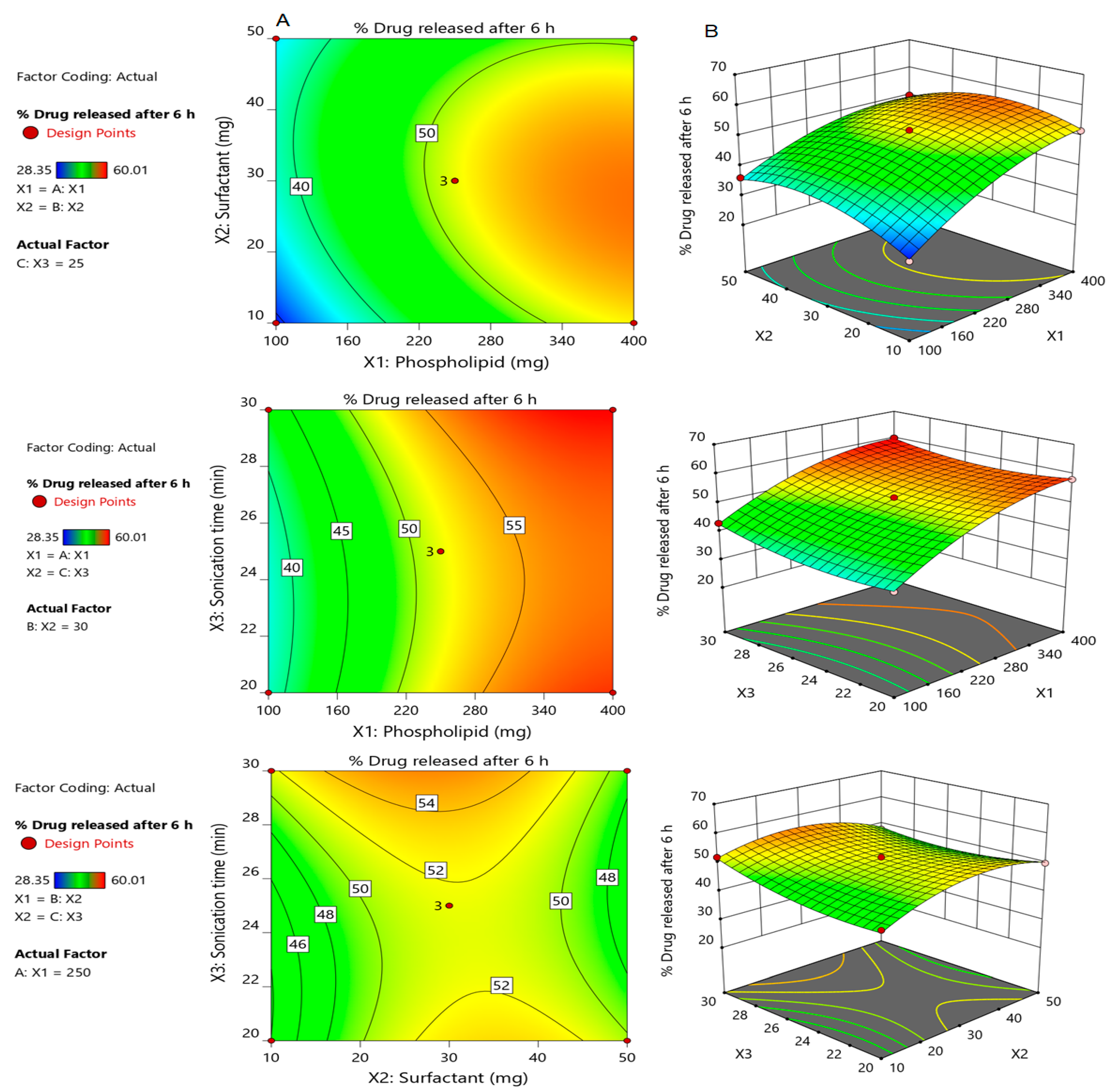
3.2.3. Effect of Independent Factors on Percentage of Drug Released after 5 h (Y2)
3.2.4. Selection of the Optimized Formulation of Silymarin Loaded Transfersomes (SmTFs)
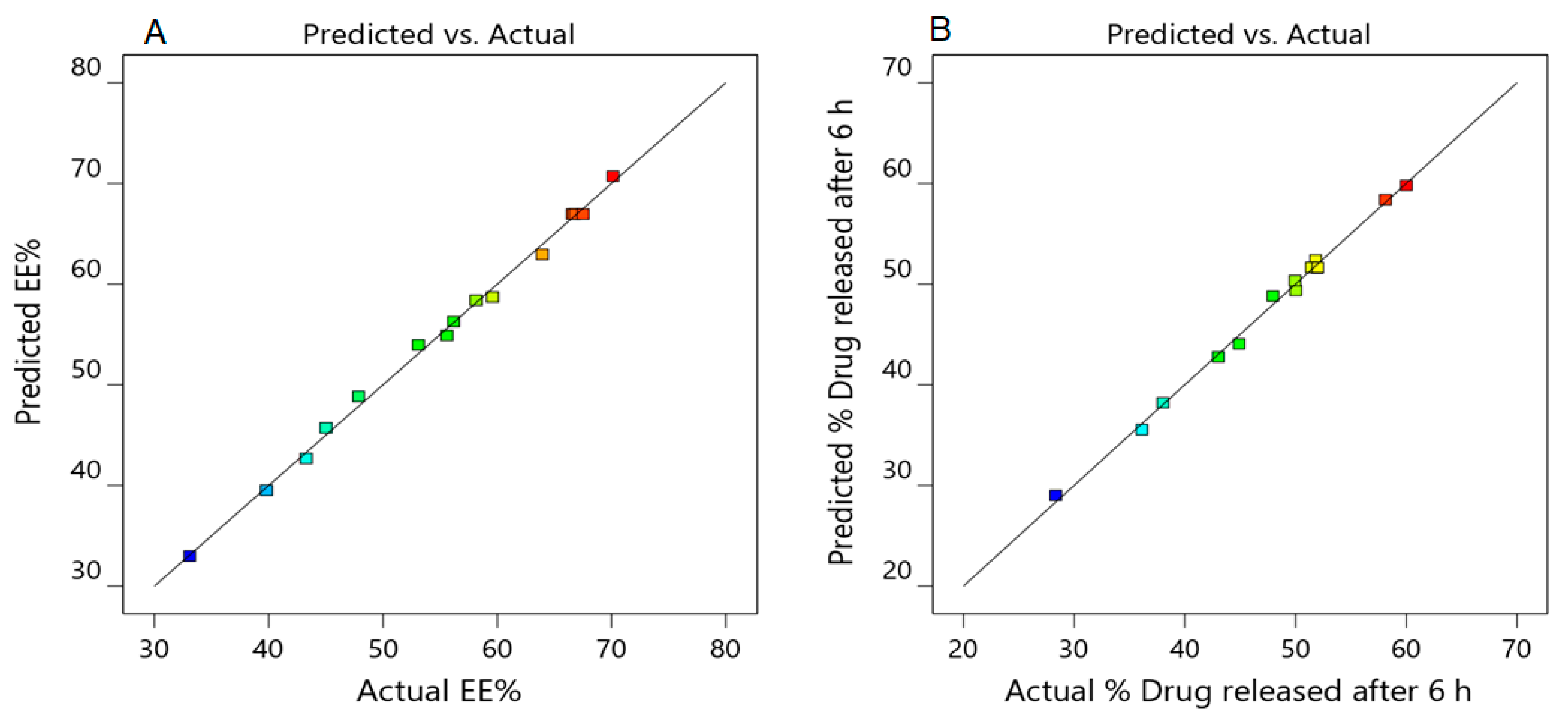
3.2.5. Validation of the Developed Response Surface Methodology (RSM) Model
3.2.6. Particle Size of the Optimized Silymarin Loaded Transfersomes Formulation
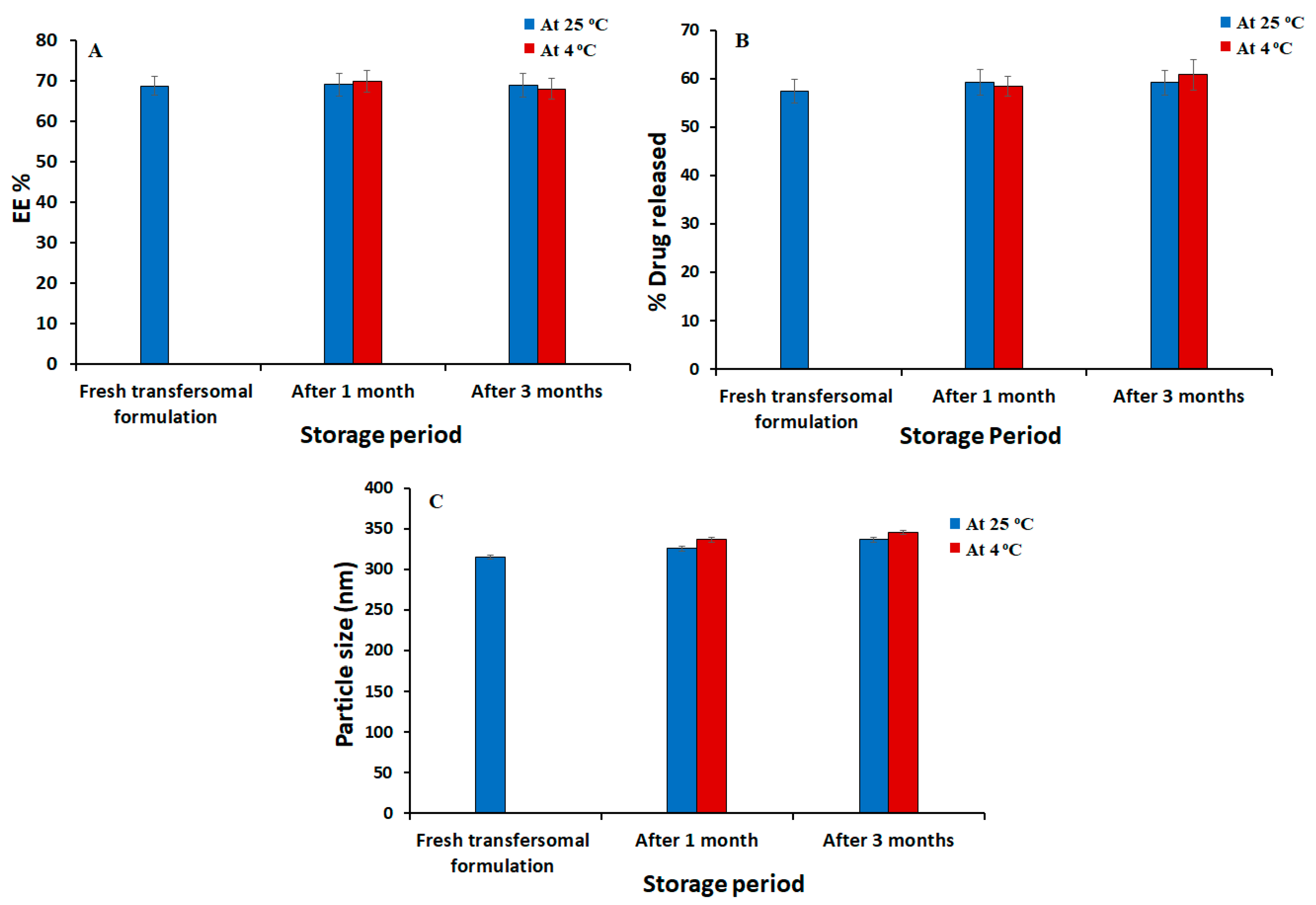
3.3. Stability Studies of the Optimized Silymarin Loaded Transfersomes Formulation
3.4. Evaluation of the Developed Silymarin Loaded Transfersomal Gel
3.5. In Vitro Release Studies
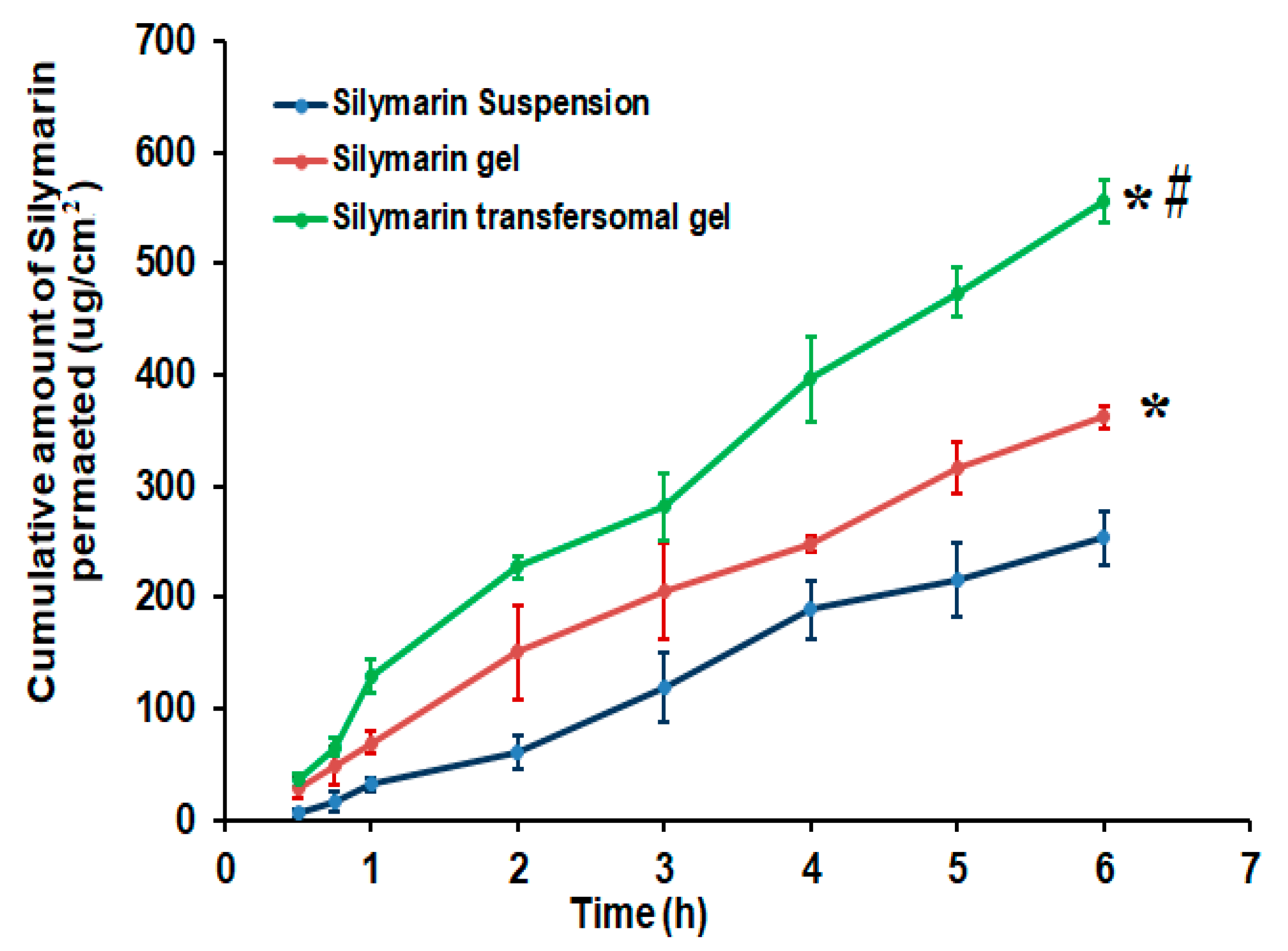
3.6. Ex Vivo Skin Permeation Investigation of Silymarin from Different Formulations
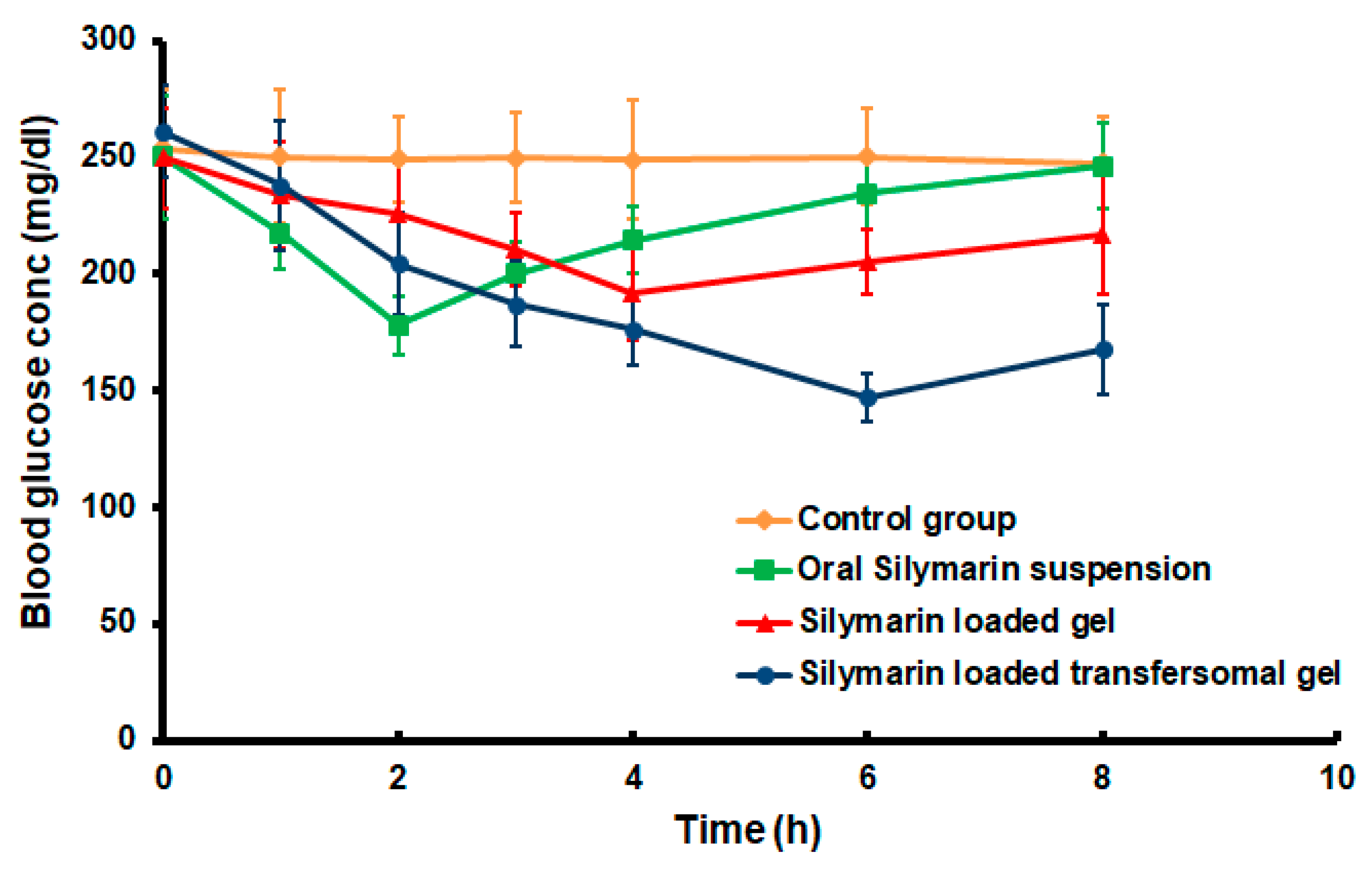
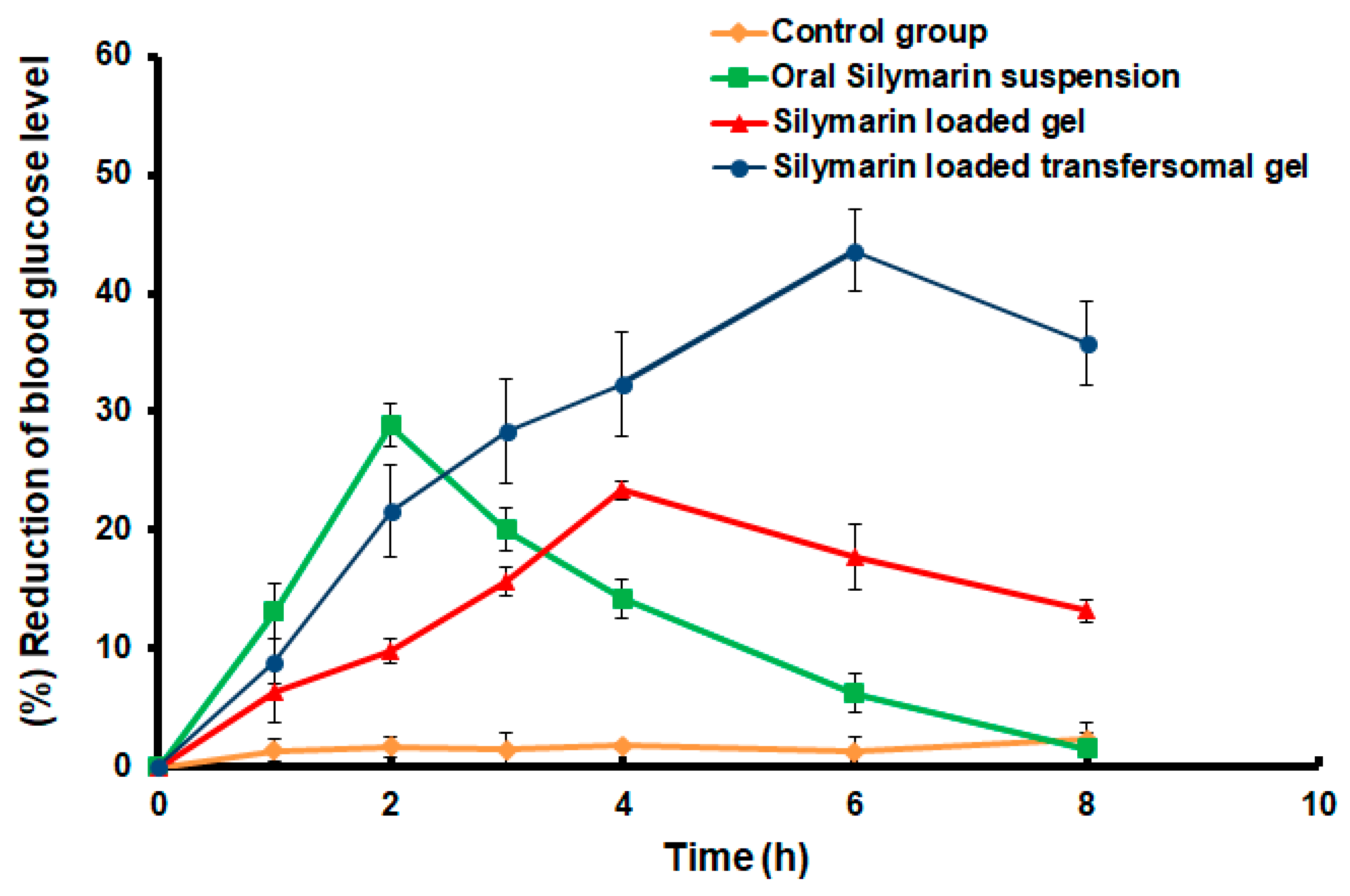
3.7. Hypoglycemic Effect of the Developed Silymarin Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef] [Green Version]
- Isaac, M.; Holvey, C. Transdermal patches: The emerging mode of drug delivery system in psychiatry. Ther. Adv. Psychopharmacol. 2012, 2, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules 2016, 21, 1719. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Elsewedy, H.S.; AbuLila, A.S.; Almansour, K.; Unissa, R.; Elghamry, H.A.; Soliman, M.S. Quality by Design for Optimizing a Novel Liposomal Jojoba Oil-Based Emulgel to Ameliorate the Anti-Inflammatory Effect of Brucine. Gels 2021, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Brucine-Loaded Ethosomal Gel: Design, Optimization, and Anti-inflammatory Activity. AAPS PharmSciTech 2021, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Gillet, A.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Skin penetration behaviour of liposomes as a function of their composition. Eur. J. Pharm. Biopharm. 2011, 79, 43–53. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.B.; Alam, M.A. Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate. Saudi Pharm. J. 2017, 25, 1040–1046. [Google Scholar] [CrossRef]
- El Maghraby, G.; Williams, A.C.; Barry, B. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Tunalı-Akbay, T.; Erkanlı, G.; Yüksel, M.; Ercan, F.; Şener, G. Silymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injury. Burns 2007, 33, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Silymarin and skin cancer prevention: Anti-inflammatory, antioxidant and immunomodulatory effects. Int. J. Oncol. 2005, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Dehmlow, C.; Erhard, J.; de Groot, H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology 1996, 23, 749–754. [Google Scholar] [CrossRef]
- Xiao, F.; Gao, F.; Zhou, S.; Wang, L. The therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction: A meta-analysis. Medicine 2020, 99, e22249. [Google Scholar] [CrossRef]
- MacDonald-Ramos, K.; Michán, L.; Martínez-Ibarra, A.; Cerbón, M. Silymarin is an ally against insulin resistance: A review. Ann. Hepatol. 2021, 23, 100255. [Google Scholar] [CrossRef]
- Abdallah, M.H. Box-behnken design for development and optimization of acetazolamide microspheres. Int. J. Pharm. Sci. Res. 2014, 5, 1228–1239. [Google Scholar]
- Abdallah, M.H. Transfersomes as a transdermal drug delivery system for enhancement the antifungal activity of nystatin. Int. J. Pharm. Pharm. Sci. 2013, 5, 560–567. [Google Scholar]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Upgrading of dissolution and anti-hypertensive effect of Carvedilol via two combined approaches: Self-emulsification and liquisolid techniques. Drug Dev. Ind. Pharm. 2018, 44, 873–885. [Google Scholar] [CrossRef]
- Younis, N.; Shaheen, M.A.; Abdallah, M.H. Silymarin-loaded Eudragit® RS100 nanoparticles improved the ability of silymarin to resolve hepatic fibrosis in bile duct ligated rats. Biomed. Pharmacother. 2016, 81, 93–103. [Google Scholar] [CrossRef]
- Husseiny, R.A.; Lila, A.S.A.; Abdallah, M.H.; Hamed, E.E.; El-ghamry, H.A. Design, in vitro/in vivo evaluation of meclizine HCl-loaded floating microspheres targeting pregnancy-related nausea and vomiting. J. Drug Deliv. Sci. Technol. 2018, 47, 395–403. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Ayoub, A.M.; Mahdy, M.A.E.; Abdallah, M.H. Solid Lipid Nanoparticles of Sulpiride: Improvement of Pharmacokinetic Properties. Int. J. Pharm. Investig. 2019, 9, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.H.; Sabry, S.A.; Hasan, A.A. Enhancing Transdermal Delivery of Glimepiride Via Entrapment in Proniosomal Gel. J. Young Pharm. 2016, 8, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021, 26, 3454. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.A.; Anwer, M.K.; Khafagy, E.-S.; Mohammad, M.; Soliman, M.S. Formulation, development and evaluation of ibuprofen loaded nano-transferosomal gel for the treatment of psoriasis. J. Pharm. Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Tawde, S.; Gawde, K.; Mahind, D.; Mhaprolkar, C.; Sawant, K.; Surve, C. GINGEL: Development and Evaluation of Anti-Arthritic Gel Containing Ginger (Zingiber officinale). Int. J. Innov. Sci. Res. Technol. 2020, 5. [Google Scholar]
- Abdallah, M.H.; Lila, A.S.A.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef]
- Abdelnabi, D.M.; Abdallah, M.H.; Elghamry, H.A. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: In vitro and animal studies. AAPS PharmSciTech 2019, 20, 1–14. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhao, W.; Dong, X.; Wu, W. Biomimetic thiamine-and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm. Sin. B 2018, 8, 97–105. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Ibrahim, M.M.; Abdallah, M.H.; Mahdy, M.A. Sulpiride microemulsions as antipsychotic nasal drug delivery systems: In-vitro and pharmacodynamic study. J. Drug Deliv. Sci. Technol. 2016, 36, 10–22. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. Effects of preparation methods on the characteristics of niosomes. Br. J. Pharm. 2019, 4, S24–S25. [Google Scholar]
- Mahmood, S.; Taher, M.; Mandal, U.K. Experimental design and optimization of raloxifene hydrochloride loaded nanotransfersomes for transdermal application. Int. J. Nanomed. 2014, 9, 4331. [Google Scholar]
- Barani, S.; Ali, M.; Amin, S. Formulation and Characterization of Flexible Phosphatydilcholine Vesicles for Systemic Delivery of Piroxicam. J. Pharm. Sci. Res. 2017, 9, 972. [Google Scholar]
- Rao, M.; Sukre, G.; Aghav, S.; Kumar, M. Optimization of metronidazole emulgel. J. Pharm. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J. Liposome Res. 2019, 29, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.; Rizq, W.Y. Finasteride nano-transferosomal gel formula for management of androgenetic alopecia: Ex vivo investigational approach. Drug Des. Dev. Ther. 2018, 12, 2259. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.S.; Imam, S.S.; Aqil, M.; Sultana, Y.; Ali, A. QbD-based carbopol transgel formulation: Characterization, pharmacokinetic assessment and therapeutic efficacy in diabetes. Drug Deliv. 2016, 23, 1047–1056. [Google Scholar] [CrossRef]


| Independent Variable | Character | Level of Variation | ||
| −1 | 0 | +1 | ||
| Phospholipid concentration (mg) | X1 | 100 | 250 | 400 |
| Surfactant concentration (mg) | X2 | 10 | 30 | 50 |
| Sonication time (min) | X3 | 20 | 25 | 30 |
| Dependent Responses | ||||
| (Y1) = Encapsulation efficiency EE% | ||||
| (Y2) = In vitro release of the drug after 6 h | ||||
| Formulation | Independent Variables | Dependent Variables | |||
|---|---|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (min) | Y1 (%) | Y2 (%) | |
| F1 | 100 | 30 | 30 | 43.27 ± 1.22 | 43.03 ± 1.09 |
| F2 | 250 | 30 | 25 | 66.80 ± 1.21 | 51.43 ± 1.13 |
| F3 | 400 | 10 | 25 | 58.13 ± 0.32 | 51.81 ± 1.22 |
| F4 | 100 | 50 | 25 | 39.80 ± 0.82 | 36.13 ± 0.70 |
| F5 | 250 | 10 | 30 | 50.03 ± 1.05 | 51.97 ± 0.96 |
| F6 | 250 | 30 | 25 | 67.50 ± 1.32 | 52.02 ± 0.66 |
| F7 | 250 | 30 | 25 | 66.58 ± 1.51 | 51.50 ± 0.77 |
| F8 | 250 | 50 | 20 | 55.03 ± 1.05 | 49.96 ± 0.87 |
| F9 | 400 | 30 | 20 | 70.13 ± 0.80 | 58.13 ± 1.56 |
| F10 | 100 | 30 | 20 | 47.87 ± 1.31 | 38.03 ± 1.56 |
| F11 | 400 | 30 | 30 | 63.93 ± 0.90 | 60.01 ± 0.59 |
| F12 | 400 | 50 | 25 | 56.17 ± 0.76 | 50.03 ± 1.13 |
| F13 | 250 | 50 | 30 | 53.10 ± 0.56 | 47.96 ± 0.78 |
| F14 | 250 | 10 | 20 | 57.17 ± 0.47 | 44.91 ± 0.82 |
| F15 | 100 | 10 | 25 | 33.10 ± 0.66 | 28.35 ± 0.28 |
| Independent Variables | Symbol | Goal |
| Phospholipid concentration (mg) | X1 | In range |
| Surfactant concentration (mg) | X2 | In range |
| Sonication time (min) | X3 | In range |
| Dependent variables | Predicted values | Observed values |
| R1 (%) | 70.13 ± 2.62 | 68.61 ± 2.36 |
| R2 (%) | 58.33 ± 1.92 | 57.33 ± 2.07 |
| Source | Y1 | Y2 | ||
| F-Value | p-Value | F-Value | p-Value | |
| Model | 317.13 | <0.0001 * | 146.99 | <0.0001 * |
| X1—Phospholipid (mg) | 1511.95 | <0.0001 * | 934.30 | <0.0001 * |
| X2—Surfactant (mg) | 6.84 | 0.0474 * | 8.36 | 0.0342 * |
| X3—Sonication time (min) | 83.96 | 0.0003 * | 24.04 | 0.0045 * |
| Lack of Fit | 3.58 | 0.2261 | 11.23 | 0.0829 |
| R2 analysis | ||||
| R² | 0.9983 | 0.9962 | ||
| Adjusted R² | 0.9951 | 0.9895 | ||
| Predicted R² | 0.9758 | 0.9427 | ||
| Adequate Precision | 58.0476 | 43.8283 | ||
| Properties | Silymarin Gel | Silymarin Transfersomal Gel |
|---|---|---|
| Visual inspection | Smooth and homogenous | Smooth and homogenous |
| pH | 6.89 ± 0.31 | 7.05 ± 0.45 |
| Spreadability (mm) | 52.9 ± 2.4 | 55.35 ± 3.03 * |
| Viscosity (Pa) | 5.96 ± 0.77 | 6.27 ± 0.63 Pa * |
| Drug content (%) | 99.13 ± 0.42 | 99.35 ± 0.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.-S.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. https://doi.org/10.3390/polym14030508
Abdallah MH, Abu Lila AS, Shawky SM, Almansour K, Alshammari F, Khafagy E-S, Makram TS. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers. 2022; 14(3):508. https://doi.org/10.3390/polym14030508
Chicago/Turabian StyleAbdallah, Marwa H., Amr S. Abu Lila, Seham Mohammed Shawky, Khaled Almansour, Farhan Alshammari, El-Sayed Khafagy, and Tarek Saad Makram. 2022. "Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin" Polymers 14, no. 3: 508. https://doi.org/10.3390/polym14030508
APA StyleAbdallah, M. H., Abu Lila, A. S., Shawky, S. M., Almansour, K., Alshammari, F., Khafagy, E.-S., & Makram, T. S. (2022). Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers, 14(3), 508. https://doi.org/10.3390/polym14030508







