Advances in Polymeric Colloids for Cancer Treatment
Abstract
:1. Introduction
2. Polymer Colloids
2.1. Micelles in Cancer Treatment
2.2. Liposomes in Cancer Treatment
2.3. Emulsions in Cancer Treatment
2.4. Cationic Carriers in Cancer Treatment
2.5. Hydrogels in Cancer Treatment
3. Targets in Cancer Therapy, Drug Delivery, and Controlled Release
4. Cancer Diagnosis
- Smaller in size
- Eco-friendly
- Low toxicity to ordinary cells
- High stability in biological conditions
- Capable of conveying the imaging agents
- Releasable for therapeutic agents easily
5. Benefits of Polymeric Colloidal Material in Cancer Treatment
- biocompatibility, non-toxicity, non-immunogenicity, and biodegradability.
- transport essential drugs.
- release the medicines at the tumor location.
- be stable in physiological conditions.
- control the effect of EPR or receptor-facilitated interfaces.
5.1. Ecological
5.2. Polymeric Micelles
5.3. Miscellaneous Functions
6. Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahbub, A.A.; Maitre, C.L.; Cross, N.A.; Jordan-Mahy, N. The effect of apigenin and chemotherapy combination treatments on apoptosis-related genes and proteins in acute leukaemia cell lines. Sci. Rep. 2022, 12, 8858. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, A.A.; Aslam, A.; Elzubier, M.E.; El-Boshy, M.; Abdelghany, A.H.; Ahmad, J.; Idris, S.; Almaimani, R.; Alsaegh, A.; El-Readi, Z.; et al. Enhanced anti-cancer effects of oestrogen and progesterone co-therapy against colorectal cancer in males. Front. Endocrinol. 2022, 13, 941834. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Refaat, B.; Almaimani, R.A.; Ahmed, H.G.; Ahmad, J.; Alhadrami, M.; El-Readi, M.Z.; Elzubier, M.E.; Alaufi, H.A.A.; Al-Amin, B.; et al. Enhanced in vitro tumoricidal effects of 5-Fluorouracil, thymoquinone, and active vitamin D3 triple therapy against colon cancer cells by attenuating the PI3K/AKT/mTOR pathway. Life Sci. 2022, 296, 120442. [Google Scholar] [CrossRef] [PubMed]
- Sharhan, N.A.A.; Messaoudi, S.A.; Babu, S.R.; Chaudhary, A.R.B.; Alsharm, A.A.; Alrefaei, A.F.; Kadasah, S.; Abu-Elmagd, M.; Assidi, M.; Buhmeida, A.; et al. Utility of Circulating Cell-Free DNA in Assessing Microsatellite Instability and Loss of Heterozygosity in Breast Cancer Using Human Identification Approach. Genes 2022, 13, 590. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of anthraquinones as anticancer agents—A systematic review of recent literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Hussain, A.; Oves, M.; Alajmi, M.F.; Hussain, I.; Amir, S.; Ahmed, J.; Rehman, M.T.; El-Seedi, H.R.; Ali, I. Biogenesis of ZnO Nanoparticles Using Pandanus Odorifer Leaf Extract: Anticancer and Antimicrobial Activities. RSC Adv. 2019, 9, 15357–15369. [Google Scholar] [CrossRef] [Green Version]
- Saleem, K.; Wani, W.A.; Haque, A.; Lone, M.N.; Hsieh, M.-F.; Jairajpuri, M.A.; Ali, I. Synthesis, DNA Binding, Hemolysis Assays and Anticancer Studies of Copper(II), Nickel(II) and Iron(III) Complexes of a Pyrazoline-Based Ligand. Future Med. Chem. 2013, 5, 135–146. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Saleem, K.; Hsieh, M.F. Anticancer metallodrugs of glutamic acid sulphonamides: In silico, DNA binding, hemolysis and anticancer studies. RSC Adv. 2014, 4, 29629–29641. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Khan, A.; Haque, A.; Ahmad, A.; Saleem, K.; Manzoor, N. Synthesis and Synergistic Antifungal Activities of a Pyrazoline Based Ligand and Its Copper(II) and Nickel(II) Complexes with Conventional Antifungals. Microb. Pathog. 2012, 53, 66–73. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Haque, A.; Saleem, K. Glutamic Acid and Its Derivatives: Candidates for Rational Design of Anticancer Drugs. Future Med. Chem. 2013, 5, 961–978. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; A Wani, W.; Saleem, K.; Wesselinova, D. Syntheses, DNA Binding and Anticancer Profiles of L-Glutamic Acid Ligand and Its Copper(II) and Ruthenium(III) Complexes. Med. Chem. 2013, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Aboul-Enein, H.Y.; Ghanem, A. Enantioselective toxicities and carcinogenesis. Curr. Pharm. Anal. 2005, 1, 109–125. [Google Scholar] [CrossRef]
- Imani, R.; Dillert, R.; Bahnemann, D.W.; Pazoki, M.; Apih, T.; Kononenko, V.; Repar, N.; Kralj-Iglic, V.; Boschloo, G.; Drobne, D.; et al. Multifunctional Gadolinium-Doped Mesoporous TiO2 Nanobeads: Photoluminescence, Enhanced Spin Relaxation, and Reactive Oxygen Species Photogeneration, Beneficial for Cancer Diagnosis and Treatment. Small 2017, 13, 1700349. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Suhail, M.; Mukhtar, S.D.; Asnin, L. Advances in Nanocarriers for Anticancer Drugs Delivery. Curr. Med. Chem. 2016, 23, 2159–2187. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The Future of Cancer Diagnosis and Therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Kari, Z.A.; Edinur, H.T.; Baishya, D.; Atanase, L.I. Development of polymer-based nanoformulations for glioblastoma brain cancer therapy and diagnosis: An update. Polymers 2021, 13, 4114. [Google Scholar] [CrossRef]
- Saeedi, T.; Alotaibi, H.F.; Prokopovich, P. Polymer colloids as drug delivery systems for the treatment of arthritis. Adv. Colloid Interf. Sci. 2020, 285, 102273. [Google Scholar] [CrossRef]
- Ali, I.; Alsehli, M.; Scotti, L.; Scotti, M.T.; Tsai, S.T.; Yu, R.S.; Hsieh, M.F.; Chen, J.C. Progress in Polymeric Nano-Medicines for Theranostic Cancer Treatment. Polymers 2020, 12, 598. [Google Scholar] [CrossRef]
- Kesharwani, P.; Choudhury, H.; Meher, J.G.; Pandey, M.; Gorain, B. Dendrimer-Entrapped Gold Nanoparticles as Promising Nanocarriers for Anticancer Therapeutics and Imaging. Prog. Mater. Sci. 2019, 103, 484–508. [Google Scholar] [CrossRef]
- Li, Z.; Tan, S.; Li, S.; Shen, Q.; Wang, K. Cancer Drug Delivery in the Nano Era: An Overview and Perspectives. Oncol. Rep. 2017, 38, 611–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, S.; Hussain, T.; Raza, Z.A.; Nazir, A. Current Applications of Electrospun Polymeric Nanofibers in Cancer Therapy. Mater. Sci. Eng. C 2019, 97, 966–977. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic Nanomedicine for Cancer Detection and Treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, K.-T.; Wang, Y.; Roy, I.; Rui, H.; Swihart, M.T.; Law, W.-C.; Kwak, S.K.; Ye, L.; Liu, J.; Mahajan, S.D.; et al. Preparation of Quantum Dot/Drug Nanoparticle Formulations for Traceable Targeted Delivery and Therapy. Theranostics 2012, 2, 681–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, J.A.; Bardhan, R. Emerging Advances in Nanomedicine with Engineered Gold Nanostructures. Nanoscale 2014, 6, 2502. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic Applications of Nanoparticles in Cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Current Advances in Polymer-Based Nanotheranostics for Cancer Treatment and Diagnosis. ACS Appl. Mater. Interf. 2014, 6, 21859–21873. [Google Scholar] [CrossRef]
- Priestley, R.D.; Prud’homme, R.K. (Eds.) Polymer Colloids Formation, Characterization and Applications; RSC Publisher: London, UK, 2020. [Google Scholar]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Cuong, N.-V.; Jiang, J.-L.; Li, Y.-L.; Chen, J.-R.; Jwo, S.-C.; Hsieh, M.-F. Doxorubicin-Loaded PEG-PCL-PEG Micelle Using Xenograft Model of Nude Mice: Effect of Multiple Administration of Micelle on the Suppression of Human Breast Cancer. Cancers 2011, 3, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Torcello-Gómez, A.; Wulff-Pérez, M.; Gálvez-Ruiz, M.J.; Martín-Rodríguez, A.; Cabrerizo-Vílchez, M.; Maldonado-Valderrama, J. Block Copolymers at Interfaces: Interactions with Physiological Media. Adv. Colloid Interface Sci. 2014, 206, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, C.; Pang, X.; Yu, Z.; Qi, Y.; Chen, X.; Liang, H.; Fang, X.; Sha, X. Adding Vitamin E-TPGS to the Formulation of Genexol-PM: Specially Mixed Micelles Improve Drug-Loading Ability and Cytotoxicity against Multidrug-Resistant Tumors Significantly. PLoS ONE 2015, 10, e0120129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, C.; Yue, X.; Li, X.; Zhou, P.; Wu, A.; Chen, C.; Qu, Y.; Zhang, C. Application advance of electrosprayed micro/nanoparticles based on natural or synthetic polymers for drug delivery system. Mater. Des. 2022, 220, 110850. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Cammas, S.; Harada, A.; Nagasaki, Y.; Kataoka, K. Poly(Ethylene Oxide- Co -β-Benzyl l -Aspartate) Block Copolymers: Influence of the Poly(Ethylene Oxide) Block on the Conformation of the Poly(β-Benzyl l -Aspartate) Segment in Organic Solvents. Macromolecules 1996, 29, 3227–3231. [Google Scholar] [CrossRef]
- Ren, J.; Fang, Z.; Yao, L.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. A Micelle-like Structure of Poloxamer–Methotrexate Conjugates as Nanocarrier for Methotrexate Delivery. Int. J. Pharm. 2015, 487, 177–186. [Google Scholar] [CrossRef]
- Tang, X.; Liang, Y.; Feng, X.; Zhang, R.; Jin, X.; Sun, L. Co-Delivery of Docetaxel and Poloxamer 235 by PLGA–TPGS Nanoparticles for Breast Cancer Treatment. Mater. Sci. Eng. C 2015, 49, 348–355. [Google Scholar] [CrossRef]
- Daraba, O.M.; Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Vochita, G. Antitumoral drug-loaded biocompatible polymeric nanoparticles obtained by non-aqueous emulsion polymerization. Polymers 2020, 12, 1018. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Lin, Z.; Wang, D.; Mei, D.; He, B.; Wang, X.; Wang, X.; Zhang, Q.; Gao, W. A Folate Modified PH Sensitive Targeted Polymeric Micelle Alleviated Systemic Toxicity of Doxorubicin (DOX) in Multi-Drug Resistant Tumor Bearing Mice. Eur. J. Pharm. Sci. 2015, 76, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, P.; Duan, Y.; Yin, X.; Wang, Q.; Liu, X.; Wang, X.; Zhou, J.; Wang, W.; Qiu, L.; et al. Specific Cell Targeting with APRPG Conjugated PEG–PLGA Nanoparticles for Treating Ovarian Cancer. Biomaterials 2014, 35, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Shieh, M.-J.; Tsai, M.-H.; Chang, C.-C.; Lai, P.-S. Self-Assembled Star-Shaped Chlorin-Core Poly(ɛ-Caprolactone)–Poly(Ethylene Glycol) Diblock Copolymer Micelles for Dual Chemo-Photodynamic Therapies. Biomaterials 2008, 29, 3599–3608. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.-V.; Li, Y.-L.; Hsieh, M.-F. Targeted Delivery of Doxorubicin to Human Breast Cancers by Folate-Decorated Star-Shaped PEG–PCL Micelle. J. Mater. Chem. 2012, 22, 1006–1020. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent Advances in Polymeric Micelles for Anti-Cancer Drug Delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric Micelles for Delivery of Poorly Soluble Drugs: Preparation and Anticancer Activity in Vitro of Paclitaxel Incorporated into Mixed Micelles Based on Poly(Ethylene Glycol)-Lipid Conjugate and Positively Charged Lipids. J. Drug Target. 2005, 13, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yang, D.; Cai, C.; Gou, J.; Zhang, Y.; Wang, L.; Zhong, H.; Tang, X. Dual-Responsive MPEG-PLGA-PGlu Hybrid-Core Nanoparticles with a High Drug Loading to Reverse the Multidrug Resistance of Breast Cancer: An in Vitro and in Vivo Evaluation. Acta Biomater. 2015, 16, 156–168. [Google Scholar] [CrossRef]
- Fouladi, F.; Steffen, K.J.; Mallik, S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug. Chem. 2017, 28, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Gregoriadis, G.; Leathwood, P.D.; Rymanet, B.E. Enzyme entrapment in liposomes. FEBS Lett. 1971, 14, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, B.; Singh, S.K.; Gulati, M.; Gupta, R.; Vaidya, Y. Application of Liposomes in Treatment of Rheumatoid Arthritis: Quo Vadis. Sci. World J. 2014, 2014, 978351. [Google Scholar] [CrossRef]
- Vanniasinghe, A.S.; Bender, V.; Manolios, N. The Potential of Liposomal Drug Delivery for the Treatment of Inflammatory Arthritis. Semin. Arthritis Rheum. 2009, 39, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, 16150477. [Google Scholar] [CrossRef] [Green Version]
- Krivak, T.; Smith, A.; Zorn, K.; Sukumvanich, P.; Olawaiye, A.B.; Kelley, J.; Berger, J. Outcomes. Analysis of an Alternative Formulation of PEGylated Liposomal Doxorubicin in Recurrent Epithelial Ovarian Carcinoma during the Drug Shortage Era. Onco. Targets. Ther. 2014, 7, 1409–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Chou, H.-H.; Lin, H. A Tale of the Two PEGylated Liposomal Doxorubicins. Onco. Targets. Ther. 2015, 8, 1719–1720. [Google Scholar] [CrossRef] [Green Version]
- Barratt, G.M. Therapeutic applications of colloidal drug carriers. PSTT 2000, 3, 163–171. [Google Scholar] [CrossRef]
- Lu, G.W.; Gao, P. Emulsions and Microemulsions for Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Goindi, S.; Narula, M.; Kalra, A. Microemulsion-Based Topical Hydrogels of Tenoxicam for Treatment of Arthritis. AAPS PharmSciTech 2016, 17, 597–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent Advances in Topical Ophthalmic Drug Delivery with Lipid-Based Nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, M.; Alkhayyal, N. Cytotoxicity of Gemcitabine-Loaded-Microemulsions in Breast and Colon Cancer Cells. Trop. J. Pharm. Res. 2014, 13, 217. [Google Scholar] [CrossRef] [Green Version]
- E Flores, S.; Isabel Rial-Hermida, M.; C Ramirez, J.; Pazos, A.; Concheiro, A.; Alvarez-Lorenzo, C.; Peralta, R.D. Microemulsions for Colorectal Cancer Treatments. General Considerations and Formulation of Methotrexate. Mini-Rev. Med. Chem. 2016, 16, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Fofaria, M.F.; Qhattal, H.S.S.; Liu, X.; Srivastava, S.K. Nanoemulsion formulations for anti-cancer agent piplartine—characterization, toxicological, pharmacokinetics and efficacy studies. Int. J. Pharm. 2016, 498, 12–22. [Google Scholar]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan Induces Apoptosis via Caspase-3 Activation in Bladder Tumor Cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E. Cationic Nanoparticles for Cancer Therapy. Expert Opin. Drug Deliv. 2010, 7, 795–809. [Google Scholar] [CrossRef]
- Putnam, D.; Gentry, C.A.; Pack, D.W.; Langer, R. Polymer-Based Gene Delivery with Low Cytotoxicity by a Unique Balance of Side-Chain Termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Han, K.O.; Han, I.K.; Cho, M.H.; Nah, J.W.; Choi, Y.J.; Cho, C.S. Degradable Polyethylenimine-Alt-Poly(Ethylene Glycol) Copolymers as Novel Gene Carriers. J. Control. Release 2005, 105, 367–380. [Google Scholar] [CrossRef]
- Fan, D.; Tian, Y.; Li, Z. Injectable hydrogels for localized cancer therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Gou, Y.; Wang, X.; Zhao, X.; Tao, L. Improving Tumor Chemotherapy Effect Using an Injectable Self-Healing Hydrogel as Drug Carrier. Polym. Chem. 2017, 8, 5071–5076. [Google Scholar] [CrossRef]
- Xie, W.; Gao, Q.; Guo, Z.; Wang, D.; Gao, F.; Wang, X.; Wei, Y.; Zhao, L. Injectable and Self-Healing Thermosensitive Magnetic Hydrogel for Asynchronous Control Release of Doxorubicin and Docetaxel to Treat Triple-Negative Breast Cancer. ACS Appl. Mater. Interf. 2017, 9, 33660–33673. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Park, J.H.; Park, S.H.; Oh, H.J.; Kim, J.H.; Min, B.H.; Park, K.; Kim, M.S. Synergistic Anti-Tumor Activity through Combinational Intratumoral Injection of an in-Situ Injectable Drug Depot. Biomaterials 2016, 85, 232–245. [Google Scholar] [CrossRef]
- Lerouge, S.; Monette, A.; Ceccaldi, C.; Cunnigham, N.; Lapointe, R. Injectable T Cell Delivery Scaffolds for Cancer Immunotherapy. Cytotherapy 2018, 20, S106–S107. [Google Scholar] [CrossRef]
- Miele, M.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Cirstea, D.; Hideshima, T.; Rodig, S.; Santo, L.; Pozzi, S.; Vallet, S.; Ikeda, H.; Perrone, G.; Gorgun, G.; Patel, K.; et al. Dual Inhibition of Akt/Mammalian Target of Rapamycin Pathway by Nanoparticle Albumin-Bound –Rapamycin and Perifosine Induces Antitumor Activity in Multiple Myeloma. Mol. Cancer Ther. 2010, 9, 963–975. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Yoo, H.; Lin, W.; Brooks, J.G.; Lippard, S.J. Pt(IV) Prodrugs Designed to Bind Non-Covalently to Human Serum Albumin for Drug Delivery. J. Am. Chem. Soc. 2014, 136, 8790–8798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barraud, L.; Merle, P.; Soma, E.; Lefrançois, L.; Guerret, S.; Chevallier, M.; Dubernet, C.; Couvreur, P.; Trépo, C.; Vitvitski, L. Increase of Doxorubicin Sensitivity by Doxorubicin-Loading into Nanoparticles for Hepatocellular Carcinoma Cells in Vitro and in Vivo. J. Hepatol. 2005, 42, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, X.; Zeng, L.; Liu, J.; Zhang, Z. A Randomized Multicenter Phase II Clinical Trial of Mitoxantrone-Loaded Nanoparticles in the Treatment of 108 Patients with Unresected Hepatocellular Carcinoma. Nanomedicine Nanotechnology Biol. Med. 2009, 5, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Clinical Translation of Nanomedicines. Curr. Opin. Solid State Mater. Sci. 2012, 16, 287–294. [Google Scholar] [CrossRef]
- Williamson, S.K.; Johnson, G.A.; Maulhardt, H.A.; Moore, K.M.; McMeekin, D.S.; Schulz, T.K.; Reed, G.A.; Roby, K.F.; Mackay, C.B.; Smith, H.J.; et al. A Phase I Study of Intraperitoneal Nanoparticulate Paclitaxel (Nanotax®) in Patients with Peritoneal Malignancies. Cancer Chemother. Pharmacol. 2015, 75, 1075–1087. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Du, H.-L.; Zhang, H.-Q.; Zhai, Y.-J.; Zhai, G.-X. Polymer–Drug Conjugates: Present State of Play and Future Perspectives. Drug Discov. Today 2013, 18, 1316–1322. [Google Scholar] [CrossRef]
- Sabir, F.; Qindeel, M.; Zeeshan, M.; Ul Ain, Q.; Rahdar, A.; Barani, M.; González, E.; Aboudzadeh, M.A. Onco-Receptors Targeting in Lung Cancer via Application of Surface-Modified and Hybrid Nanoparticles: A Cross-Disciplinary Review. Processes 2021, 9, 621. [Google Scholar] [CrossRef]
- Steere, A.N.; Byrne, S.L.; Chasteen, N.D.; Mason, A.B. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta 2012, 20, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.S.-G.; Liu, H.-J. Structural Analysis and Degradation Behavior in Polyethylene Glycol/Poly(L-Lactide) Copolymers. J. Appl. Polym. Sci. 1994, 51, 473–482. [Google Scholar] [CrossRef]
- Shiaw-Guang Hu, D.; Liu, H.-J. Effect of Soft Segment on Degradation Kinetics in Polyethylene Glycol/Poly(l-Lactide) Block Copolymers. Polym. Bull. 1993, 30, 669–676. [Google Scholar] [CrossRef]
- Bazile, D.; Prud’homme, C.; Bassoullet, M.; Marlard, M.; Spenlehauer, G.; Veillard, M. Stealth Me. PEG-PLA Nanoparticles Avoid Uptake by the Mononuclear Phagocytes System. J. Pharm. Sci. 1995, 84, 493–498. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A Review of the Formation and Classification of Amphiphilic Block Copolymer Nanoparticulate Structures: Micelles, Nanospheres, Nanocapsules and Polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, S.; Liao, Z.; Shi, W.; Wang, Y.; Zhao, J.; Hu, Y.; Yang, J.; Chen, J.; Mei, H.; et al. Targeting Fibronectins of Glioma Extracellular Matrix by CLT1 Peptide-Conjugated Nanoparticles. Biomaterials 2014, 35, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gao, X.; Gu, G.; Kang, T.; Tu, Y.; Liu, Z.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Glioma Therapy Using Tumor Homing and Penetrating Peptide-Functionalized PEG–PLA Nanoparticles Loaded with Paclitaxel. Biomaterials 2013, 34, 5640–5650. [Google Scholar] [CrossRef]
- Hu, Q.; Gu, G.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; Yao, L.; et al. F3 Peptide-Functionalized PEG-PLA Nanoparticles Co-Administrated with TLyp-1 Peptide for Anti-Glioma Drug Delivery. Biomaterials 2013, 34, 1135–1145. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Daman, Z.; Ostad, S.; Amini, M.; Gilani, K. Preparation, Optimization and in Vitro Characterization of Stearoyl-Gemcitabine Polymeric Micelles: A Comparison with Its Self-Assembled Nanoparticles. Int. J. Pharm. 2014, 468, 142–151. [Google Scholar] [CrossRef]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of Polymer Micelles for Imaging and Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef]
- Lee, E. Polymeric Micelle for Tumor PH and Folate-Mediated Targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent Trends in Protein and Peptide-Based Biomaterials for Advanced Drug Delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, A.L.; Pangua, C.; Reboredo, C.; Campión, R.; Morales-Gracia, J.; Irache, J.M. Protein-Based Nanoparticles for Drug Delivery Purposes. Int. J. Pharm. 2020, 581, 119289. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive Polymers: Classification and Some Fine Potential Applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Katoh, Y.; Ohshima, H. Colloidal stability of aqueous polymeric dispersions: Effect of pH and salt concentration. Colloids Surf. B Biointerf. 2005, 42, 53–58. [Google Scholar] [CrossRef]
- Jaquet, B.; Wei, D.; Reck, B.; Reinhold, F.; Zhang, X.; Wu, H.; Morbidelli, M. Stabilization of polymer colloid dispersions with pH-sensitive poly-acrylic acid brushes. Colloid Polym. Sci. 2013, 291, 1659–1667. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowski, K.; Gutarowicz, M.; Janke, K.; Jurek, I. Colloidal Stability of Positively Charged Dispersions of Styrene and Acrylic Copolymers in the Presence of TiO2 and CaCO3. Colloids Interf. 2019, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Aseyev, V.O.; Tenhu, H.; Winnik, F.M. Temperature dependence of the colloidal stability of neutral amphiphilic polymers in water. Adv. Polym. Sci. 2006, 196, 1–85. [Google Scholar]
- Korshak, V.V.; Vinogradova, S.V. Dependence of thermal stability of polymers on their chemical structur. Russ. Chem. Revs. 2007, 37, 885. [Google Scholar] [CrossRef]
- Hu, H. Recent advances of polymeric phase change composites for flexible electronics and thermal energy storage system. Compos. Part B Eng. 2020, 195, 108094. [Google Scholar] [CrossRef]
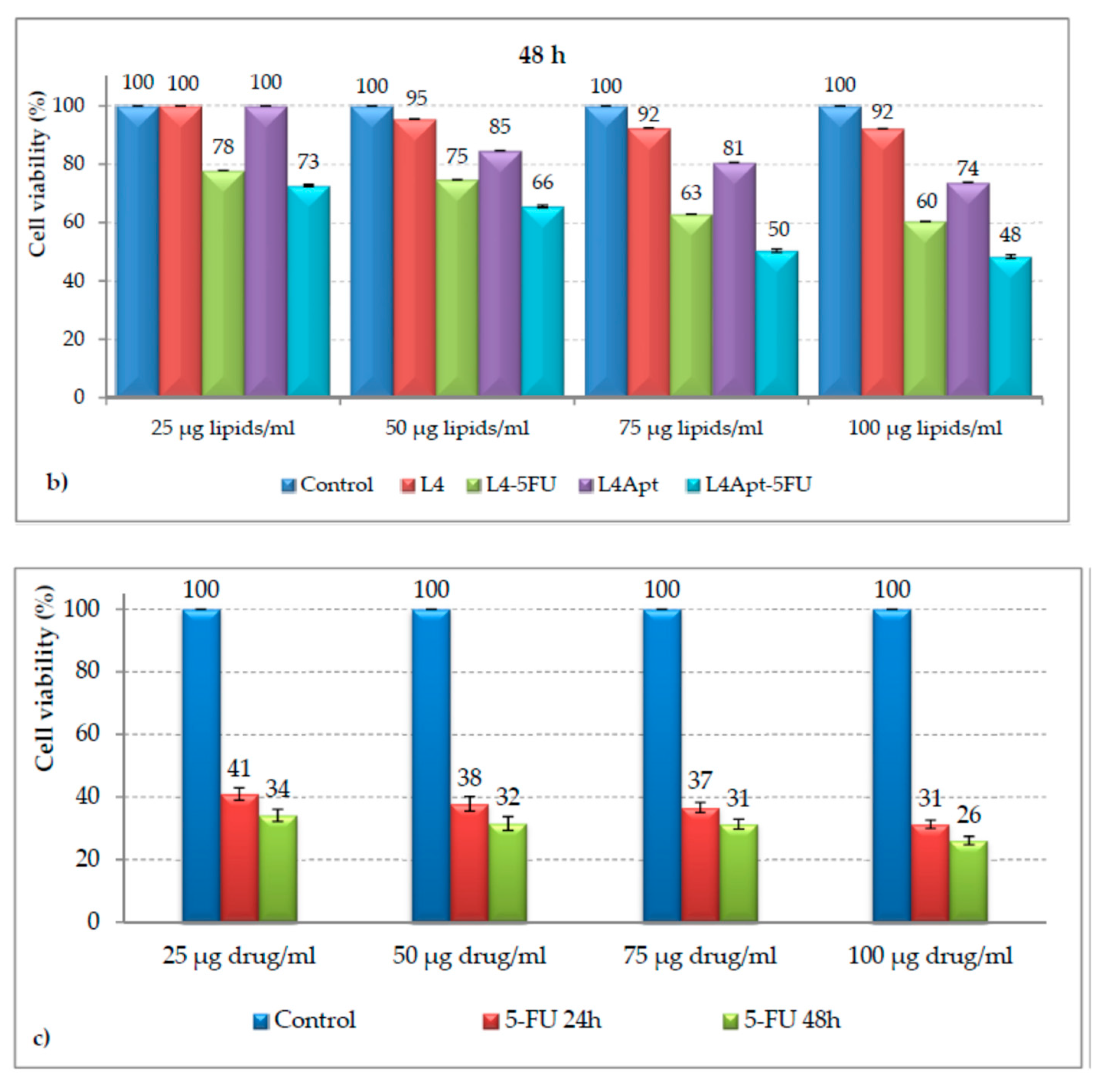
- Rață, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.M.; Gherghe, D.; Popa, M. In vitro behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C 2019, 103, 109828. [Google Scholar] [CrossRef] [PubMed]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-functionalized liposomes as a potential treatment for basal cell carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Mihai, C.T.; Bacaita, S.E.; Popa, M. Formulations Based on Drug Loaded Aptamer-Conjugated Liposomes as a Viable Strategy for the Topical Treatment of Basal Cell Carcinoma—In Vitro Tests. Pharmaceutics 2021, 13, 866. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa Mihai, C.T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.G.; Song, W. Intelligent poly(L-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.G.; Kim, I. Wenliang Song, Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Barash, O.; Peled, N.; Hirsch, F.R.; Haick, H. Sniffing the unique “odor print” of non-small-cell lung cancer with gold nanoparticles. Small 2009, 5, 2618–2624. [Google Scholar] [CrossRef]
- Assmar, M.; Yeganeh, S.; Mansourghanaei, F.; Amirmozafari, N. Combined Evaluation of AFP, CA15-3, CA125, CA19-9, and CEA Tumor Markers in Patients with Hepatitis B and C. Iran. J. Public Health 2016, 45, 1645–1651. [Google Scholar]
- Feng, F.; Tian, Y.; Xu, G.; Liu, Z.; Liu, S.; Zheng, G.; Guo, M.; Lian, X.; Fan, D.; Zhang, H. Diagnostic and prognostic value of CEA, CA19–9, AFP and CA125 for early gastric cancer. MC Cancer 2017, 17, 737. [Google Scholar] [CrossRef] [PubMed]
- Boyes, S.G.; Rowe, M.D.; Serkova, N.J.; Kim, F.J.; Lambert, J.R.; Werahera, P.N. Polymer-modified gadolinium nanoparticles for targeted magnetic resonance imaging and therapy. Nano Life 2010, 01, 263–275. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, L.; Kuang, Y.; Xiong, D.; Pei, R. Gadolinium-Based Nanoscale MRI Contrast Agents for Tumor Imaging. J. Mater. Chem. B 2017, 5, 3431–3461. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Dewi, N.; Yanagie, H.; Kokuryo, D.; Suzuki, M.; Sakurai, Y.; Li, Y.; Aoki, I.; Ono, K.; Takahashi, H.; et al. Hybrid Calcium Phosphate-Polymeric Micelles Incorporating Gadolinium Chelates for Imaging-Guided Gadolinium Neutron Capture Tumor Therapy. ACS Nano 2015, 9, 5913–5921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuu, K.; Xie, J.; McDonald, M.A.; Bernardo, M.; Hunter, F.; Zhang, Y.; Li, K.; Bednarski, M.; Guccione, S. Gadolinium-Rhodamine Nanoparticles for Cell Labeling and Tracking via Magnetic Resonance and Optical Imaging. Bioconjug. Chem. 2005, 16, 995–999. [Google Scholar] [CrossRef]
- Mitra, A.; Nan, A.; Line, B.; Ghandehari, H. Nanocarriers for Nuclear Imaging and Radiotherapy of Cancer. Curr. Pharm. Des. 2006, 12, 4729–4749. [Google Scholar] [CrossRef]
- Park, K.; Lee, S.; Kang, E.; Kim, K.; Choi, K.; Kwon, I.C. New Generation of Multifunctional Nanoparticles for Cancer Imaging and Therapy. Adv. Funct. Mater. 2009, 19, 1553–1566. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and Drug Delivery Using Theranostic Nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Wang, Q.; Zhang, Y.; Zhu, Z.; Xu, X.; Zhang, J.; Wang, A. Colorful Superamphiphobic Coatings with Low Sliding Angles and High Durability Based on Natural Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 1941–1952. [Google Scholar] [CrossRef]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A.; et al. Theranostic Application of Mixed Gold and Superparamagnetic Iron Oxide Nanoparticle Micelles in Glioblastoma Multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Nayak, T.R.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Theranostic Unimolecular Micelles Based on Brush-Shaped Amphiphilic Block Copolymers for Tumor-Targeted Drug Delivery and Positron Emission Tomography Imaging. ACS Appl. Mater. Interfaces 2014, 6, 21769–21779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Hu, Y.; Yang, J.; Sun, W.; Cai, H.; Wei, P.; Sun, Y.; Zhang, G.; Shi, X.; Shen, M. Facile Synthesis of Folic Acid-Functionalized Iron Oxide Nanoparticles with Ultrahigh Relaxivity for Targeted Tumor MR Imaging. J. Mater. Chem. B 2015, 3, 5720–5730. [Google Scholar] [CrossRef]
- Wang, Y.; Strohm, E.M.; Sun, Y.; Wang, Z.; Zheng, Y.; Wang, Z.; Kolios, M.C. Biodegradable Polymeric Nanoparticles Containing Gold Nanoparticles and Paclitaxel for Cancer Imaging and Drug Delivery Using Photoacoustic Methods. Biomed. Opt. Express 2016, 7, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yu, Y.; Lu, X.; Ding, H.; Ma, Y. Designing a Nanoparticle-Containing Polymeric Substrate for Detecting Cancer Cells by Computer Simulations. Nanoscale 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hoang, B.; Fonge, H.; Reilly, R.M.; Allen, C. In Vivo Distribution of Polymeric Nanoparticles at the Whole-Body, Tumor, and Cellular Levels. Pharm. Res. 2010, 27, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Lee, H.; Reilly, R.M.; Allen, C. Noninvasive Monitoring of the Fate of 111 In-Labeled Block Copolymer Micelles by High Resolution and High Sensitivity MicroSPECT/CT Imaging. Mol. Pharm. 2009, 6, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Kawano, K.; Minowa, T.; Maitani, Y.; Yokoyama, M. Preparation and in Vivo Imaging of PEG-Poly(L-Lysine)-Based Polymeric Micelle MRI Contrast Agents. J. Control. Release 2009, 136, 14–20. [Google Scholar] [CrossRef]
- Talelli, M.; Rijcken, C.J.F.; Lammers, T.; Seevinck, P.R.; Storm, G.; van Nostrum, C.F.; Hennink, W.E. Superparamagnetic Iron Oxide Nanoparticles Encapsulated in Biodegradable Thermosensitive Polymeric Micelles: Toward a Targeted Nanomedicine Suitable for Image-Guided Drug Delivery. Langmuir 2009, 25, 2060–2067. [Google Scholar] [CrossRef]
- Nasongkla, N.; Bey, E.; Ren, J.; Ai, H.; Khemtong, C.; Guthi, J.S.; Chin, S.-F.; Sherry, A.D.; Boothman, D.A.; Gao, J. Multifunctional Polymeric Micelles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems. Nano Lett. 2006, 6, 2427–2430. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, X. Dendrimer-Based Molecular Imaging Contrast Agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Yang, J.; Luo, Y.; Xu, Y.; Li, J.; Zhang, Z.; Wang, H.; Shen, M.; Shi, X.; Zhang, G. Conjugation of Iron Oxide Nanoparticles with RGD-Modified Dendrimers for Targeted Tumor MR Imaging. ACS Appl. Mater. Interfaces 2015, 7, 5420–5428. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Kante, B.; Lenaerts, V.; Scailteur, V.; Roland, M.; Speiser, P. Tissue Distribution of Antitumor Drugs Associated with Polyalkylcyanoacrylate Nanoparticles. J. Pharm. Sci. 1980, 69, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable Nanoparticles for Drug and Gene Delivery to Cells and Tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, Y.M. Taxol-Loaded Block Copolymer Nanospheres Composed of Methoxy Poly(Ethylene Glycol) and Poly(ε-Caprolactone) as Novel Anticancer Drug Carriers. Biomaterials 2001, 22, 1697–1704. [Google Scholar] [CrossRef]
- Hawker, C.J.; Frechet, J.M.J. Preparation of Polymers with Controlled Molecular Architecture. A New Convergent Approach to Dendritic Macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications—Reflections on the Field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N.; Gao, Z.; Papahadjopoulos-Sternberg, B. Immunomicelles: Targeted Pharmaceutical Carriers for Poorly Soluble Drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 6039–6044. [Google Scholar] [CrossRef] [Green Version]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising Vehicle for Bioactive Drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval Duhyeong. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric Mixed Micelles as Nanomedicines: Achievements and Perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Oses, J.K.; García, M.C.; Feitosa, V.A.; Pachioni-Vasconcelos, J.A.; Gomes-Filho, S.M.; Lourenço, F.R.; Cerize, N.N.P.; Bassères, D.S.; Rangel-Yagui, C.O. Development and Characterization of Miltefosine-Loaded Polymeric Micelles for Cancer Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lan, Y.; Cao, M.; Ma, X.; Cao, A.; Sun, Y.; Yang, J.; Li, L.; Liu, Y. Glycyrrhetinic Acid-Conjugated Polymeric Prodrug Micelles Co-Delivered with Doxorubicin as Combination Therapy Treatment for Liver Cancer. Colloids Surf. B Biointerfaces 2019, 175, 106–115. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhang, J.; Wang, L.; Ou, C.; Shu, Y.; Wu, Q.; Ma, G.; Gong, C. Gambogic Acid-Encapsulated Polymeric Micelles Improved Therapeutic Effects on Pancreatic Cancer. Chin. Chem. Lett. 2019, 30, 885–888. [Google Scholar] [CrossRef]
- Volsi, A.L.; Fiorica, C.; D’Amico, M.; Scialabba, C.; Palumbo, F.S.; Giammona, G.; Licciardi, M. Hybrid Gold/Silica/Quantum-Dots Supramolecular-Nanostructures Encapsulated in Polymeric Micelles as Potential Theranostic Tool for Targeted Cancer Therapy. Eur. Polym. J. 2018, 105, 38–47. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.-S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.-W.; Kim, I.-S. Tumor-Homing Multifunctional Nanoparticles for Cancer Theragnosis: Simultaneous Diagnosis, Drug Delivery, and Therapeutic Monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Park, K.; Kim, J.-H.; Nam, Y.S.; Lee, S.; Nam, H.Y.; Kim, K.; Park, J.H.; Kim, I.-S.; Choi, K.; Kim, S.Y.; et al. Effect of Polymer Molecular Weight on the Tumor Targeting Characteristics of Self-Assembled Glycol Chitosan Nanoparticles. J. Control. Release 2007, 122, 305–314. [Google Scholar] [CrossRef]
- Gao, Z.-G.; Fain, H.D.; Rapoport, N. Controlled and Targeted Tumor Chemotherapy by Micellar-Encapsulated Drug and Ultrasound. J. Control. Release 2005, 102, 203–222. [Google Scholar] [CrossRef]
- Lammers, T.; Subr, V.; Peschke, P.; Kühnlein, R.; Hennink, W.E.; Ulbrich, K.; Kiessling, F.; Heilmann, M.; Debus, J.; Huber, P.E.; et al. Image-Guided and Passively Tumour-Targeted Polymeric Nanomedicines for Radiochemotherapy. Br. J. Cancer 2008, 99, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef] [PubMed]
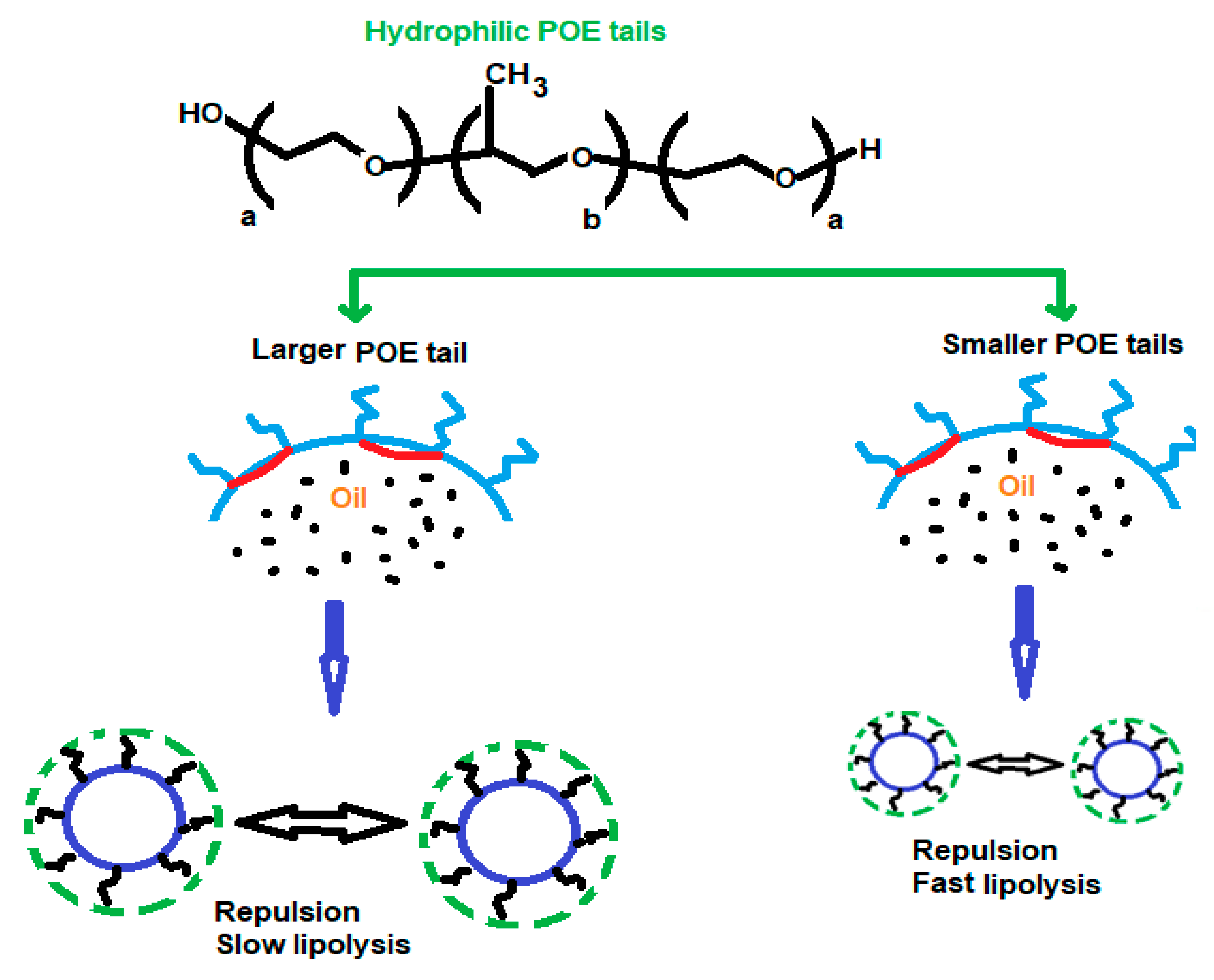
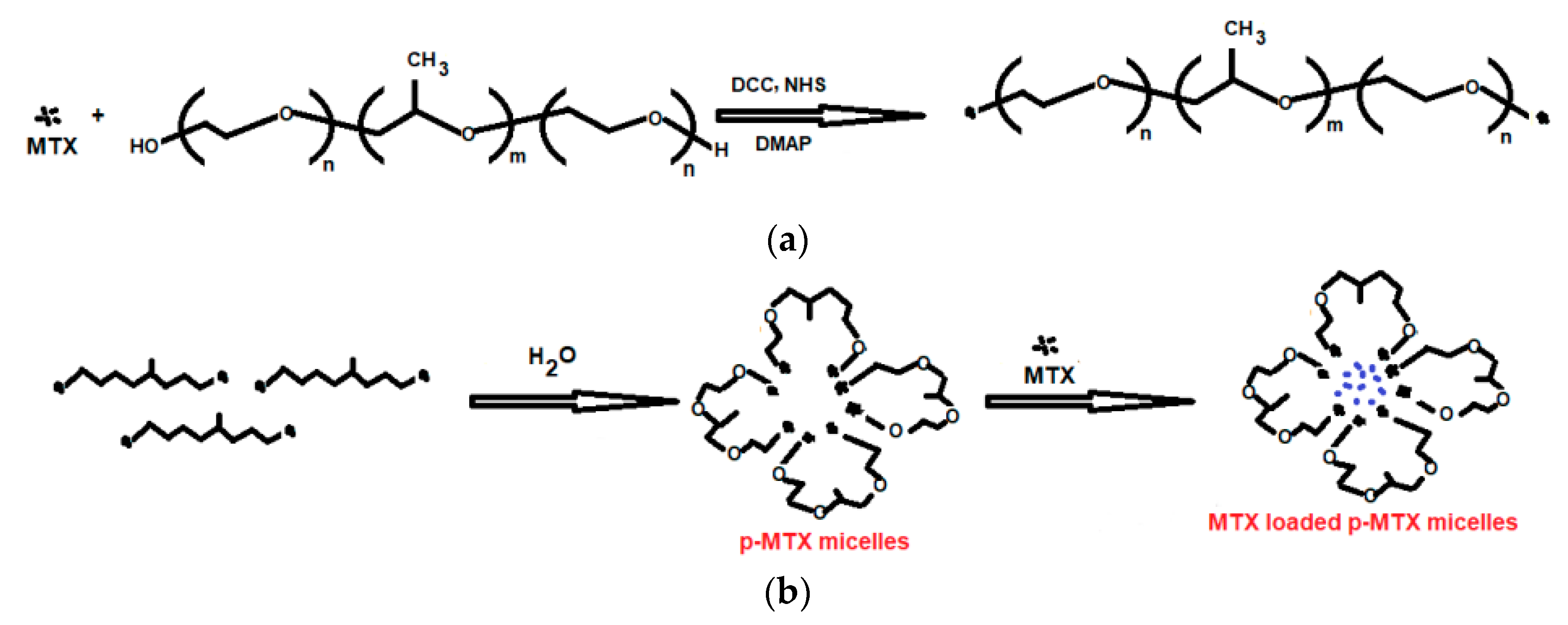
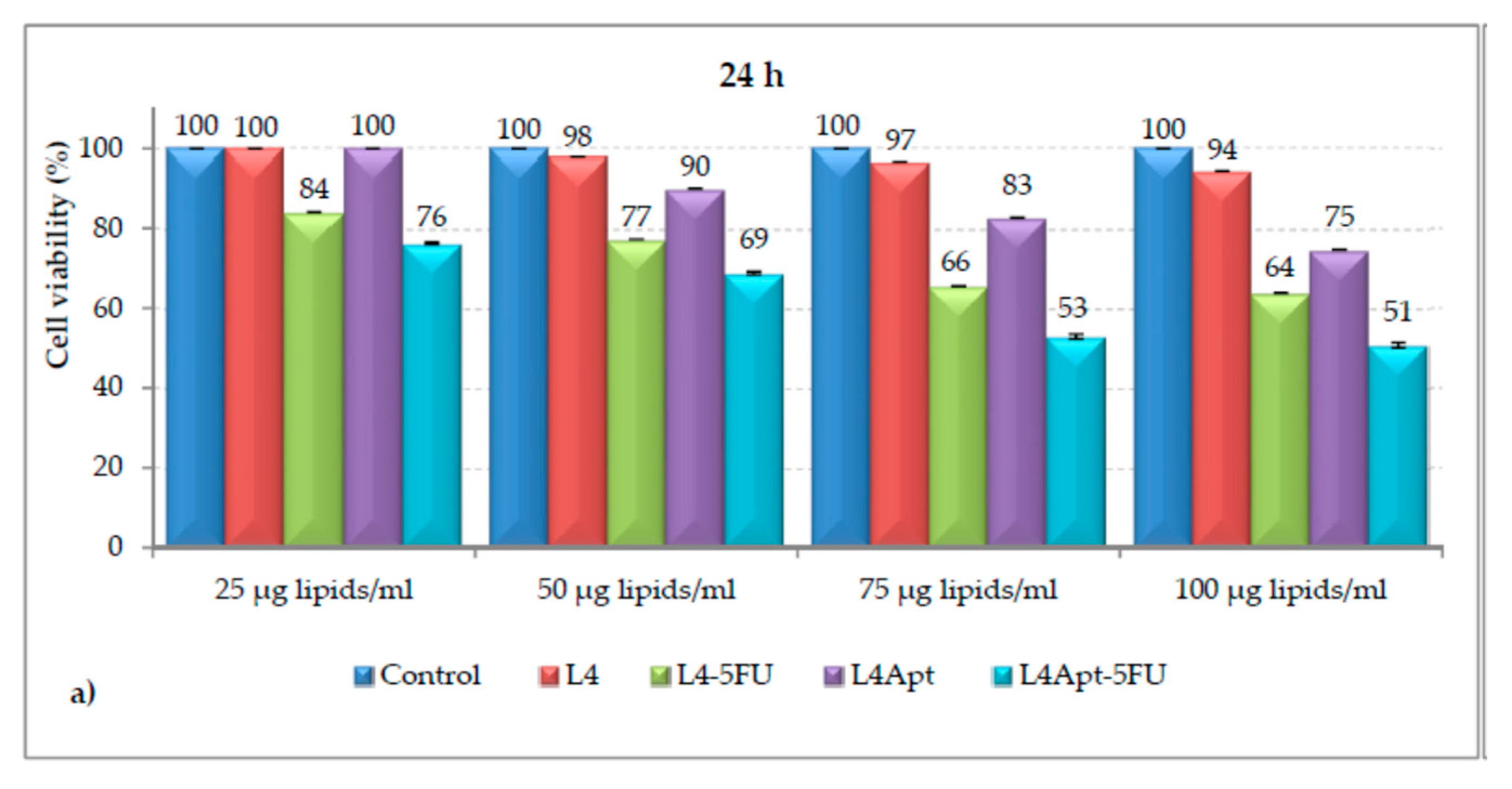


| Class | Polymeric Unit | Combining Units | Obtained Skeleton | Refs. |
|---|---|---|---|---|
| Pluronics | poly(propylene oxide) | propylene oxide and ethylene oxide | (PEO)a-(PPO)b-(PEO)a | [34] |
| None | Poloxamer and MTX | p-MTX | [40] | |
| TPGS (D-α-tocopheryl polyethylene glycol Succinate) | Poloxamer 427 and 27 and vitamin E TPGS | PLGA-TPGS/Poloxamer 235 | [41] | |
| PEG | PLA | Dodecanol, folic acid and PEG-PLA | Dol-PLA-PEG-FA | [43] |
| PLA | Ala-Pro-Arg-Pro-Gly and | peptide-maleimide-PEG-PLA | [44] | |
| acid-chloride of PCL | PCL-COCl, PEG and TAPC | mPEG-b-PCL copolymer | [45] | |
| PCL | PEG-PCL and folic acid | Folate-PEG-PCL | [46] | |
| poly(ethylene glycol)-distearoyl phosphoethanolamine | Lipofectin and PEG-PE | Lip-PEG-PE | [47] | |
| poly(ethylene glycol)-distearoyl phosphoethanolamine | PEG-PE, Lipofectin lipids and paclitaxel | PEG-PE/ST/LL | [48] | |
| PLGA | PLGA, PEG, Dox and nanoparticle | Dox-NP | [49] |
| Name of Drugs | Status | Compony |
|---|---|---|
| Doxorubicin | Doxil | Sequus (Alza) |
| Daunorubicin | DaunoXomeTM | NeXstar (Gilead Sciences) |
| Edolfosine | Phase I | The Liposome Company |
| Tretinoin | Phase I/II | Aronex |
| Cisplatin | Phase II | Aronex |
| Annamycine | Phase I/II | Aronex |
| Drugs | Grade | Tumor types | Refs. |
|---|---|---|---|
| Paclitaxel | Accepted | Lung, breast, and pancreatic | [74] |
| Rapamycin | Under clinical trial | Solid | [75] |
| Docetaxel | Under clinical trial | Prostrate and breast | [76] |
| Doxorubicin | Under clinical trial | Hepatocellular | [77] |
| Mitoxantrone | Under clinical trial | Hepatocellular | [78] |
| Docetaxel | Under clinical trial | Solid | [79] |
| Paclitaxel | Under clinical trial | Neoplasms | [80] |
| DACHPt | Under clinical trial | Ovarian | [81] |
| Materials | Names | Indication | Year(s) Approved |
|---|---|---|---|
| Liposome-PEG | Doxorubicin | Metastatic breast cancer, metastatic ovarian cancer | 1995 |
| PLGA | Leuprolide acetate | Prostate Cancer | 2002 |
| Albumin | Nab-paclitaxel | Metastatic breast cancer | 2005 |
| Pancreatic cancer | 2013 | ||
| mPEG-PLA | Paclitexal | Metastatic breast cancer | 2007 |
| Liposome | Lrinotecan | Pancreatic cancer | 2015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, I.; Althakfi, S.H.; Suhail, M.; Locatelli, M.; Hsieh, M.-F.; Alsehli, M.; Hameed, A.M. Advances in Polymeric Colloids for Cancer Treatment. Polymers 2022, 14, 5445. https://doi.org/10.3390/polym14245445
Ali I, Althakfi SH, Suhail M, Locatelli M, Hsieh M-F, Alsehli M, Hameed AM. Advances in Polymeric Colloids for Cancer Treatment. Polymers. 2022; 14(24):5445. https://doi.org/10.3390/polym14245445
Chicago/Turabian StyleAli, Imran, Sara H. Althakfi, Mohammad Suhail, Marcello Locatelli, Ming-Fa Hsieh, Mosa Alsehli, and Ahmed M. Hameed. 2022. "Advances in Polymeric Colloids for Cancer Treatment" Polymers 14, no. 24: 5445. https://doi.org/10.3390/polym14245445







