Role of Macrodiols in the Synthesis and Thermo-Mechanical Behavior of Anti-Tack Water Borne Polyurethane Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
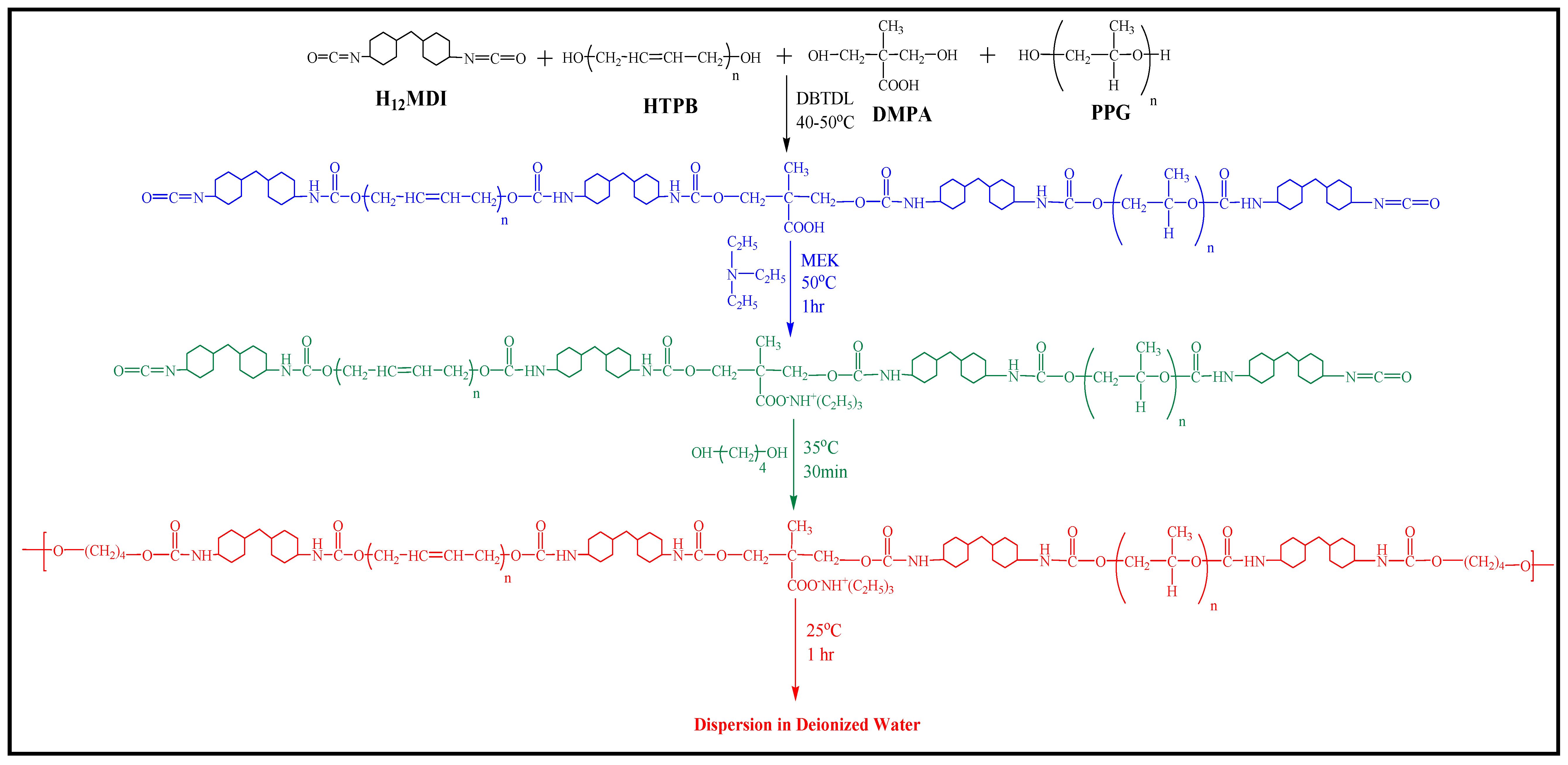
2.2. Preparation of WBPUD
2.3. Characterization
2.3.1. FTIR Analysis
2.3.2. Probe Tack Adhesion Test
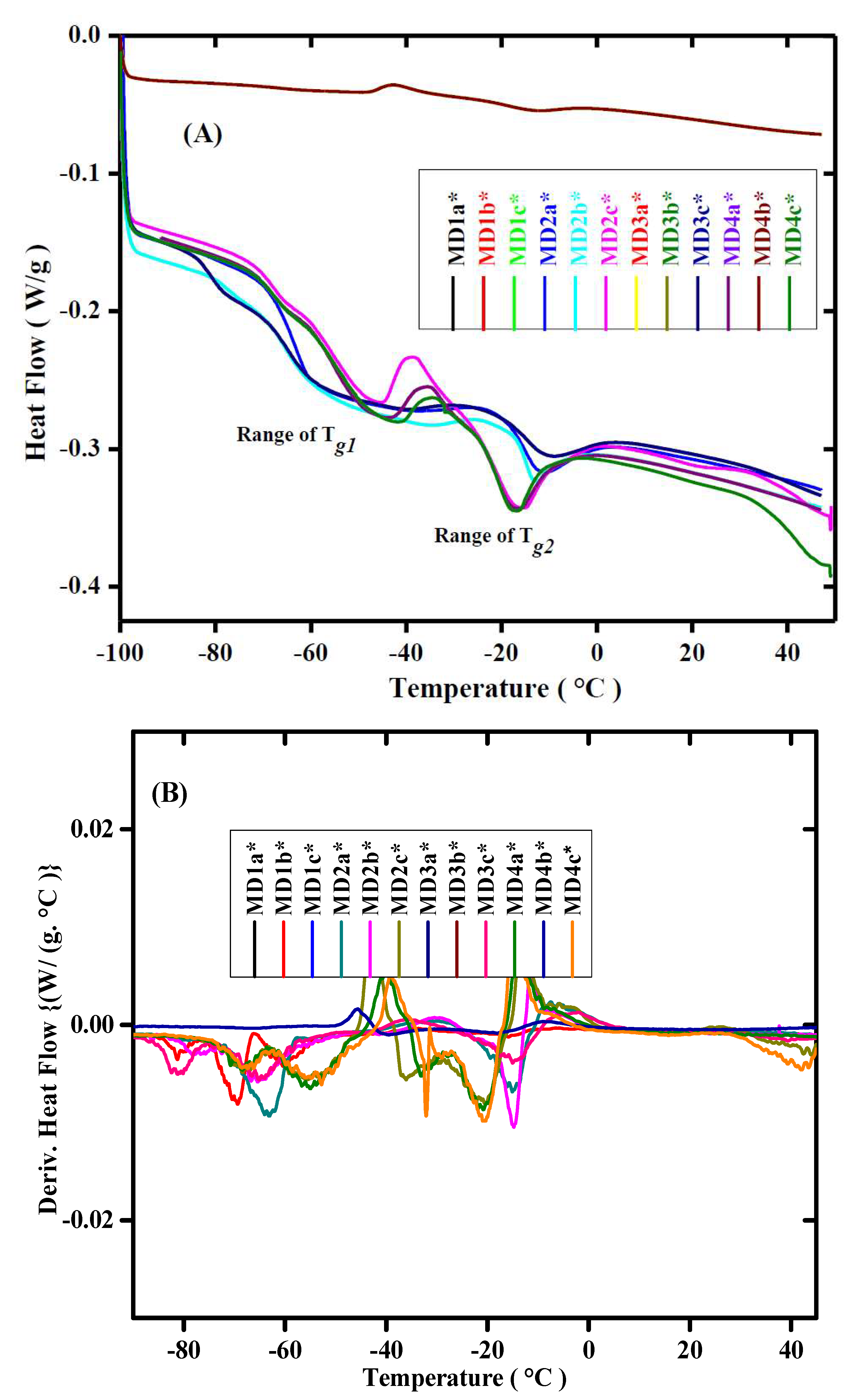
2.3.3. Differential Scanning Calorimetry
2.3.4. Dynamic Mechanical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
3.2. Probe Tack Adhesion Analysis and Debonding Mechanism
3.3. Thermal Stability of WBPUD Thin Films
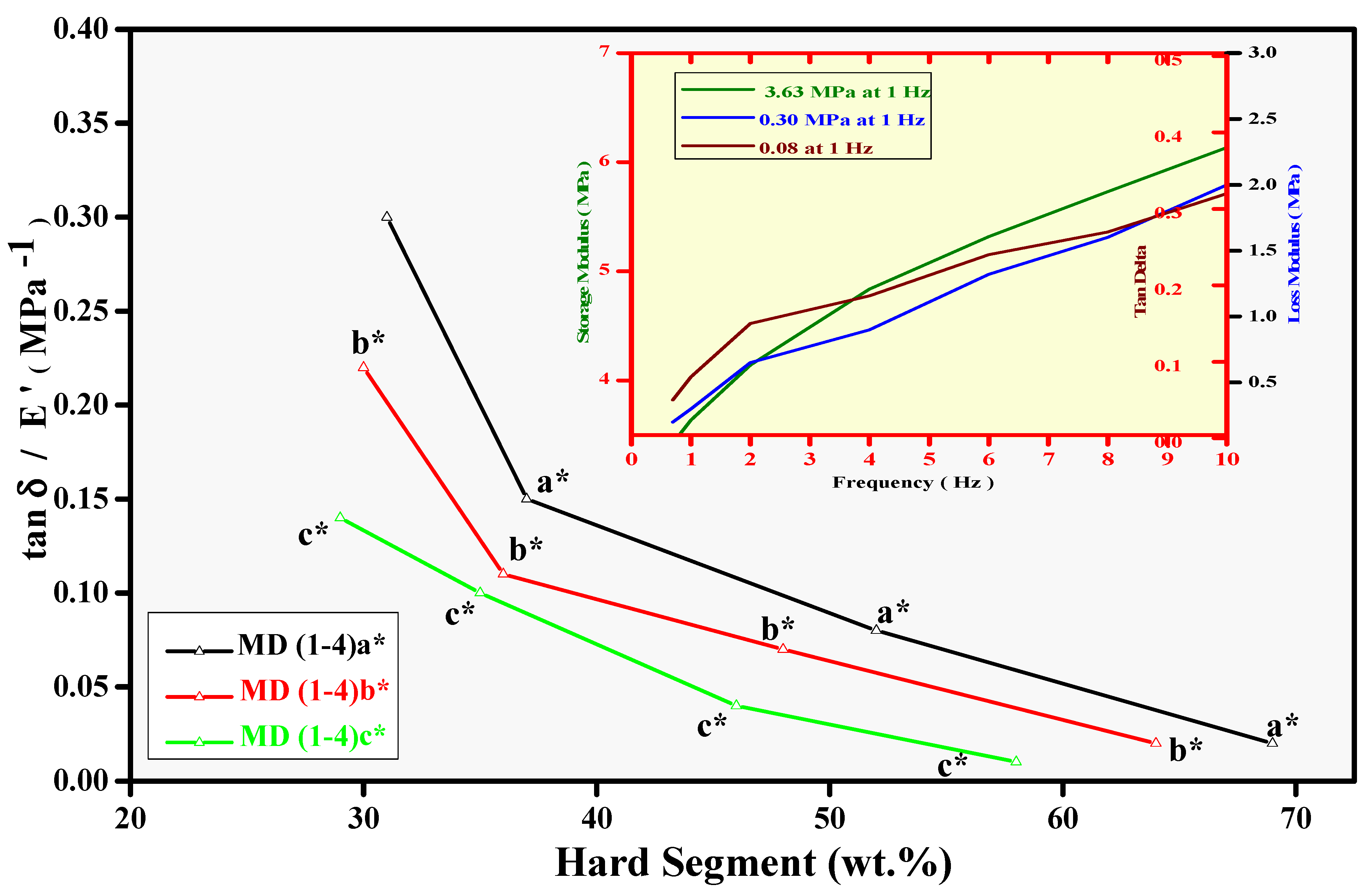
3.4. Mechanical Analysis and Relative Adhesion Trend (DMA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, M.-G.; Jo, K.-I.; Kim, E.; Park, J.-H.; Ko, J.-W.; Lee, J.H. Preparation of Polydimethylsiloxane-Modified Waterborne Polyurethane Coatings for Marine Applications. Polymers 2021, 13, 4283. [Google Scholar] [CrossRef] [PubMed]
- Lacruz, A.; Salvador, M.; Blanco, M.; Vidal, K.; Goitandia, A.M.; Martinková, L.; Kyselka, M.; de Ilarduya, A.M. Biobased Waterborne Polyurethane-Ureas Modified with POSS-OH for Fluorine-Free Hydrophobic Textile Coatings. Polymers 2021, 13, 3526. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, L.; Li, G. Properties of waterborne polyurethane conductive coating with low MWCNTs content by electrostatic spraying. Polymers 2018, 10, 1406. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.K.; Bach, A.; Hassager, O.; Skov, A.L. Linear rheology of cross-linked polypropylene oxide as a pressure sensitive adhesive. Int. J. Adhes. Adhes. 2009, 29, 687–693. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Basri, M.; Jusoh, E.R.; Mamat, S.; Osman Al Edrus, S.S. Chemical and Thermo-Mechanical Properties of Waterborne Polyurethane Dispersion Derived from Jatropha Oil. Polymers 2021, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Colver, P.J.; Bon, S.A.F.; Keddie, J.L. Soft polymer and nano-clay supracolloidal particles in adhesives: Synergistic effects on mechanical properties. Soft Matter. 2009, 5, 3842–3849. [Google Scholar] [CrossRef] [Green Version]
- Rivals, I.; Personnaz, L.; Creton, C.; Simal, F.; Roose, P. A Statistical Method for the Prediction of the Loop Tack and the Peel of PSAs from Probe test Measurements. Meas. Sci. Technol. 2005, 16, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Baron, A.; Hernandez, J.R.; Ibarboure, E.; Derail, C.; Papon, E. Adhesives based on polyurethane graft multiblock copolymers: Tack, rheology and first morphological analyses. Int. J. Adhes. Adhes. 2009, 29, 1–8. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and poss/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Cakic, S.M.; Stamenkovic, J.V.; Djordjevic, D.M.; Ristic, I.S. Synthesis and Degradation Profile of Cast Films of PPG–DMPA–IPDI Aqueous Polyurethane Dispersions Based on Selective Catalysts. Polym. Degrad. Stab. 2009, 94, 2015–2022. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Li, W.; Chen, L.; Wang, W. Synthesis of Waterborne Polyurethane by the Telechelic α,ω-Di(hydroxy)poly(n-butyl acrylate). Polymers 2018, 10, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanović, I.S.; Džunuzović, J.V.; Džunuzović, E.S.; Brzić, S.J.; Jasiukaityte-Grojzdek, E.; Basagni, A.; Marega, C. Tailoring the properties of waterborne polyurethanes by incorporating different content of poly (dimethylsiloxane). Prog. Org. Coat. 2021, 161, 106474. [Google Scholar] [CrossRef]
- Creton, C.; Hooker, J.; Shull, R.K. Bulk and Interfacial Contributions to the Debonding Mechanisms of Soft Adhesives: Extension to Large Strains. Langmuir 2001, 17, 4948–4954. [Google Scholar] [CrossRef]
- Crosby, A.J.; Shulla, K.R.; Lakrout, H.; Creton, C. Deformation and failure modes of adhesively bonded elastic layers. J. Appl. Phys. 2000, 88, 2956–2966. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Doi, M. Debonding dynamics of pressure-sensitive adhesives: 3D block model. Eur. Phys. J. 2006, 21, 331–339. [Google Scholar] [CrossRef]
- Tao, Y.; Hasan, A.; Deeb, G.; Hu, C.; Han, H. Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane. Polymers 2016, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Nagle, D.J.; Celina, M.; Rintoul, L.; Fredericks, P.M. Infrared micro spectroscopic study of the thermo-oxidative degradation of hydroxy-terminated polybutadiene/isophorone diisocyanate polyurethane rubber. Polym. Degrad. Stab. 2007, 92, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.; Gurney, R.S.; Keddie, J.L.; Zuber, M.; Ishaq, M. Influence of Polyol molecular weight and type on the tack and peel properties of waterborne polyurethane pressure-sensitive adhesives. Macromol. React. Eng. 2013, 7, 493–503. [Google Scholar] [CrossRef]
- Han, Y.; Hu, J.; Xin, Z. In-Situ Incorporation of Alkyl-Grafted Silica into Waterborne Polyurethane with High Solid Content for Enhanced Physical Properties of Coatings. Polymers 2018, 10, 514. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Li, X.; Luo, Y. Synthesis and Characterization of Multifunctional Two-Component Waterborne Polyurethane Coatings: Fluorescence, Thermostability and Flame Retardancy. Polymers 2017, 9, 492. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.; Zia, K.M.; Saeed, M.; Usman, M.; Khan, W.G. Role of isophorone diisocyanate in the optimization of adhesion tendency of polyurethane pressure sensitive adhesives. J. Appl. Polym. Sci. 2019, 136, 47124. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Iqbal, S.; Shoaib, M.; ur Rahman, M.S.; Bashir, M.I.; Asghar, M.; Ali, M.A.; Mahmood, T. Biocompatible waterborne polyurethane-urea elastomer as intelligent anticancer drug release matrix: A sustained drug release study. React. Funct. Polym. 2017, 119, 57–63. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Saeed, M.; Usman, M.; Khan, W.G.; Bashir, M.A. Investigation of non-adhesive behaviour of waterborne polyurethane dispersions. J. Polym. Res. 2019, 26, 45. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Saeed, M.; Usman, M.; Saleem, S. Impact of macrodiols on the adhesion strength of polyurethane pressure-sensitive adhesives. J. Appl. Polym. Sci. 2018, 135, 46635. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Sattar, R.; Tabassum, S.; Saeed, M. Thermomechanical investigation of hydroxyl-terminated polybutadiene-based linear polyurethane elastomers. J. Appl. Polym. Sci. 2019, 136, 47289. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Saeed, M.; Mansha, A.; Khan, W.G. Morphological studies of polyurethane based pressure sensitive adhesives by tapping mode atomic force microscopy. J. Polym. Res. 2018, 25, 194. [Google Scholar] [CrossRef]
- Diana, F.; Victória, M.; Carlos, A.F.; Clara, M.G.; Ana, C.; Nuria, G.; Maria, J.S.; Marta, C.; Otávio, B. Effect of chain extender on the morphology, thermal, viscoelastic, and dielectric behavior of soybean polyurethane. J. Appl. Polym. Sci. 2021, 138, 50709. [Google Scholar]
- Manuel, A.; Victor, C.; Andrés, N.; Otávio, B.; Clara, M.G. Tunable Structure and Properties of Segmented Thermoplastic Polyurethanes as a Function of Flexible Segment. Polymers 2019, 11, 1910. [Google Scholar] [CrossRef] [Green Version]




| Sample Code | Composition (Mole) | Hard and Soft Segment Contents (%) | ||||
|---|---|---|---|---|---|---|
| PPG | HTPB | H12MDI | TEA | HS | SS | |
| MD1 a,* | 0.95 | 0.05 | 3 | 1 | 69 | 39 |
| MD1 b,* | 0.90 | 0.10 | 3 | 1 | 64 | 36 |
| MD1 c,* | 0.85 | 0.15 | 3 | 1 | 58 | 42 |
| MD2 a,* | 0.95 | 0.05 | 3 | 1 | 52 | 58 |
| MD2 b,* | 0.90 | 0.10 | 3 | 1 | 48 | 52 |
| MD2 c,* | 0.85 | 0.15 | 3 | 1 | 46 | 54 |
| MD3 a,* | 0.95 | 0.05 | 3 | 1 | 37 | 63 |
| MD3 b,* | 0.90 | 0.10 | 3 | 1 | 36 | 64 |
| MD3 c,* | 0.85 | 0.15 | 3 | 1 | 35 | 65 |
| MD4 a,* | 0.95 | 0.05 | 3 | 1 | 31 | 69 |
| MD4 b,* | 0.90 | 0.10 | 3 | 1 | 30 | 70 |
| MD4 c,* | 0.85 | 0.15 | 3 | 1 | 29 | 71 |
| Sample Code | σmax (MPa) | ε at σmax | ε max | Wadh (J/m−2) | Tg1 (°C) | Tg2 (°C) |
|---|---|---|---|---|---|---|
| MD1 a,* | 1.14 | 0.69 | 0.75 | 30.8 ± 1.5 | −65 | −6 |
| MD1 b,* | 1.17 | 0.72 | 0.82 | 34.2 ± 1.2 | −65 | −14 |
| MD1 c,* | 1.26 | 0.81 | 0.93 | 37.1 ± 1.2 | −66 | −15 |
| MD2 a,* | 1.44 | 0.71 | 0.78 | 44.8 ± 2.0 | −56 | −20 |
| MD2 b,* | 1.63 | 0.81 | 0.97 | 59.8 ± 1.8 | −63 | −14 |
| MD2 c,* | 1.64 | 0.93 | 1.00 | 61.3 ± 1.3 | −65 | −10 |
| MD3 a,* | 1.61 | 0.59 | 0.60 | 70.4 ± 2.0 | −43 | −14 |
| MD3 b,* | 1.74 | 0.83 | 0.85 | 73.3 ± 1.8 | −45 | −16 |
| MD3 c,* | 1.82 | 0.87 | 0.94 | 80.7 ± 1.5 | −45 | −19 |
| MD4 a,* | 2.97 | 0.83 | 0.94 | 102.0 ± 1.7 | −41 | −20 |
| MD4 b,* | 3.82 | 1.10 | 1.12 | 120.0 ± 2.5 | −43 | −12 |
| MD4 c,* | 4.06 | 1.14 | 1.23 | 127.0 ± 2.8 | −45 | −18 |
| Sample Code | E′ (Mpa) | E″ (MPa) | Tanδ | tanδ/E′ (MPa−1) |
|---|---|---|---|---|
| MD1 a,* | 3.63 | 0.30 | 0.08 | 0.02 |
| MD1 b,* | 3.75 | 0.29 | 0.07 | 0.02 |
| MD1 c,* | 3.97 | 0.28 | 0.07 | 0.01 |
| MD2 a,* | 2.25 | 0.38 | 0.17 | 0.08 |
| MD2 b,* | 2.26 | 0.36 | 0.16 | 0.07 |
| MD2 c,* | 2.91 | 0.35 | 0.12 | 0.04 |
| MD3 a,* | 2.10 | 0.65 | 0.31 | 0.15 |
| MD3 b,* | 2.40 | 0.65 | 0.27 | 0.11 |
| MD3 c,* | 2.66 | 0.63 | 0.24 | 0.10 |
| MD4 a,* | 1.66 | 0.82 | 0.49 | 0.30 |
| MD4 b,* | 1.82 | 0.74 | 0.41 | 0.22 |
| MD4 c,* | 1.97 | 0.54 | 0.27 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, N.; Saeed, M.; Usman, M. Role of Macrodiols in the Synthesis and Thermo-Mechanical Behavior of Anti-Tack Water Borne Polyurethane Dispersions. Polymers 2022, 14, 572. https://doi.org/10.3390/polym14030572
Akram N, Saeed M, Usman M. Role of Macrodiols in the Synthesis and Thermo-Mechanical Behavior of Anti-Tack Water Borne Polyurethane Dispersions. Polymers. 2022; 14(3):572. https://doi.org/10.3390/polym14030572
Chicago/Turabian StyleAkram, Nadia, Muhammad Saeed, and Muhammad Usman. 2022. "Role of Macrodiols in the Synthesis and Thermo-Mechanical Behavior of Anti-Tack Water Borne Polyurethane Dispersions" Polymers 14, no. 3: 572. https://doi.org/10.3390/polym14030572







