In Situ Construction of Thermotropic Shape Memory Polymer in Wood for Enhancing Its Dimensional Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Performance Evaluation of MMA/PEGDA Copolymer (PMP)
2.3. Construction of PMP in Wood
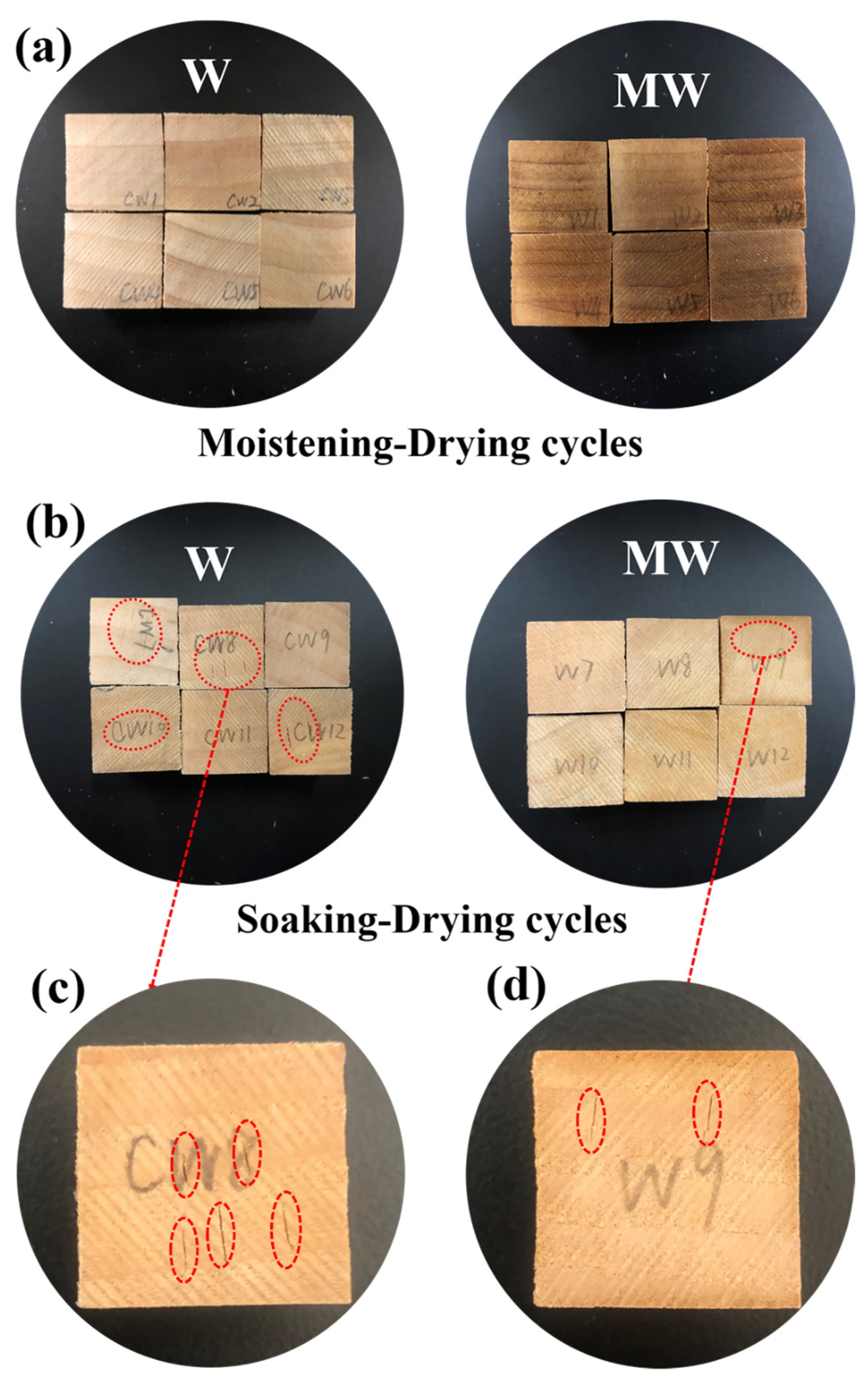
2.4. Dimensional Stability of Wood
2.5. Effect of the Shape Memory Properties of PMP on Wood Dimensional Stability
2.6. Characterizations
3. Results and Discussion
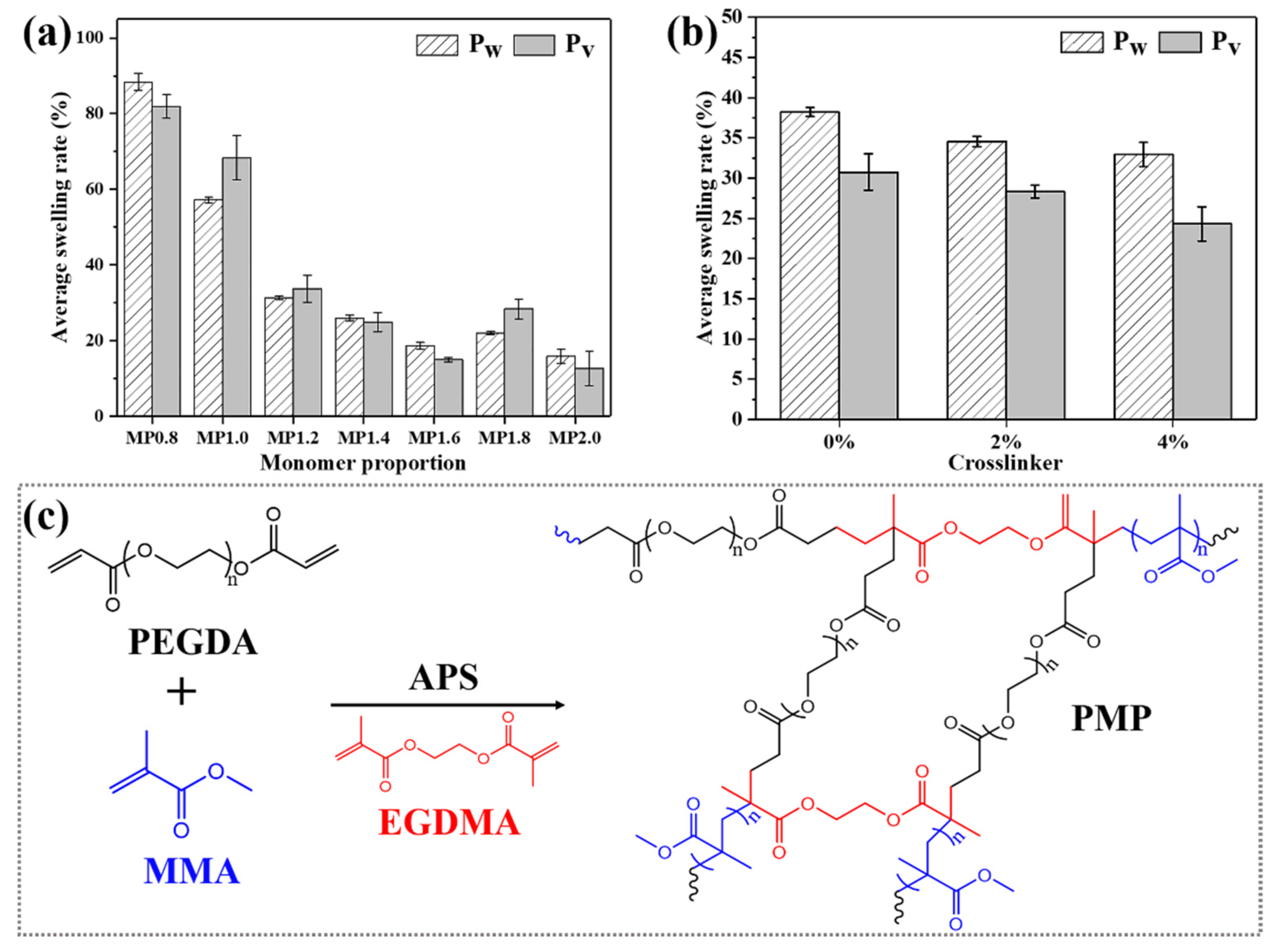
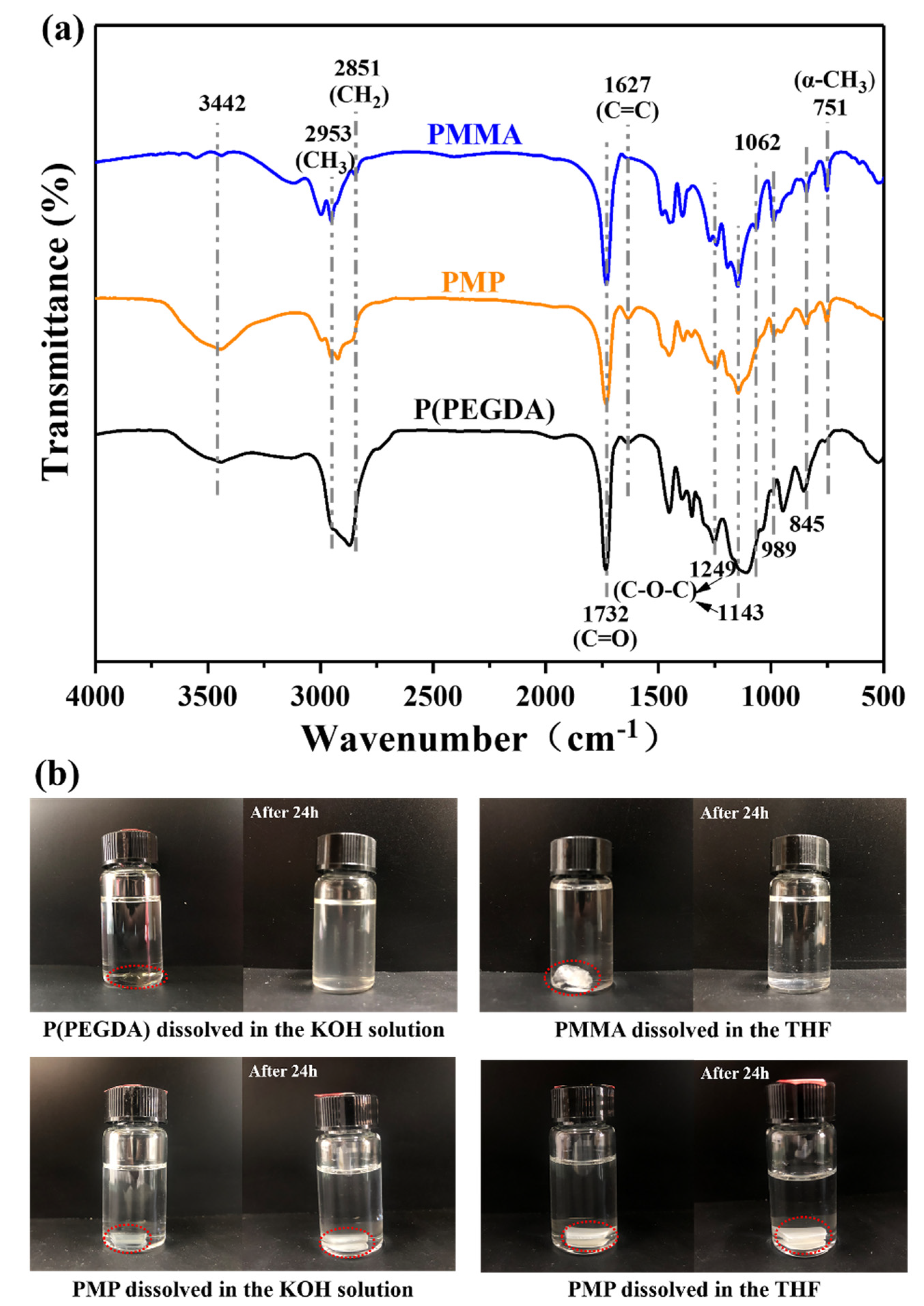
3.1. Polymerization of MMA and PEGDA
3.2. Shape Memory Behavior of PMP
3.3. In Situ Construction of PMP in Wood
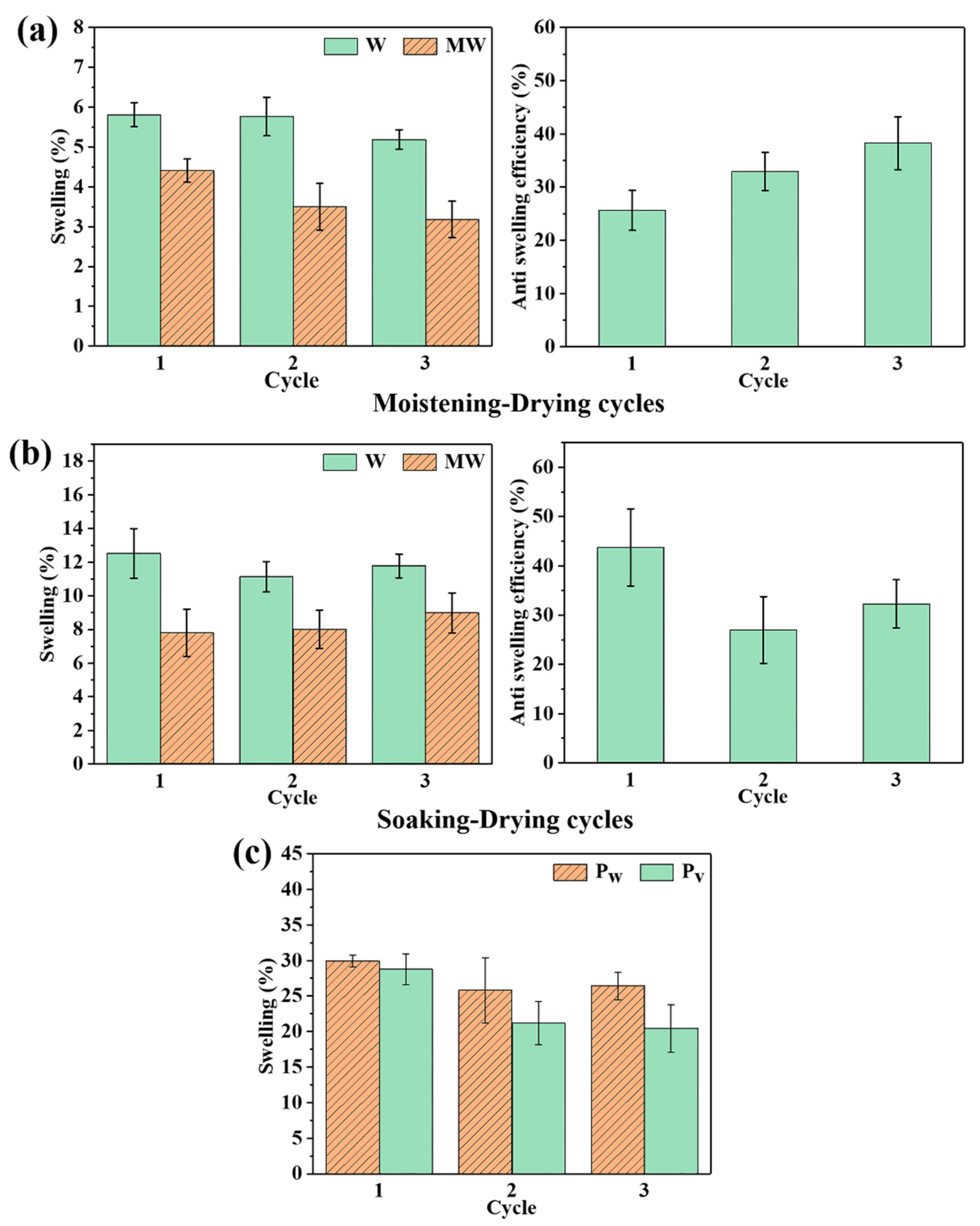
3.4. Dimensional Stability of the Wood
3.5. Mechanism of Action of SMP on Wood Dimensional Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, X.; Zhuo, X.; Wei, J.; Zhang, G.; Li, Y. Wood-Based Nanocomposite Derived by In Situ Formation of Organic-Inorganic Hybrid Polymer within Wood via a Sol-Gel Method. ACS Appl. Mater. Interfaces 2017, 9, 9070–9078. [Google Scholar] [CrossRef]
- Van Hai, L.; Muthoka, R.M.; Panicker, P.S.; Agumba, D.O.; Pham, H.D.; Kim, J. All-biobased transparent-wood: A new approach and its environmental-friendly packaging application. Carbohydr. Polym. 2021, 264, 118012. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zou, M.; Gao, K.; Chang, L.; Gao, L.; Guo, W. Laminating Delignified Wood Veneers toward High-Strength, Flame-Retardant Composites for Structural Applications. ACS Sustain. Chem. Eng. 2021, 9, 10717–10726. [Google Scholar] [CrossRef]
- Usmani, S.M.; Stephan, I.; Hübert, T.; Kemnitz, E. Nano Metal Fluorides for Wood Protection against Fungi. ACS Appl. Nano Mater. 2018, 1, 1444–1449. [Google Scholar] [CrossRef]
- Huang, C.; Chui, Y.; Gong, M.; Chana, F. Mechanical behaviour of wood compressed in radial direction: Part II. Influence of temperature and moisture content. J. Bioresour. Bioprod. 2020, 5, 266–275. [Google Scholar] [CrossRef]
- Chen, H.; Miao, X.; Feng, Z.; Pu, J. In Situ Polymerization of Phenolic Methylolurea in Cell Wall and Induction of Pulse-Pressure Impregnation on Green Wood. Ind. Eng. Chem. Res. 2014, 53, 9721–9727. [Google Scholar] [CrossRef]
- Autengruber, M.; Lukacevic, M.; Gröstlinger, C.; Füssl, J. Finite-element-based prediction of moisture-induced crack patterns for cross sections of solid wood and glued laminated timber exposed to a realistic climate condition. Constr. Build. Mater. 2021, 271, 121775. [Google Scholar] [CrossRef]
- Mi, R.; Chen, C.; Keplinger, T.; Pei, Y.; He, S.; Liu, D.; Li, J.; Dai, J.; Hitz, E.; Yang, B.; et al. Scalable Aesthetic Transparent Wood for Energy Efficient Buildings. Nat. Commun. 2020, 11, 3836. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.-D.; Mustata, F.; Rusu, T.; Rosu, D.; Rosca, I.; Tudorachi, N.; Teacă, C.-A. Enhancing the Thermal and Fungal Resistance of Wood Treated with Natural and Synthetic Derived Epoxy Resins. ACS Sustain. Chem. Eng. 2018, 6, 5470–5478. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Masic, A.; Koetz, J.; Burgert, I. Flavonoid insertion into cell walls improves wood properties. ACS Appl. Mater. Interfaces 2012, 4, 5782–5789. [Google Scholar] [CrossRef]
- Ding, W.-D.; Koubaa, A.; Chaala, A. Mechanical Properties of MMA-Hardened Hybrid Poplar Wood. Ind. Crops Prod. 2013, 46, 304–310. [Google Scholar] [CrossRef]
- Wang, D.; Ling, Q.; Nie, Y.; Zhang, Y.; Zhang, W.; Wang, H.; Sun, F. In-Situ Cross-Linking of Waterborne Epoxy Resin Inside Wood for Enhancing Its Dimensional Stability, Thermal Stability, and Decay Resistance. ACS Appl. Polym. Mater. 2021, 3, 6265–6273. [Google Scholar] [CrossRef]
- Devi, R.R.; Maji, T.K. Effect of Nano-ZnO on Thermal, Mechanical, UV Stability, and Other Physical Properties of Wood Polymer Composites. Ind. Eng. Chem. Res. 2012, 51, 3870–3880. [Google Scholar] [CrossRef]
- Kong, L.; Guan, H.; Wang, X. In Situ Polymerization of Furfuryl Alcohol with Ammonium Dihydrogen Phosphate in Poplar Wood for Improved Dimensional Stability and Flame Retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, K.; Li, J.; Zhang, S.; Shi, S.Q. Environmentally Benign Wood Modifications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 3532–3540. [Google Scholar] [CrossRef]
- Wang, L.; Luo, B.; Wu, D.; Liu, Y.; Li, L.; Liu, H. Fabrication and Characterization of Thermal-responsive Biomimetic Small-scale Shape Memory Wood Composites with High Tensile Strength, High Anisotropy. Polymers 2019, 11, 1892. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, e2000713. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Gall, K. Shape-Memory Polymers for Biomedical Applications. Adv. Polym. Sci. 2009, 226, 147–175. [Google Scholar]
- Rodriguez, J.N.; Zhu, C.; Duoss, E.B.; Wilson, T.S.; Spadaccini, C.M.; Lewicki, J.P. Shape-morphing composites with designed micro-architectures. Sci. Rep. 2016, 6, 27933. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Zhou, B.; Xue, S. A Viscoelastic-Plastic Constitutive Model of Shape Memory Polymer. J. Mech. 2019, 35, 601–611. [Google Scholar] [CrossRef]
- Kun, W.; Guangming, Z.; Lei, N.; Yongkun, W.; Zhe, L. Shape Memory Effect and Mechanical Properties of Cyanate Ester-Polybutadiene Epoxy Copolymer. J. Polym. Res. 2014, 21, 385. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gall, K.; Dunn, M.L.; Greenberg, A.R.; Diani, J. Thermomechanics of Shape Memory Polymers: Uniaxial Experiments and Constitutive Modeling. Int. J. Plast. 2006, 22, 279–313. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, K.; Zhou, J.; Ni, L.; Shan, G.; Bao, Y.; Pan, P. Stress-Free Two-Way Shape Memory Effects of Semicrystalline Polymer Networks Enhanced by Self-Nucleated Crystallization. ACS Macro Lett. 2020, 9, 1325–1331. [Google Scholar] [CrossRef]
- Wu, H.; Chen, P.; Yan, C.; Cai, C.; Shi, Y. Four-dimensional printing of a novel acrylate-based shape memory polymer using digital light processing. Mater. Des. 2019, 171, 107704. [Google Scholar] [CrossRef]
- Samuel, C.; Barrau, S.; Lefebvre, J.-M.; Raquez, J.-M.; Dubois, P. Designing Multiple-Shape Memory Polymers with Miscible Polymer Blends: Evidence and Origins of a Triple-Shape Memory Effect for Miscible PLLA/PMMA Blends. Macromolecules 2014, 47, 6791–6803. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Z.; Wang, M.; Zhang, L.; Liang, Y.; Zhang, Z.; Ren, L.; Ren, L. 4D Printing of PLA/PCL Shape Memory Composites with Controllable Sequential Deformation. Bio-Des. Manuf. 2021, 4, 867–878. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chuang, W.T.; Jeng, U.S.; Hsu, S.H. Preparation, Characterization, and Mechanism for Biodegradable and Biocompatible Polyurethane Shape Memory Elastomers. ACS Appl. Mater. Interfaces 2017, 9, 5419–5429. [Google Scholar] [CrossRef]
- Yu, R.; Yang, X.; Zhang, Y.; Zhao, X.; Wu, X.; Zhao, T.; Zhao, Y.; Huang, W. Three-Dimensional Printing of Shape Memory Composites with Epoxy-Acrylate Hybrid Photopolymer. ACS Appl. Mater. Interfaces 2017, 9, 1820–1829. [Google Scholar] [CrossRef]
- Kajita, H.; Furuno, T.; Imamura, Y. The Modification of Wood by Treatment with Low Molecular Weight Phenol-Formaldehyde Resin: A Properties Enhancement with Neutralized Phenolic-Resin and Resin Penetration into Wood Cell Walls. Wood Sci. Technol. 2004, 37, 349–361. [Google Scholar] [CrossRef]
- Altgen, M.; Awais, M.; Altgen, D.; Kluppel, A.; Makela, M.; Rautkari, L. Distribution and curing reactions of melamine formaldehyde resin in cells of impregnation-modified wood. Sci. Rep. 2020, 10, 3366. [Google Scholar] [CrossRef] [Green Version]
- Fortino, S.; Metsäjoki, J.; Ronkainen, H.; Bjurhager, I.; Heinemann, S.; Salminen, L.I. Scratch Resistance of PEG-Impregnated Green Wood: A Method for Evaluation of Wwollen Wood Properties. Wood Sci. Technol. 2020, 54, 715–735. [Google Scholar] [CrossRef]
- Trey, S.M.; Netrval, J.; Berglund, L.; Johansson, M. Electron-Beam-Initiated Polymerization of Poly(ethylene glycol)-Based Wood Impregnants. ACS Appl. Mater. Interfaces 2010, 2, 3352–3362. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, K.; Yan, Y.; Zhang, S.; Li, J. Grafting Polyethylene Glycol Dicrylate (PEGDA) to Cell Walls of Poplar Wood in Two Steps for Improving Dimensional Stability and Durability of the Wood Polymer Composite. Holzforschung 2016, 70, 919–926. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Safranski, D.; Ortega, A.M.; Sassaman, K.; Gall, K. Strong, Tailored, Biocompatible Shape-Memory Polymer Networks. Adv. Funct. Mater. 2008, 18, 2428–2435. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Cao, Y.; Ke, L.; Ye, X.; Huang, X.; Shi, B. Plant Polyphenols as Multifunctional Platforms To Fabricate Three-Dimensional Superhydrophobic Foams for Oil/Water and Emulsion Separation. Ind. Eng. Chem. Res. 2018, 57, 16442–16450. [Google Scholar] [CrossRef]
- Sha, D.; Yang, X.; Wang, B.; Liu, X.; Li, C.; Tang, Y.; Wei, M.; Liang, Z.; Wei, H.; Xu, J.; et al. Surface Grafting of a Quaternary Ammonium Salt on Macroporous Polyvinyl Alcohol-Formaldehyde Sponges and Their Highly Efficient Antibacterial Performance. ACS Appl. Polym. Mater. 2020, 2, 4936–4942. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, W.; Wang, J.; Zhang, Y.; Wang, H.; Sun, F.; Cai, L. Improved Dimensional Stability and Mold Resistance of Bamboo via In Situ Growth of Poly(Hydroxyethyl Methacrylate-N-Isopropyl Acrylamide). Polymers 2020, 12, 1584. [Google Scholar] [CrossRef]
- Reggente, M.; Masson, P.; Dollinger, C.; Palkowski, H.; Zafeiratos, S.; Jacomine, L.; Passeri, D.; Rossi, M.; Vrana, N.E.; Pourroy, G.; et al. Novel Alkali Activation of Titanium Substrates to Grow Thick and Covalently Bound PMMA Layers. ACS Appl. Mater. Interfaces 2018, 10, 5967–5977. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, C.; Li, A.; Yang, X.; Lu, L.; Wang, X. Preparation and Characterization of Mesoporous Zirconia Made by Using a Poly (methyl methacrylate) Template. Nanoscale Res. Lett. 2008, 3, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Pullagura, B.K.; Amarapalli, S.; Gundabala, V. Coupling Electrohydrodynamics with Photopolymerization for Microfluidics-Based Generation of Polyethylene Glycol Diacrylate (PEGDA) Microparticles and Hydrogels. Colloids Surf. A 2021, 608, 125586. [Google Scholar] [CrossRef]
- Klimchuk, S.; Shang, M.; Samuel, M.S.; Niu, J. Robust Hybrid Hydrophilic Coating on a High-Density Polyethylene Surface with Enhanced Mechanical Property. ACS Appl. Mater. Interfaces 2020, 12, 32017–32022. [Google Scholar] [CrossRef] [PubMed]
- Bingol, B.; Altuncu, S.; Duman, F.D.; Ak, A.; Gulyuz, U.; Acar, H.Y.; Okay, O.; Avci, D. One-Step Injectable and Bioreducible Poly(β-Amino Ester) Hydrogels as Controlled Drug Delivery Platforms. ACS Appl. Polym. Mater. 2019, 1, 1724–1734. [Google Scholar] [CrossRef]
- Grubjesic, S.; Seifert, S.; Firestone, M.A. Cytoskeleton Mimetic Reinforcement of a Self-Assembled N,N′-Dialkylimidazolium Ionic Liquid Monomer by Copolymerization. Macromolecules 2009, 42, 5461–5470. [Google Scholar] [CrossRef]
- Piedrahita, C.R.; Yue, P.; Cao, J.; Lee, H.; Rajapaksha, C.P.; Feng, C.; Jakli, A.; Kyu, T. Flexoelectricity in Flexoionic Polymer Electrolyte Membranes: Effect of Thiosiloxane Modification on Poly(ethylene glycol) Diacrylate and Ionic Liquid Electrolyte Composites. ACS Appl. Mater. Interfaces 2020, 12, 16978–16986. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, S. A Review of Actively Moving Polymers in Textile Applications. J. Mater. Chem. 2010, 20, 3346–3355. [Google Scholar] [CrossRef]
- Wang, K.; Jia, Y.-G.; Zhu, X. Biocompound-Based Multiple Shape Memory Polymers Reinforced by Photo-Cross-Linking. ACS Biomater. Sci. Eng. 2015, 1, 855–863. [Google Scholar] [CrossRef]
- Zhu, Y.; Radlauer, M.R.; Schneiderman, D.K.; Shaffer, M.S.P.; Hillmyer, M.A.; Williams, C.K. Multiblock Polyesters Demonstrating High Elasticity and Shape Memory Effects. Macromolecules 2018, 51, 2466–2475. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, K.; Wang, Z. A Novel Heat-Triggered Shape-Memory Polymer Based on Ethylene-Vinyl Acetate Copolymer/Nitrile-Butadiene Rubber Thermoplastic Vulcanizates. J. Thermoplast. Compos. Mater. 2019, 33, 1217–1233. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Wang, X.; Li, X.; Sun, J. Thermally and Near-Infrared Light-Induced Shape Memory Polymers Capable of Healing Mechanical Damage and Fatigued Shape Memory Function. ACS Appl. Mater. Interfaces 2019, 11, 9470–9477. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F.; Wang, H.; Ren, W.; Cao, M.; Yu, Y. Highly Stable Wood Material with Low Resin Consumption via Vapor Phase Furfurylation in Cell Walls. ACS Sustain. Chem. Eng. 2020, 8, 13924–13933. [Google Scholar] [CrossRef]
- Islam, M.S.; Hamdan, S.; Jusoh, I.; Rahman, M.R.; Talib, Z.A. Dimensional Stability and Dynamic Young’s Modulus of Tropical Light Hardwood Chemically Treated with Methyl Methacrylate in Combination with Hexamethylene Diisocyanate Cross-Linker. Ind. Eng. Chem. Res. 2011, 50, 3900–3906. [Google Scholar] [CrossRef]
- Izawa, H.; Okuda, N.; Moriyama, A.; Miyazaki, Y.; Ifuku, S.; Morimoto, M.; Saimoto, H. Biobased Wrinkled Surfaces Induced by Wood Mimetic Skins upon Drying: Effect of Mechanical Properties on Wrinkle Morphology. Langmuir 2016, 32, 12799–12804. [Google Scholar] [CrossRef] [PubMed]
- Diawanich, P.; Matan, N.; Kyokong, B. Evolution of Internal Stress During Drying, Cooling and Conditioning of Rubberwood Lumber. Eur. J. Wood Wood Prod. 2009, 68, 1–12. [Google Scholar] [CrossRef]

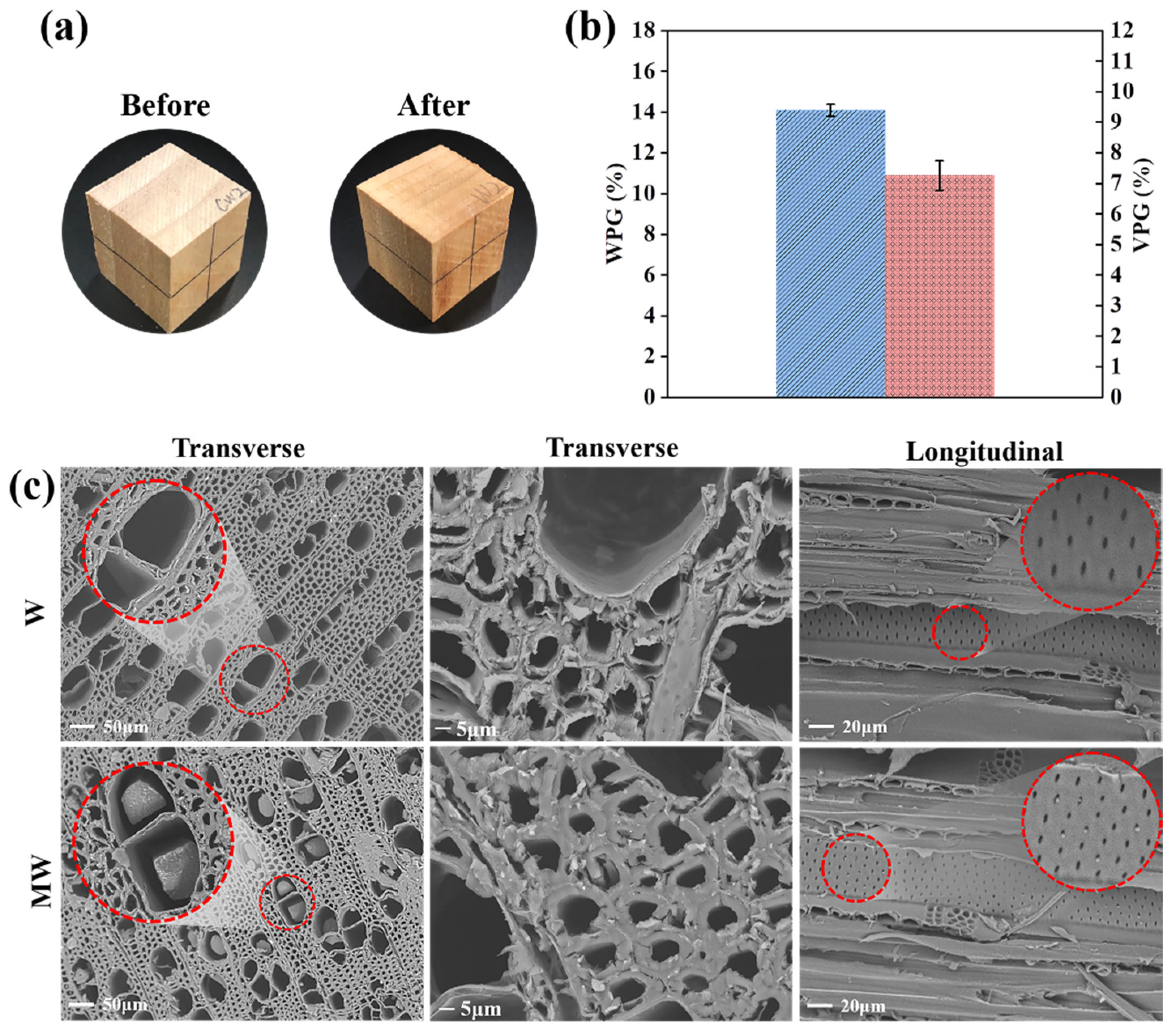

| Samples | MC0 (%) | V0 (cm3) | MC1 (%) | V1 (cm3) | Volumetric Change (%) |
|---|---|---|---|---|---|
| W | 0.50 | 8.21 ± 0.26 | 28.63 | 9.35 ± 0.27 | 13.90 |
| MW | 0.61 | 8.45 ± 0.23 | 32.75 | 9.24 ± 0.24 | 9.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhou, J.; Cao, Z.; Wu, X.; Wang, H.; Han, S.; Zhang, Y.; Sun, F.; Zhang, T. In Situ Construction of Thermotropic Shape Memory Polymer in Wood for Enhancing Its Dimensional Stability. Polymers 2022, 14, 738. https://doi.org/10.3390/polym14040738
Zhang W, Zhou J, Cao Z, Wu X, Wang H, Han S, Zhang Y, Sun F, Zhang T. In Situ Construction of Thermotropic Shape Memory Polymer in Wood for Enhancing Its Dimensional Stability. Polymers. 2022; 14(4):738. https://doi.org/10.3390/polym14040738
Chicago/Turabian StyleZhang, Wenhao, Jianchao Zhou, Zhijin Cao, Xinxing Wu, Hui Wang, Shuaibo Han, Yan Zhang, Fangli Sun, and Ting Zhang. 2022. "In Situ Construction of Thermotropic Shape Memory Polymer in Wood for Enhancing Its Dimensional Stability" Polymers 14, no. 4: 738. https://doi.org/10.3390/polym14040738






