Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
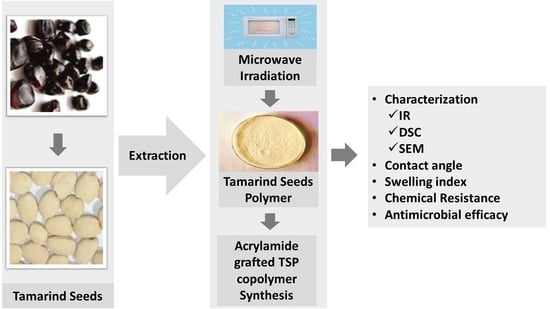
2.2.1. Synthesis of Polyacrylamide Grafted Tamarind Seed Polysaccharide
Factorial Design
3. Results and Discussion
3.1. Factorial Design
3.2. Effect of Initiator on Grafting
3.3. Effect of Time of Exposure on Grafting
3.4. Contact Angle Determination
3.5. DSC Analysis of Polymers
3.6. Surface Morphology
3.7. Swelling Index
3.8. Swelling and Deswelling Study
3.9. Chemical Resistance
3.10. Antimicrobial Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, D.; Nayak, A.K. Plant polysaccharides-blended ionotropically-gelled alginate multiple-unit systems for sustained drug release. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Wiley-Scrivener: Beverly, MA, USA, 2017; Volume 6, pp. 399–400. [Google Scholar]
- Nayak, A.K.; Pal, D. Plant-derived polymers: Ionically gelled sustained drug release systems. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; Taylor & Francis Group: New York, NY, USA, 2016; Volume VIII, pp. 6002–6017. [Google Scholar] [CrossRef]
- Avachat, A.M.; Dash, R.R.; Shrotriya, S.N. Recent investigations of plant based natural gums, mucilages and resins in novel drug delivery systems. Indian J. Pharm. Educ. Res. 2011, 45, 86–99. [Google Scholar]
- Choudhary, P.D.; Pawar, H.A. Recently investigated gums and mucilages as pharmaceutical excipients: An overview. J. Pharm. 2014, 2014, 204849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, V.J.; Thakur, M.K. Biodegradable polymers. In Handbook of Polymers for Pharmaceutical Technologies; Technology & Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 3, p. 608. [Google Scholar]
- R Bhosale, R.; Gangadharappa, H.V.; Moin, A.; Gowda, D.V.; Osmani, A.M. Grafting Technique with Special Emphasis on Natural Gums: Applications and Perspectives in Drug Delivery. Nat. Prod. J. 2015, 5, 124–139. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Okamoto, M. Recent Progress on the Design and Applications of Polysaccharide-Based Graft Copolymer Hydrogels as Adsorbents for Wastewater Purification. Macromol. Mater. Eng. 2016, 301, 496–522. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A Review on the Modification of Polysaccharide Through Graft Copolymerization for Various Potential Applications. Open Med. Chem. J. 2017, 11, 109. [Google Scholar] [CrossRef]
- Boppana, R.; Kulkarni, R.V.; Mohan, G.K.; Mutalik, S.; Aminabhavi, T.M. In vitro and in vivo assessment of novel pH-sensitive interpenetrating polymer networks of a graft copolymer for gastro-protective delivery of ketoprofen. RSC Adv. 2016, 6, 64344–64356. [Google Scholar] [CrossRef]
- Jampala, S.N.; Manolache, S.; Gunasekaran, S.; Denes, F.S. Plasma-enhanced modification of xanthan gumand its effect on rheological properties. J. Agric. Food. Chem. 2005, 53, 3618–3625. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, D.K.; Bihari, K. Synthesis of partially hydrolyzed graft co-polymer (H-partially carboxymethylated guar gum-g-methacrylic acid): A superabsorbing material. Carbohydr. Polym. 2011, 85, 29. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, S. Synthesis and characterization of psyllium-NVP based drug delivery system through radiation crosslinking polymerization. Nucl. Instrum. Methods Phys. Res. B 2008, 31, 3417–3430. [Google Scholar] [CrossRef]
- Rondall, R.C.; Philips, G.O.; Williams, P.A. The role of proteincaceous component on the emulsifying properties of gum Arabic. Food Hydrocoll. 1988, 2, 131–140. [Google Scholar] [CrossRef]
- Sarkar, S.; Gupta, B.S.; Variyar, P.S.; Sharma, A.; Singhal, R.S. Hydrophobic derivatives of guar gum hydrolyzate and gum Arabic as matrices for microencapsulation of mint oil. Carbohydr. Polym. 2013, 95, 177–182. [Google Scholar] [CrossRef]
- Sarkar, S.; Singhal, R.S. Esterification of guar gum hydrolyzate and gum Arabic with n-octenyl siccinic anhydride and oleic acid and its evaluation as wall material in microencapsulation. Carbohydr. Polym. 2011, 86, 1723–1731. [Google Scholar]
- Wang, H.A.O.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsifying properties of dodecenyl succinic anhydride derivatives of gum Arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Srinivasan, R.; Dubey, R. Flocculation of textile wastewater by Plantago psyllium mucilage. Macromol. Mater. Eng. 2002, 1, 592–596. [Google Scholar] [CrossRef]
- Dholakia, A.B.; Patel, K.H.; Trivedi, H.C. Photo-induced graft copolymerization of acrylonitrile onto sodium salt of partially carboxymethylated Psyllium. Chem. Sin. 2011, 2, 106–116. [Google Scholar]
- Maji, B.; Maiti, S. Chemical modification of xanthan gum through graft copolymerization: Tailored properties and potential applications in drug delivery and wastewater treatment. Carbohydr. Polym. 2021, 251, 117095. [Google Scholar] [CrossRef]
- El-Siddig, K.E.; Gunasena, H.P.M.; Prasad, B.A.; Pushpakumar, D.K.; Ramana, K.V.R.; Vijayanand, P. Tamarind, Tamarindus Indica; Southampton Centre for Underutilised Crops: Southampton, UK, 2006. [Google Scholar]
- Freitas, R.A.; Martin, S.; Santos, G.L.; Valenga, F.; Buckeridge, M.S.; Reicher, F. Physico-chemical properties of seed xyloglucans from different sources. Carbohydr. Polym. 2005, 60, 507–514. [Google Scholar] [CrossRef]
- Wang, Q.; Ellis, P.R.; Ross-Murphy, S.B.; Burchard, W. Solution characteristics of the xyloglucan extracted from Detarium senegalense Gmelin. Carbohydr. Polym. 1997, 33, 115–124. [Google Scholar] [CrossRef]
- Malviya, R.; Raj, S.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.; Kumari, U.; Unnikrishnan Meenakshi, D.; Porwal, O.; Hari Kumar, D.; Singh, A.; et al. Evaluation of Antitumor Efficacy of Chitosan-Tamarind Gum Polysaccharide Polyelectrolyte Complex Stabilized Nanoparticles of Simvastatin. Int. J. Nanomed. 2021, 16, 2533–2553. [Google Scholar] [CrossRef]
- Malviya, R.; Jha, S.; Fuloria, N.K.; Subramaniyan, V.; Chakravarthi, S.; Sathasivam, K.; Kumari, U.; Meenakshi, D.U.; Porwal, O.; Sharma, A.; et al. Determination of Temperature-Dependent Coefficients of Viscosity and Surface Tension of Tamarind Seeds (Tamarindus indica L.) Polymer. Polymers 2021, 13, 610. [Google Scholar] [CrossRef]
- Malviya, R.; Sundram, S.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Sekar, M.; Kumar, D.H.; Chakravarthi, S.; Porwal, O.; et al. Evaluation and Characterization of Tamarind Gum Polysaccharide: The Biopolymer. Polymers 2021, 13, 3023. [Google Scholar] [CrossRef]
- Nauib, H.F.J. Chemically induced graft copolymerization of itaconic acid onto sisal fibers. Polym. Res. 2002, 9, 207. [Google Scholar]
- Yoshida, T.; Hattori, K.; Swada, Y.; Choi, Y.; Uryu, T. Graft copolymerization of methyl methacrylate onto curdlan. J. Polym. Sci. Polym. Chem. 1996, 34, 3053–3060. [Google Scholar] [CrossRef]
- Durcilene, A.D.S.; Regina, C.M.P.; Feitosa, J.P.A. Graft copolymerisation of acrylamide onto cashew gum. Eur. Polym. J. 2007, 43, 2620–2629. [Google Scholar]
- Grassieker, N. Thermal Stability of Polymers; Conley Marcel Dekker: New York, NY, USA, 1970; Volume 1. [Google Scholar]
- Singh, R.V. Poly (acrylonitrile) grafted Cassia pudibunda seed gum: A potential commercial gum from renewable source. J. Appl. Polym. Sci. 2006, 99, 619–627. [Google Scholar] [CrossRef]
- Jatav, P.; Aggarwal, N. Synthesis and characterization of graft copolymer of methacrylamide onto psyllium. Int. J. Latest Res. Sci. Technol. 2015, 4, 1–7. [Google Scholar]
- Nayak, A.K.; Bera, H.; Saquib Hasnain, M.; Pal, D.K. Chapter 1—Synthesis and Characterization of Graft Copolymers of Plant Polysaccharides. In Biopolymer Grafting; Thakur, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–62. ISBN 9780323481045. [Google Scholar] [CrossRef]
- Biswal, J.; Kumar, V.; Bhardwaj, Y.K.; Goel, N.K.; Dubey, A.K.; Chaudhari, C.V.; Sabharwal, S. Radiation-induced grafting of acrylamide onto guar gum in aqueous medium: Synthesis and characterization of grafted polymer guar-g-acrylamide. Radiat. Phys. Chem. 2006, 76, 1624–1630. [Google Scholar] [CrossRef]
- Jour Linhardt, R.J.; Azmeera Adhikary, V.; Krishnamoorthi, S. Synthesis and Characterization of Graft Copolymer of Dextran and 2-Acrylamido-2-methylpropane Sulphonic Acid. Int. J. Carbohydr. Chem. 2012, 209085, 1687–9341. [Google Scholar] [CrossRef]
- Dodi, G.; Hritcu, D.; Popa, M. Carboxymethylation of guar gum: Synthesis and characterization. Cellul. Chem. Technol. 2011, 45, 171–176. [Google Scholar]
- Ikhuoria, E.U.; Folayan, A.S.; Okieimen. Studies in the graft copolymerization of acrylonitrile onto cassava starch by ceric ion induced initiation. Int. J. Biotechnol. Mole Biol. Res. 2011, 1, 10. [Google Scholar]
- Nandi, G.; Changder, A.; Ghosh, L.K. Graft-copolymer of polyacrylamide-tamarind seed gum: Syn-thesis, characterization and evaluation of flocculating potential in peroral paracetamol suspension. Carbohydr. Polym. 2019, 215, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Lele, V.; Kumari, S. Synthesis and Characterization of Graft Copolymer of Sago Starch- g- Poly (Acrylamide) Using Potassium Persulphate Initiator. J. Sci. Res. 2021, 65, 2. [Google Scholar] [CrossRef]
- Pati, M.K.; Nayak, P. Grafting vinyl monomers onto chitosan: IV: Graft copolymerized of acrylicacid onto chitosan using ceric ammonium nitrate as the initiator-characterization and antimicrobial activities. Mater. Sci. Appl. 2011, 2, 1741. [Google Scholar] [CrossRef] [Green Version]
- Malviya, R.; Sharma, P.K.; Dubey, S.K. Microwave-assisted preparation of biodegradable, hemocompatible, and antimicrobial neem gum–grafted poly (acrylamide) hydrogel using (3)2 factorial design. Emergent Mater. 2019, 2, 95–112. [Google Scholar] [CrossRef]
- Shi, Z.; Jia, C.; Wang, D.; Deng, J.; Xu, G.; Wu, C.; Dong, M.; Guo, Z. Synthesis and characterization of porous tree gum grafted copolymer derived from Prunus cerasifera gum polysaccharide. Int. J. Biol. Macromol. 2019, 133, 964–970. [Google Scholar] [CrossRef]
- Sen, G.; Singh, R.P.; Pal, S. Microwave-initiated synthesis of polyacrylamide grafted sodium alginate: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 115, 63–71. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]






| S. No. | Batch | Quantity of CAN (gm) | Microwave Exposure |
|---|---|---|---|
| 1 | F1 | 0.5 | 16 |
| 2 | F2 | 0.3 | 16 |
| 3 | F3 | 0.4 | 16 |
| 4 | F4 | 0.5 | 13 |
| 5 | F5 | 0.3 | 13 |
| 6 | F6 | 0.4 | 13 |
| 7 | F7 | 0.5 | 10 |
| 8 | F8 | 0.3 | 10 |
| 9 | F9 | 0.4 | 10 |
| Batch | Grafting (%) | Grafting Efficiency | Conversion (%) | Swelling Index (%) | Chemical Resistance (0.1N HCl) | Chemical Resistance (1N NaOH) |
|---|---|---|---|---|---|---|
| F1 | 667.8 | 111.2 | 152 | 97 | 3.9 | 2.5 |
| F2 | 464 | 77.4 | 104 | 73.10 | 4.2 | 3.9 |
| F3 | 587 | 53.4 | 124 | 89 | 5.7 | 5.1 |
| F4 | 558 | 93.9 | 119 | 58 | 7.7 | 3.8 |
| F5 | 385.5 | 64.2 | 63.5 | 87 | 6.5 | 5.2 |
| F6 | 209 | 34.9 | 53 | 92 | 6.4 | 4.1 |
| F7 | 531 | 88.5 | 102 | 90.6 | 5.7 | 2.6 |
| F8 | 196 | 32.7 | 50 | 88.4 | 3.8 | 7.6 |
| F9 | 140 | 23.4 | 35 | 91.1 | 4.2 | 5.5 |
| Formulation | Concentration in mg/mL | Zone of Inhibition in mm | |
|---|---|---|---|
| E. coli | A. niger | ||
| N1 | 0.25 | 0.116 ± 0.001 | 0.109 ± 0.003 |
| 0.5 | 0.120 ± 0.003 | 0.121 ± 0.003 | |
| 1 | 0.126 ± 0.003 | 0.128 ± 0.002 | |
| F1 | 0.25 | 0.289 ± 0.002 | 0.218 ± 0.002 |
| 0.5 | 0.326 ± 0.002 | 0.318 ± 0.002 | |
| 1 | 0.427 ± 0.001 | 0.420 ± 0.003 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, S.; Malviya, R.; Fuloria, S.; Sundram, S.; Subramaniyan, V.; Sekar, M.; Sharma, P.K.; Chakravarthi, S.; Wu, Y.S.; Mishra, N.; et al. Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide. Polymers 2022, 14, 1037. https://doi.org/10.3390/polym14051037
Jha S, Malviya R, Fuloria S, Sundram S, Subramaniyan V, Sekar M, Sharma PK, Chakravarthi S, Wu YS, Mishra N, et al. Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide. Polymers. 2022; 14(5):1037. https://doi.org/10.3390/polym14051037
Chicago/Turabian StyleJha, Sheetal, Rishabha Malviya, Shivkanya Fuloria, Sonali Sundram, Vetriselvan Subramaniyan, Mahendran Sekar, Pradeep Kumar Sharma, Srikumar Chakravarthi, Yuan Seng Wu, Neelesh Mishra, and et al. 2022. "Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide" Polymers 14, no. 5: 1037. https://doi.org/10.3390/polym14051037
APA StyleJha, S., Malviya, R., Fuloria, S., Sundram, S., Subramaniyan, V., Sekar, M., Sharma, P. K., Chakravarthi, S., Wu, Y. S., Mishra, N., Meenakshi, D. U., Bhalla, V., Djearamane, S., & Fuloria, N. K. (2022). Characterization of Microwave-Controlled Polyacrylamide Graft Copolymer of Tamarind Seed Polysaccharide. Polymers, 14(5), 1037. https://doi.org/10.3390/polym14051037