Influence of the Integration of Geopolymer Wastes on the Characteristics of Binding Matrices Subjected to the Action of Temperature and Acid Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Constituents
- -
- Aluminosilicate powders: a commercial metakaolin (MK, from Imerys-France) and fly-ash from coal combustion (FA, from the Jarf Lasfar thermal power station in Morocco).
- -
- An alkaline activating solution (AAS) with SiO2/Na2O molar ratio = 1.2 and 63% by weight of water. This solution is prepared from a mixture of sodium hydroxide (98% purity), commercial sodium silicate (SiO2/Na2O molar ratio of 2) and water.
- -
- Hydrochloric acid (37% HCl) was mixed with distilled water to prepare the acid solutions.
2.2. Material Manufacturing
- The first stage is preparing the reference geopolymer binders. To this end, two types of materials were developed:
- -
- A metakaolin-based geopolymer, MKref, obtained by mixing metakaolin powder (MK) and alkaline activating solution (AAS) at a Liquid/Solid ratio (L/S) ratio of 0.83. This mixture is hardened at 60 °C for 5 h.
- -
- A fly-ash-based geopolymer, FAref, obtained by mixing fly-ash powder (FA) and alkaline activation solution (AAS) at an L/S ratio of 0.58. This mixture is cured at 80 °C for 20 h.
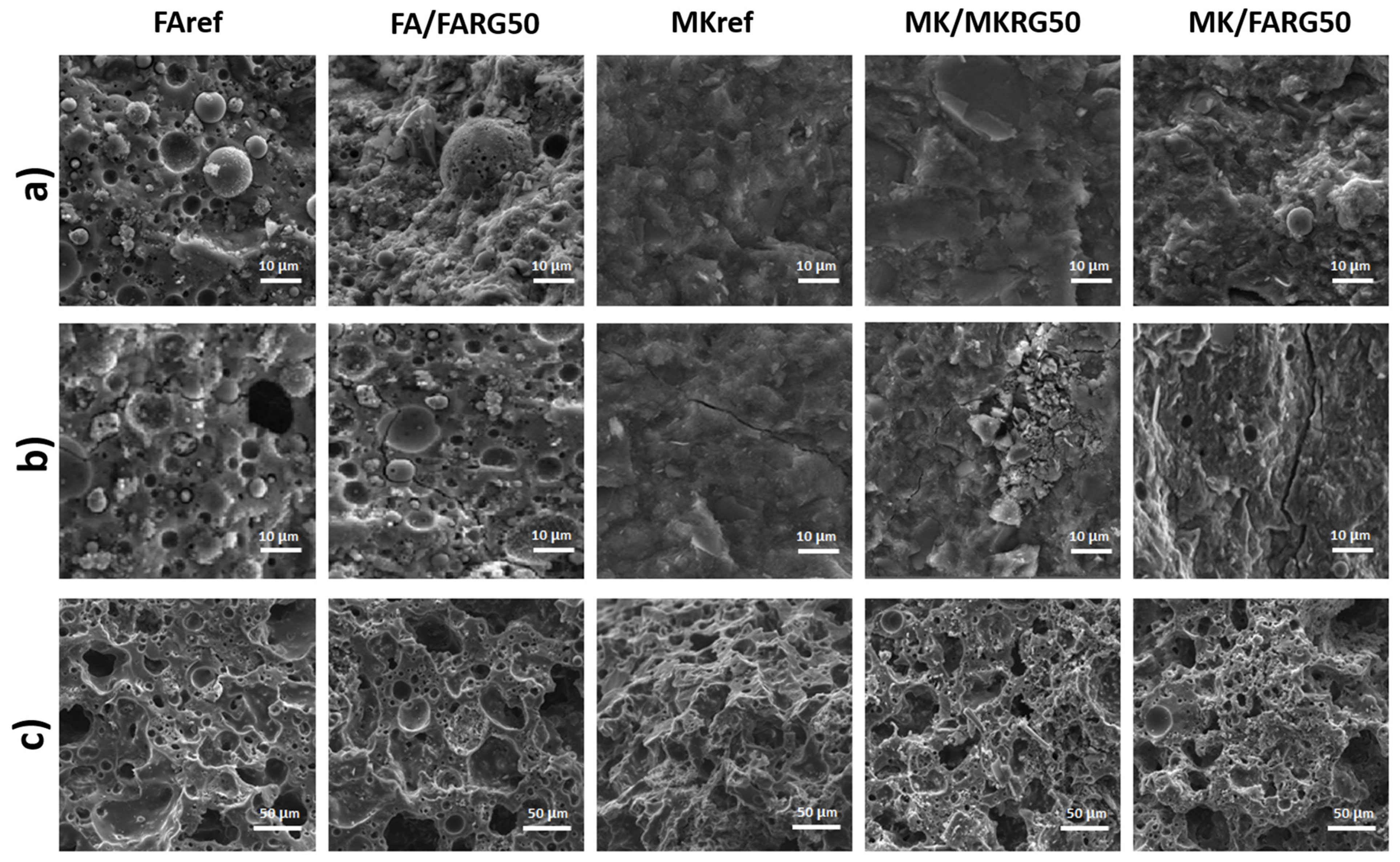
- To simulate geopolymer waste, the manufactured materials (MKref and FAref) are stored for one year in the open air at room temperature, then they are ground to obtain a fine powder D < 80 µm (Figure 3) referenced MKRG for materials based on metakaolin and FARG for those based on fly-ash.
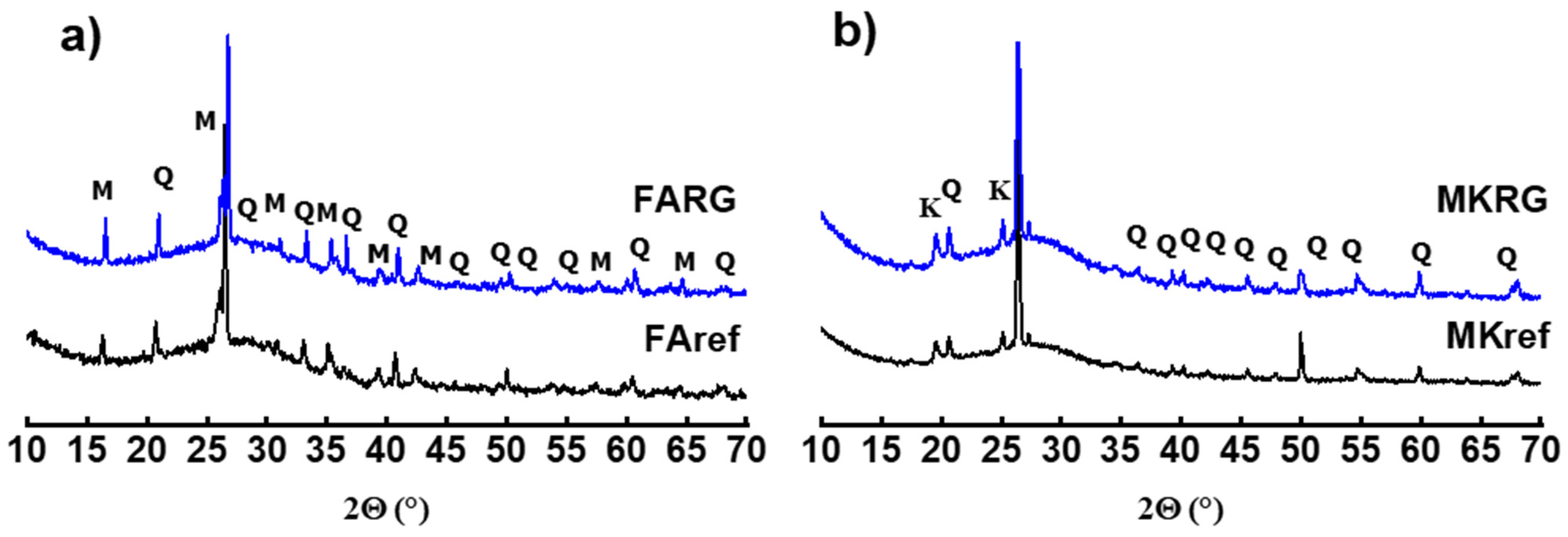
- The third stage consists in preparing the new matrices by substituting the starting aluminosilicates (fly-ash and metakoalin) with the waste (MKRG and FARG) up to a mass rate of 50%. These mixtures are then subjected to the action of the activation solution under the conditions described in stage 1. Three systems were, thus, prepared: FA/FARG, MK/MKRG and MK/FARG. Note that the materials referenced MKref and FAref are fresh matrices with 0% waste. Table 2 summarizes the data for the manufacturing of the materials.
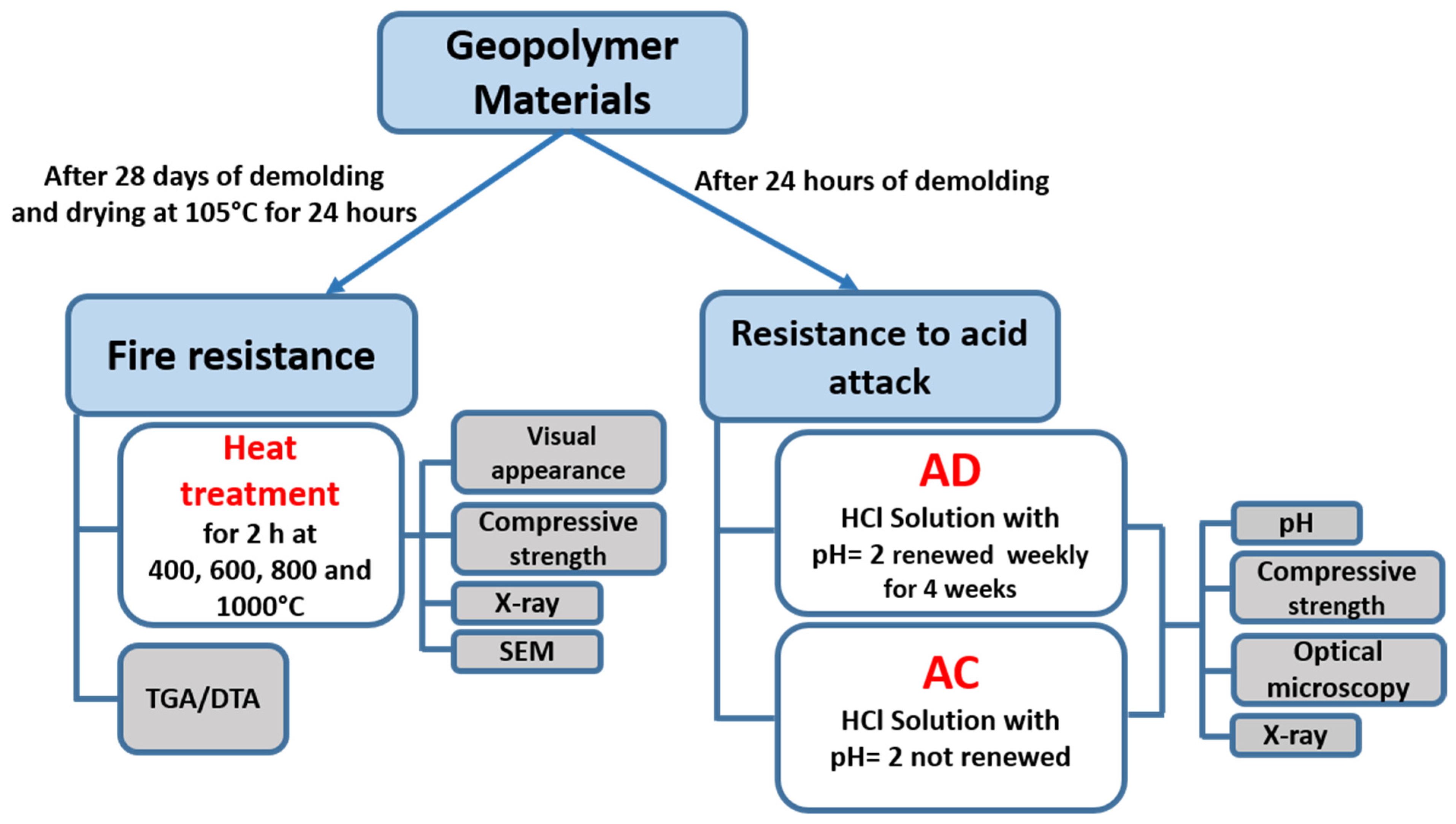
2.3. Fire Resistance
2.4. Resistane to Acid Attack
- -
- HCl solution (AD) with pH = 2. This solution was renewed weekly for 4 weeks.
- -
- HCl solution (AC) with pH = 2. This solution was not renewed during the treatment period.
2.5. Characterization Methods
- -
- The basic chemical composition of the powders was determined by X-ray fluorescence by using a Thermo ARL 9800XP spectrometer equipped with an X-ray tube with 3 kW maximum power (30 kV and 80 mA).
- -
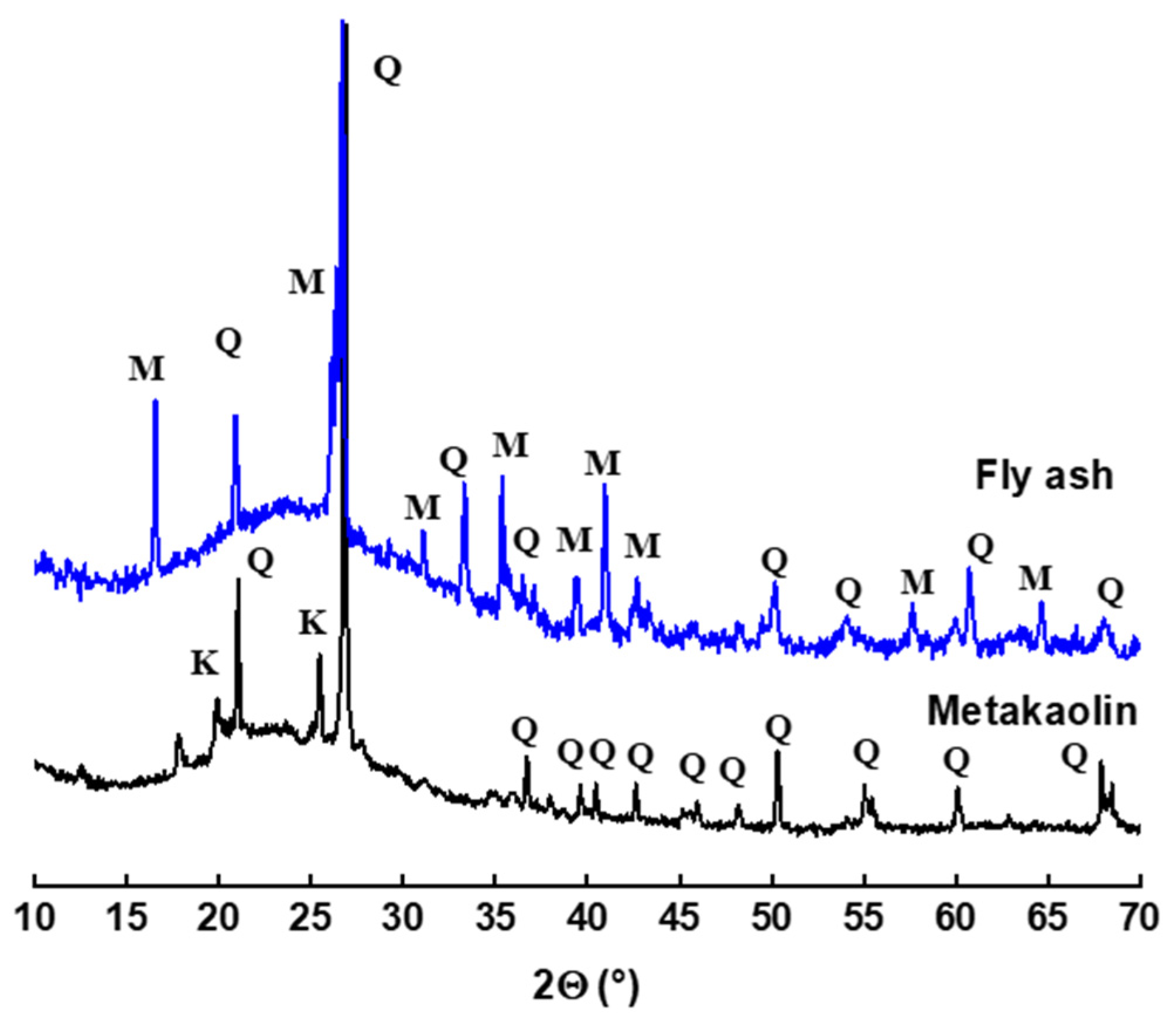
- The mineralogical composition of the materials was determined using XRD on powder, using a Bruker D8 copper anticathode diffractometer (CuKα 2θ (λKα = 1.5418 Å) operating at 40 mA and 40 kV. The results were collected for 2θ in the range 10 to 70° with an increment of 0.059°. The identification of the crystalline phases was made using version 2.1 of the DIFFRAC EVA software.
- -
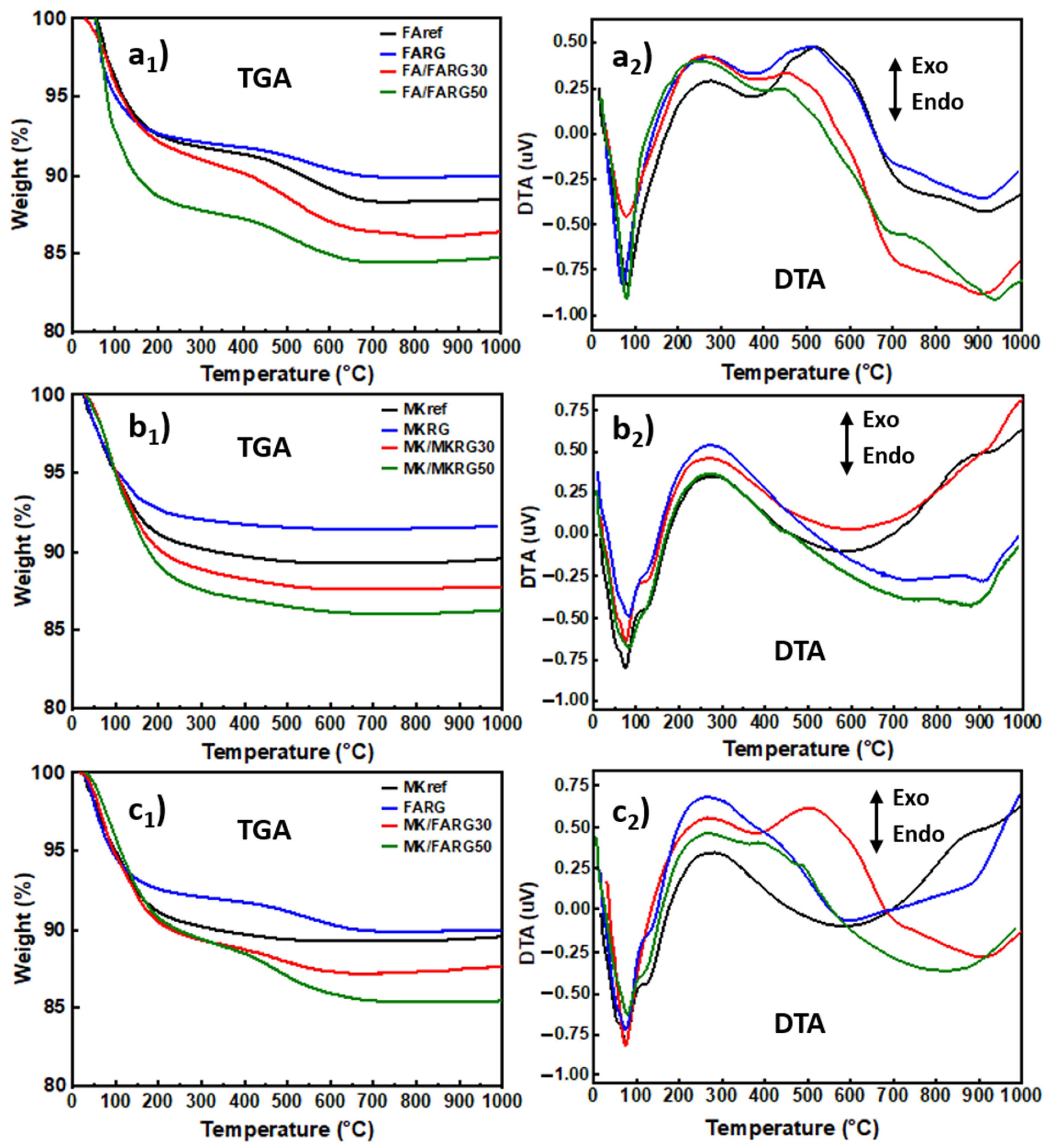
- Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were performed by using a Shimadzu DTG-60 H instrument operating at a heating rate of 6.67 °C/min.
- -
- The fracture surfaces of the materials before and after the chemical treatment were analyzed using a Tescan VEGA 3 scanning electron microscope and Keyence ZHX-700 type digital optical microscope.
- -
- Compressive strength testing was conducted on a Perrier type tensile machine, equipped with a 200 kN capacity loading cell. Four tests were performed for each material formulation.
- -
- The pH of the AC and AD acid media was determined using a standard pH-measurement device. Three pH measurements were performed for each material formulation in each medium.
3. Results
3.1. Fire Resistance
DTA-TGA
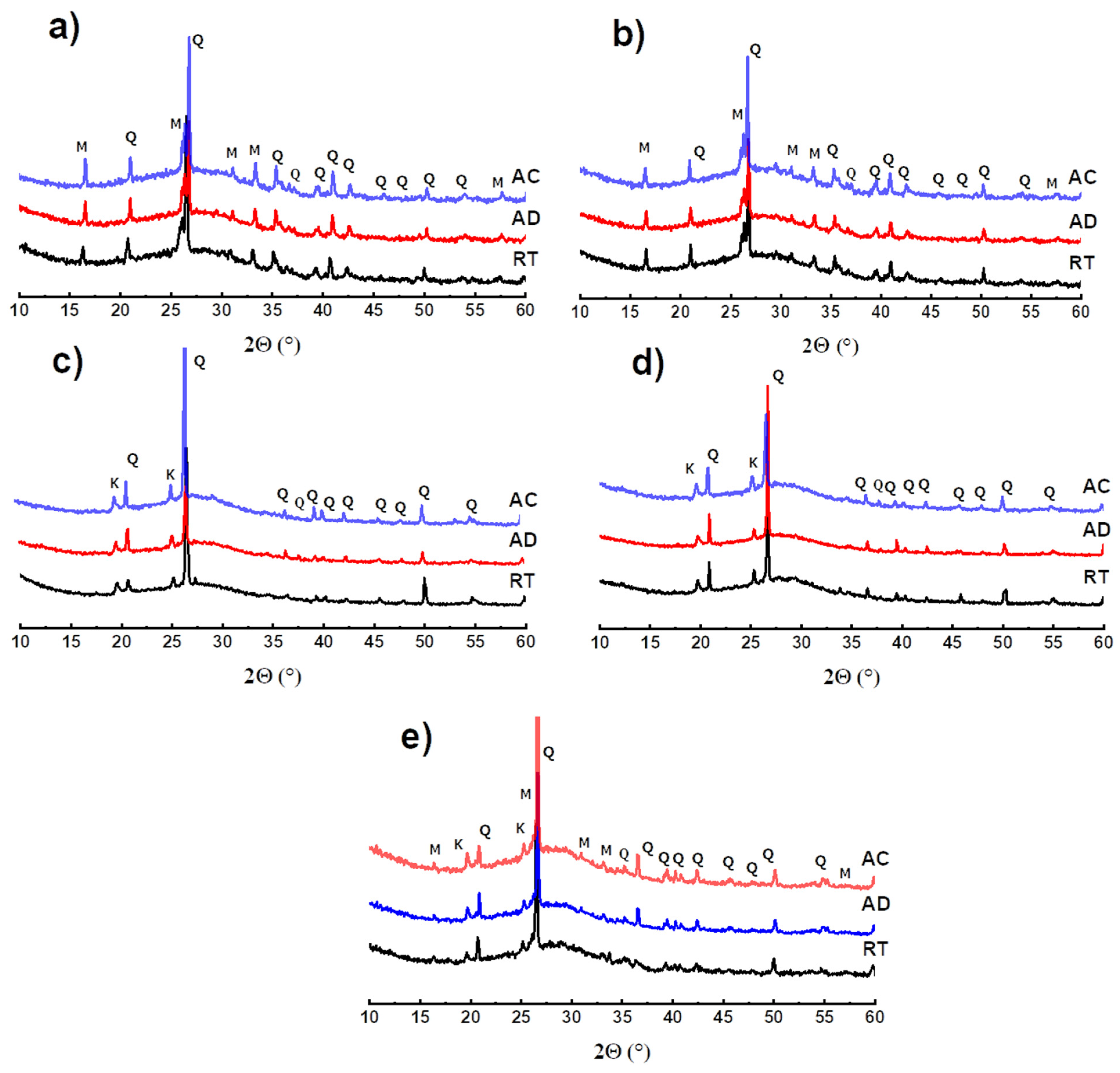
3.2. Mineralogical Analysis
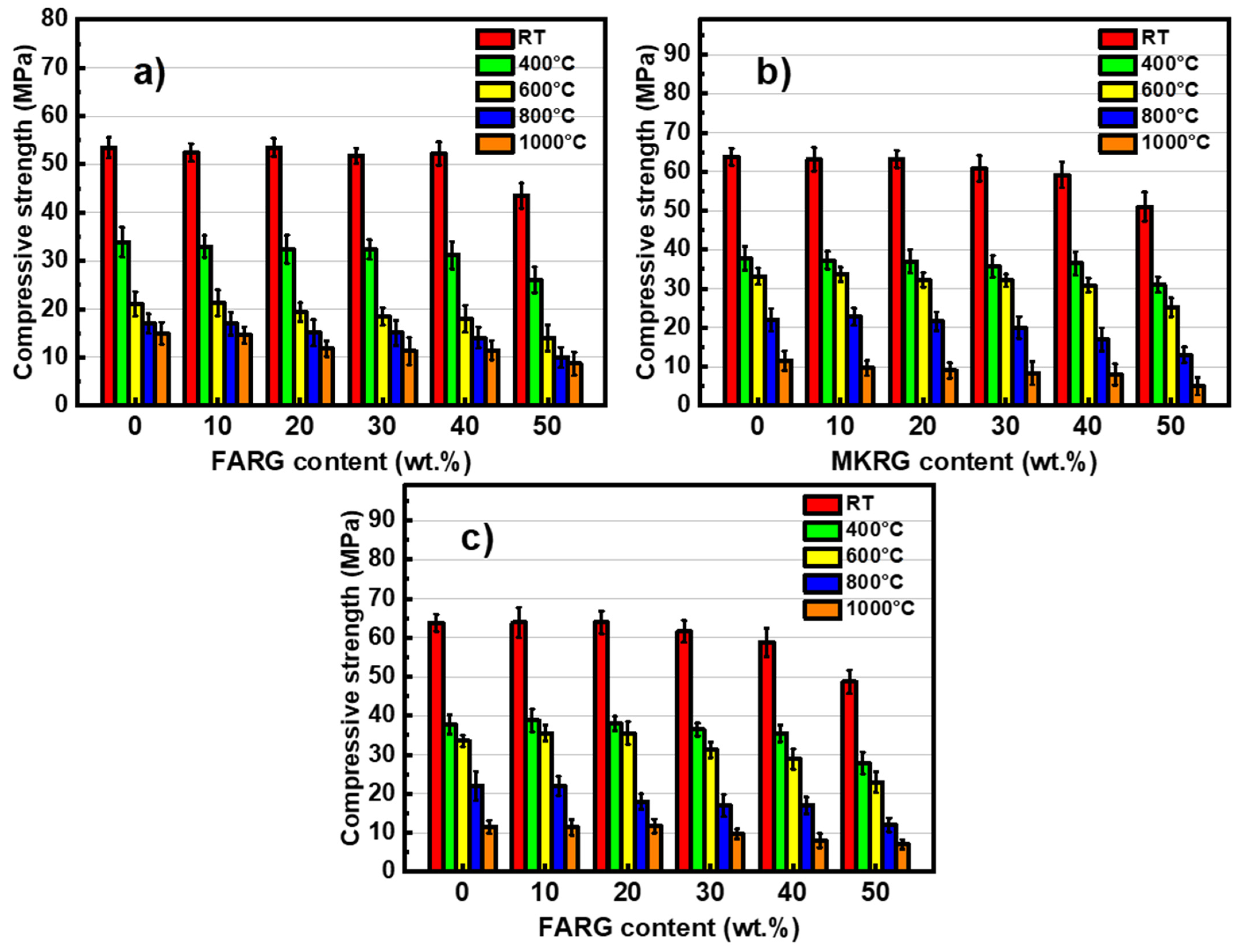
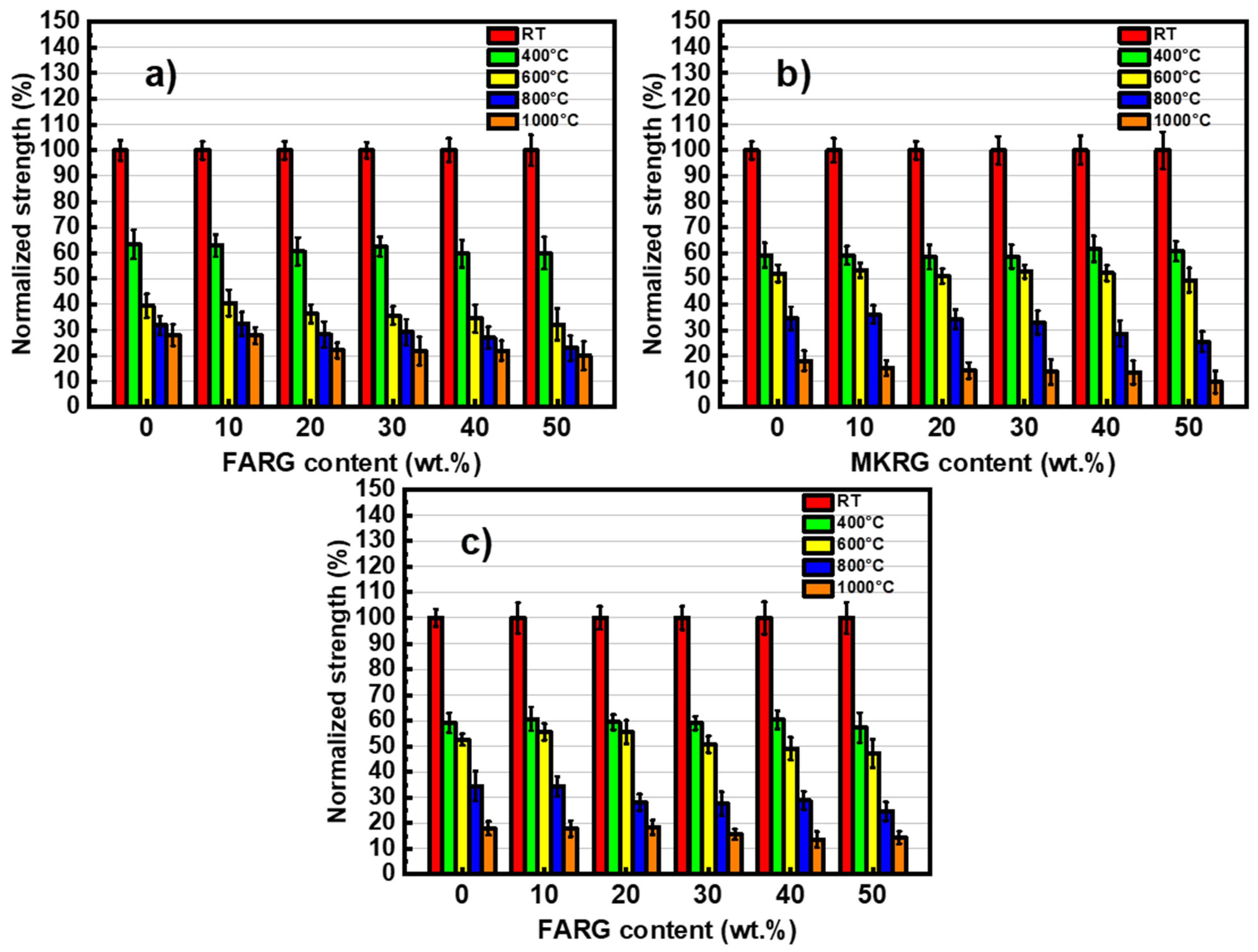
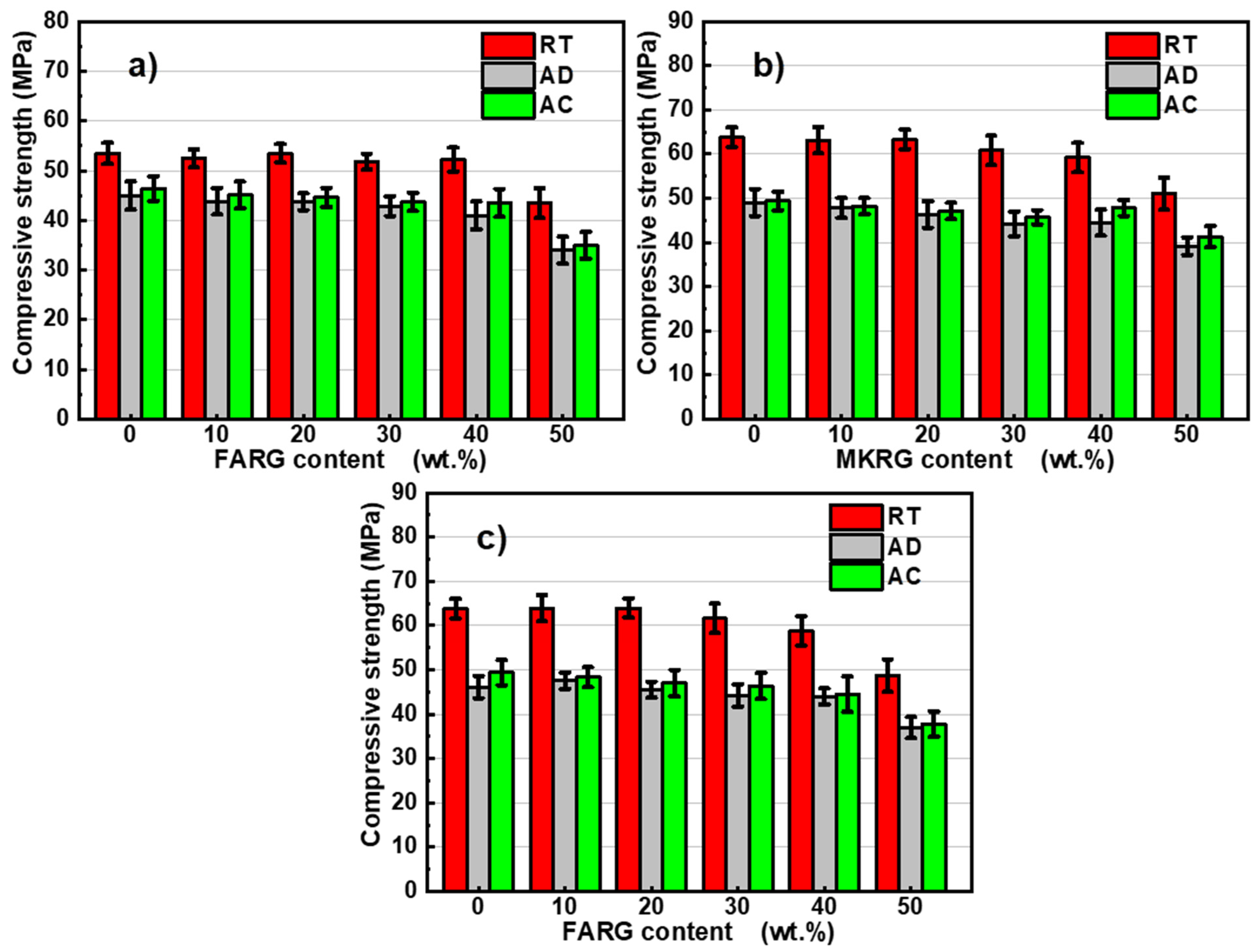
3.3. Mechanical Behavior
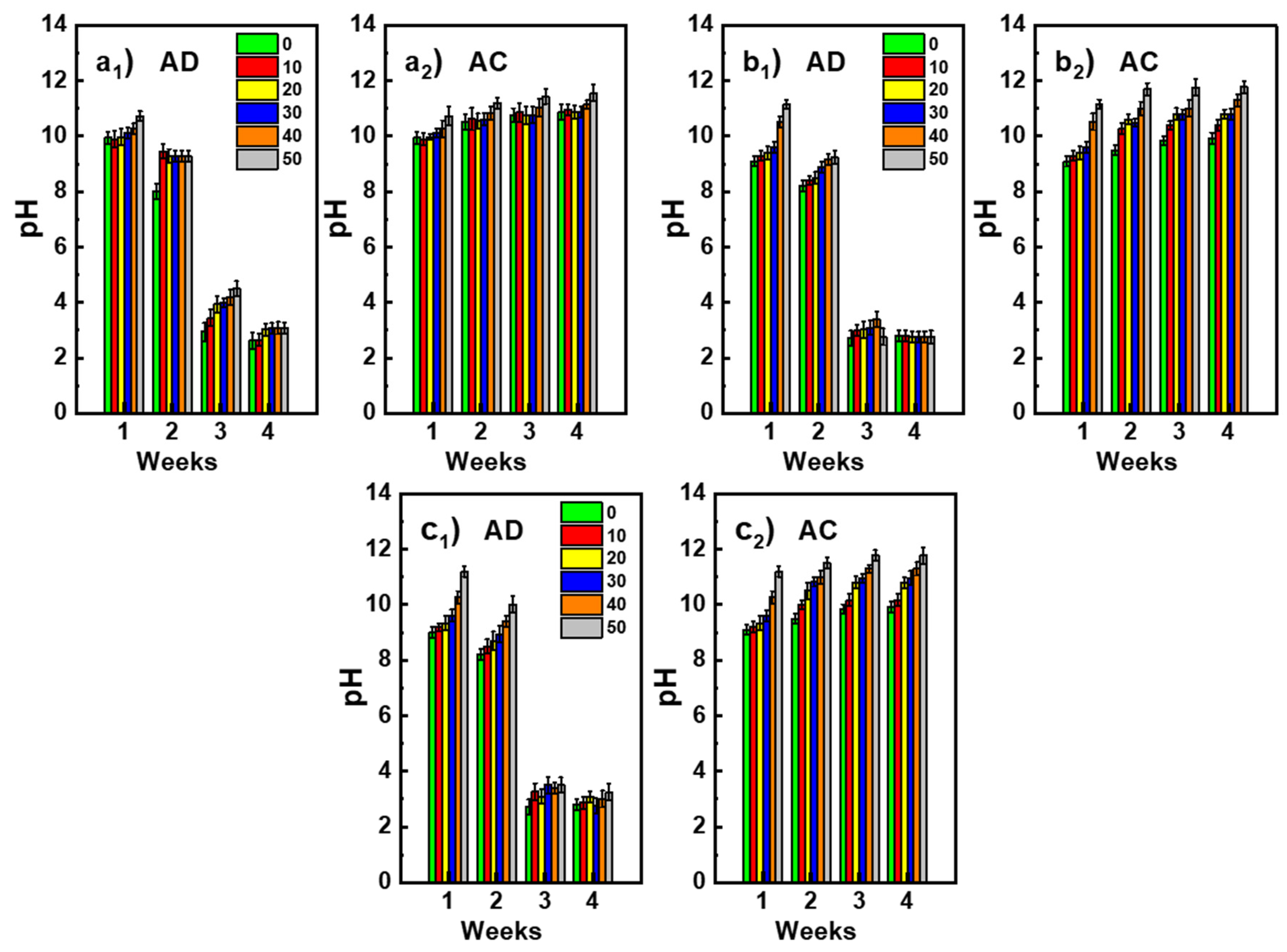
3.4. Resistane to Acid Attack
4. Conclusions
- -
- Regardless of the substitution rate, the hardened materials showed, at room temperature (RT), good mechanical performance with compressive strengths greater than 45 MPa.
- -
- Regardless of the waste substitution rate, the materials showed good fire resistance similar to that of waste-free materials. They maintained compressive strength greater than 20 MPa after treatment at 600 °C. The degradations observed included increased porosity resulting from the release of residual water and the crystallization of nepheline, which depletes the binding phase.
- -
- The crystallization of nepheline was earlier (600 °C) in the fly-ash-based materials FA/FARG. This is due to the high content of Fe2O3, K2O and Na2O oxides in the starting fly-ash, known for their role as fluxes and mineralizing agents. However, this phenomenon was only observed from 800 °C for the metakaolin-based materials MK/MKRG and MK/FARG.
- -
- Regardless of the waste substitution rate, the materials demonstrated very good resistance to acid attack (pH = 2) with resistance losses not exceeding 28%.
- -
- The increase in the pH of the attack baths confirms the mechanism of release of sodium ions present in excess in the materials, which enriches the baths in alkaline species by their combination with atmospheric CO2.
- -
- The materials were chemically stable in the media studied. Indeed, no significant impact on the mineralogical composition nor on the mass losses was recorded.
- -
- The increase in the rate of substitution of geopolymer waste had the effect of increasing the concentration of excess sodium ions in the material and, thus, that of the pH.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structures, Processing, Properties and Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781845694494. [Google Scholar]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer-An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; Franco de Carvalho, J.M.; Ribeiro, J.C.L. Application of eco-friendly alternative activators in alkali-activated materials: A review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Le, V.S.; Louda, P.; Tran, H.N.; Nguyen, P.D.; Bakalova, T.; Buczkowska, K.E.; Dufkova, I. Study on Temperature-Dependent Properties and Fire Resistance of Metakaolin-Based Geopolymer Foams. Polymers 2020, 12, 2994. [Google Scholar] [CrossRef]
- Górski, M.; Wielgus, N.; Loska, K.; Kozioł, M.; Landrat, M.; Ścierski, W.; Pikoń, K. Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers 2021, 13, 1149. [Google Scholar] [CrossRef]
- Khater, H.M.; Ghareib, M. Optimization of geopolymer mortar incorporating heavy metals in producing dense hybrid composites. J. Build. Eng. 2020, 32, 101684. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Li, P.; Cui, H.; Zhai, J. Immobilization of simulated radionuclide 133Cs+ by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262, 325–331. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. Do geopolymers actually contain nanocrystalline zeolites? a reexamination of existing results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali-metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Hattaf, R.; Aboulayt, A.; Samdi, A.; Lahlou, N.; Touhami, M.O.; Gomina, M.; Moussa, R. Metakaolin and Fly Ash-based Matrices for Geopolymer Materials: Setting Kinetics and Compressive Strength. Silicon 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation kinetics. 2. Reaction kinetic modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Muñiz-Villarreal, M.S.; Manzano-Ramírez, A.; Sampieri-Bulbarela, S.; Gasca-Tirado, J.R.; Reyes-Araiza, J.L.; Rubio-Ávalos, J.C.; Pérez-Bueno, J.J.; Apatiga, L.M.; Zaldivar-Cadena, A.; Amigó-Borrás, V. The effect of temperature on the geopolymerization process of a metakaolin-based geopolymer. Mater. Lett. 2011, 65, 995–998. [Google Scholar] [CrossRef]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; Cherki El Idrissi, A.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Aboulayt, A.; Souayfan, F.; Roziere, E.; Jaafri, R.; Cherki El Idrissi, A.; Moussa, R.; Justino, C.; Loukili, A. Alkali-activated grouts based on slag-fly ash mixtures: From early-age characterization to long-term phase composition. Constr. Build. Mater. 2020, 260, 120510. [Google Scholar] [CrossRef]
- Quiatchon, P.R.J.; Dollente, I.J.R.; Abulencia, A.B.; De Guzman Libre, R.G.; Villoria, M.B.D.; Guades, E.J.; Promentilla, M.A.B.; Ongpeng, J.M.C. Investigation on the Compressive Strength and Time of Setting of Low-Calcium Fly Ash Geopolymer Paste Using Response Surface Methodology. Polymers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Tan, J.; Vandevyvere, B. Thermal and compressive behaviors of fly ash and metakaolin-based geopolymer. J. Build. Eng. 2020, 30, 101307. [Google Scholar] [CrossRef]
- Durak, U.; İlkentapar, S.; Karahan, O.; Uzal, B.; Atiş, C.D. A new parameter influencing the reaction kinetics and properties of fly ash based geopolymers: A pre-rest period before heat curing. J. Build. Eng. 2020, 35, 102023. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwon, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- European Commission. Communication from the EU Commission, Closing the Loop-An EU Action Plan for the Circular Economy; COM/2015/0614 Final; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Hattaf, R.; Aboulayt, A.; Samdi, A.; Lahlou, N.; Ouazzani Touhami, M.; Gomina, M.; Moussa, R. Reusing Geopolymer Waste from Matrices Based on Metakaolin or Fly Ash for the Manufacture of New Binder Geopolymeric Matrices. Sustainability 2021, 13, 8070. [Google Scholar] [CrossRef]
- Hattaf, R.; Benchikhi, M.; Azzouzi, A.; El Ouatib, R.; Gomina, M.; Samdi, A.; Moussa, R. Preparation of Cement Clinker from Geopolymer-Based Wastes. Materials 2021, 14, 6534. [Google Scholar] [CrossRef] [PubMed]
- Akbarnezhad, A.; Huan, M.; Mesgari, S.; Castel, A. Recycling of geopolymer concrete. Constr. Build. Mater. 2015, 101, 152–158. [Google Scholar] [CrossRef]
- Zhu, P.; Hua, M.; Liu, H.; Wang, X.; Chen, C. Interfacial evaluation of geopolymer mortar prepared with recycled geopolymer fine aggregates. Constr. Build. Mater. 2020, 259, 119849. [Google Scholar] [CrossRef]
- Gharzouni, A.; Vidal, L.; Essaidi, N.; Joussein, E.; Rossignol, S. Recycling of geopolymer waste: Influence on geopolymer formation and mechanical properties. Mater. Des. 2016, 94, 221–229. [Google Scholar] [CrossRef]
- Mesgari, S.; Akbarnezhad, A.; Xiao, J.Z. Recycled geopolymer aggregates as coarse aggregates for Portland cement concrete and geopolymer concrete: Effects on mechanical properties. Constr. Build. Mater. 2020, 236, 117571. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin-fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Rashad, A.M.; Zeedan, S.R. The effect of activator concentration on the residual strength of alkali-activated fly ash pastes subjected to thermal load. Constr. Build. Mater. 2011, 25, 3098–3107. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Pastor, J.Y.; Martín, A. New cementitious materials based on alkali-activated fly ash: Performance at high temperatures. J. Am. Ceram. Soc. 2008, 91, 3308–3314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Kodur, V.; Wu, B.; Cao, L.; Wang, F. Thermal behavior and mechanical properties of geopolymer mortar after exposure to elevated temperatures. Constr. Build. Mater. 2016, 109, 17–24. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, H.; Zhang, Z.; Wu, Q. Effect of rice husk ash addition on the compressive strength and thermal stability of metakaolin based geopolymer. Constr. Build. Mater. 2019, 222, 872–881. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Zhang, H.; Li, M.; Wu, Y.; Guo, L.; Wang, W.; Duan, P.; Zhang, W.; Zhang, Z. Thermal stability and microstructure of metakaolin-based geopolymer blended with rice husk ash. Appl. Clay Sci. 2020, 196, 105769. [Google Scholar] [CrossRef]
- Choi, Y.C.; Park, B. Effects of high-temperature exposure on fractal dimension of fly-ash-based geopolymer composites. J. Mater. Res. Technol. 2020, 9, 7655–7668. [Google Scholar] [CrossRef]
- Hemra, K.; Aungkavattana, P. Effect of cordierite addition on compressive strength and thermal stability of metakaolin based geopolymer. Adv. Powder Technol. 2016, 27, 1021–1026. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; Van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A comparative study on geopolymers synthesized by different classes of fly ash after exposure to elevated temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Kohout, J.; Koutník, P.; Hájková, P.; Kohoutová, E.; Soukup, A. Effect of K/Al Molar Ratio on the Thermo-Mechanical Properties of Metakaolinite-Based Geopolymer Composites. Polymers 2021, 13, 3754. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Lahoti, M.; Wijaya, S.F.; Tan, K.H.; Yang, E.H. Tailoring sodium-based fly ash geopolymers with variegated thermal performance. Cem. Concr. Compos. 2020, 107, 103507. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Tan, K.H.; Yang, E.H. Effect of alkali cation type on strength endurance of fly ash geopolymers subject to high temperature exposure. Mater. Des. 2018, 154, 8–19. [Google Scholar] [CrossRef]
- Hosan, A.; Haque, S.; Shaikh, F. Compressive behaviour of sodium and potassium activators synthetized fly ash geopolymer at elevated temperatures: A comparative study. J. Build. Eng. 2016, 8, 123–130. [Google Scholar] [CrossRef]
- Bakharev, T. Thermal behaviour of geopolymers prepared using class F fly ash and elevated temperature curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Pastor, J.Y.; Martín, A.; Palomo, A. High-Temperature Resistance in Alkali-Activated Cement. J. Am. Ceram. Soc. 2010, 93, 3411–3417. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Koenders, E. Effect of Silica Fume on Metakaolin Geopolymers’ Sulfuric Acid Resistance. Materials 2021, 14, 5396. [Google Scholar] [CrossRef]
- Gao, X.X.; Michaud, P.; Joussein, E.; Rossignol, S. Behavior of metakaolin-based potassium geopolymers in acidic solutions. J. Non-Cryst. Solids 2013, 380, 95–102. [Google Scholar] [CrossRef]
- Bouguermouh, K.; Bouzidi, N.; Mahtout, L.; Pérez-Villarejo, L.; Martínez-Cartas, M.L. Effect of acid attack on microstructure and composition of metakaolin-based geopolymers: The role of alkaline activator. J. Non-Cryst. Solids 2017, 463, 128–137. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Kwasny, J.; Aiken, T.A.; Soutsos, M.N.; McIntosh, J.A.; Cleland, D.J. Sulfate and acid resistance of lithomarge-based geopolymer mortars. Constr. Build. Mater. 2018, 166, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirt, P.; Rattanasak, U.; Taebuanhuad, S. Resistance to acid and sulfate solutions of microwave-assisted high calcium fly ash geopolymer. Mater. Struct. Constr. 2013, 46, 375–381. [Google Scholar] [CrossRef]
- Rashad, A.M.; Khalil, M.H. A preliminary study of alkali-activated slag blended with silica fume under the effect of thermal loads and thermal shock cycles. Constr. Build. Mater. 2013, 40, 522–532. [Google Scholar] [CrossRef]
- Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Paya, J.; Monzo, J.M.; Borrachero, M.V. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem. Concr. Compos. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Bernal, S.A.; Rodríguez, E.D.; Mejía De Gutiérrez, R.; Gordillo, M.; Provis, J.L. Mechanical and thermal characterisation of geopolymers based on silicate-activated metakaolin/slag blends. J. Mater. Sci. 2011, 46, 5477–5486. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Kealley, C.S.; Van Riessen, A. Thermally induced microstructural changes in fly ash geopolymers: Experimental results and proposed model. J. Am. Ceram. Soc. 2015, 98, 929–939. [Google Scholar] [CrossRef]
- Celik, A.; Yilmaz, K.; Canpolat, O.; Al-mashhadani, M.M.; Aygörmez, Y.; Uysal, M. High-temperature behavior and mechanical characteristics of boron waste additive metakaolin based geopolymer composites reinforced with synthetic fibers. Constr. Build. Mater. 2018, 187, 1190–1203. [Google Scholar] [CrossRef]






| Oxide (wt.%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| MK | 56.27 | 36.39 | 1.38 | 0.42 | 0.35 | 0.19 | 0.17 | 0.79 | 0.06 | 2.04 |
| MKRG | 54.94 | 25.59 | 1.10 | 1.23 | 0.29 | 0.31 | 8.14 | 0.68 | 0.10 | 8.57 |
| FA | 50.99 | 22.36 | 5.80 | 4.75 | 2.19 | 0.51 | 0.89 | 1.77 | 0.85 | 9.90 |
| FARG | 46.23 | 17.04 | 5.39 | 5.36 | 2.09 | 0.55 | 6.52 | 1.64 | 0.57 | 12.88 |
| AAS | Liquid/Solid Ratio (L/S) | Curing Temperature (°C) | Curing Duration (h) | |
|---|---|---|---|---|
| FA/FARG | 1.2 | 0.58 | 80 | 20 |
| MK/MKRG | 1.2 | 0.83 | 60 | 5 |
| MK/FARG | 1.2 | 0.83 | 60 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattaf, R.; Aboulayt, A.; Lahlou, N.; Touhami, M.O.; Gomina, M.; Samdi, A.; Moussa, R. Influence of the Integration of Geopolymer Wastes on the Characteristics of Binding Matrices Subjected to the Action of Temperature and Acid Environments. Polymers 2022, 14, 917. https://doi.org/10.3390/polym14050917
Hattaf R, Aboulayt A, Lahlou N, Touhami MO, Gomina M, Samdi A, Moussa R. Influence of the Integration of Geopolymer Wastes on the Characteristics of Binding Matrices Subjected to the Action of Temperature and Acid Environments. Polymers. 2022; 14(5):917. https://doi.org/10.3390/polym14050917
Chicago/Turabian StyleHattaf, Rabii, Abdelilah Aboulayt, Nouha Lahlou, Mohamed Ouazzani Touhami, Moussa Gomina, Azzeddine Samdi, and Redouane Moussa. 2022. "Influence of the Integration of Geopolymer Wastes on the Characteristics of Binding Matrices Subjected to the Action of Temperature and Acid Environments" Polymers 14, no. 5: 917. https://doi.org/10.3390/polym14050917






