Abstract
Biomolecules are attractive building blocks with self-assembly ability, structural diversity, and excellent functionality for creating artificial materials. Heparin and protamine, a clinically relevant pair of biomolecules used in cardiac and vascular surgery, have been shown to coassemble into particulate polyelectrolyte complexes in vitro. The resulting heparin–protamine particles exhibit adhesive properties that enable advantageous interactions with proteins, cells, and various other substances and have been employed as functional materials for biomedical applications. In this review article, we summarize recent progress in research on the use of heparin–protamine particles as drug carriers, cell adhesives, and cell labels. Studies have demonstrated that heparin–protamine particles are potentially versatile in biomedical fields from drug delivery and regenerative medicine to plastic surgery.
1. Introduction
Biomolecules, such as proteins, nucleic acids, lipids and carbohydrates, form sophisticated assemblies via intermolecular interactions and constitute living organisms in nature. The self-assembly ability, structural diversity, and excellent functionality of biomolecules make them attractive as building blocks for creating artificial materials [1,2,3,4,5,6,7,8,9,10]. In fact, assembled biomolecular materials have found practical applications; clinical examples include collagen sponges used for hemostasis and wound healing [11,12] and lipid nanoparticles constituting drug products (e.g., COVID-19 vaccines) [13,14,15,16]. In addition to these clinically used materials composed of a single class of biomolecules, multicomponent assemblies have been explored to access new material properties and morphologies. For instance, the combination of proteins and nucleic acids is of interest in nanotechnology [17,18], while composites of lipid nanoparticles and polysaccharide hydrogels have been investigated as drug carriers [19]. These studies demonstrate that multicomponent biomolecular systems offer opportunities to generate a broad spectrum of functional materials.
Heparin and protamine are a clinically relevant pair of biomolecules. Heparin is a mixture of linear anionic polysaccharides with many sulfate groups and has been used clinically as an anticoagulant for more than 70 years [20,21,22,23]. In cardiac and vascular surgery, the use of the anticoagulant is followed by the administration of protamine, a small arginine-rich cationic protein, to neutralize the heparin [23,24,25,26]. The fact that pharmaceutical grade heparin and protamine are commercially available and clinically used in cardiac and vascular surgery makes these biomolecules attractive as building blocks for in vivo applications. The neutralization effect is a consequence of complex formation between cationic protamine and anionic heparin via electrostatic interactions and other intermolecular interactions. The complex formation in blood indicates that heparin and protamine can coassemble robustly even in crowded environments. Furthermore, the fact that the complexes form in patients’ blood suggests that heparin–protamine coassemblies have good biocompatibility, which encouraged us to explore this multicomponent system for the production of biomaterials.
Studies have explored the in vitro coassembly of heparin and protamine and demonstrated their coassembly into nanometer- or micrometer-sized particles that are useful for biomedical applications [27,28,29,30,31,32,33]. For particle preparation, low-molecular-weight heparin (several kDa), which is fractionated from heparin (10–20 kDa), is frequently used instead of heparin due to the lower risk of bleeding with the low-molecular-weight form [25,34,35]. In fact, we have developed particles from low-molecular-weight heparin as a drug delivery carrier with low bleeding risk for in vivo administration [28]. Heparin–protamine particles tend to have a net negative charge, as heparin is generally used in excess of protamine in terms of charge; a high fraction of protamine causes precipitation rather than particle formation [29]. Despite a net negative charge, polyelectrolyte complexes can exhibit attraction not only to positively charged substances but also to negatively charged proteins and surfaces through electrostatic interactions [36], partly due to the patchiness of charges on the protein surface and the charge regulation for proteins by counterpart polyelectrolytes [37]. In addition, the particles exhibit strong interactions with heparin-binding proteins; notably, the bioactivities of such proteins tend to be enhanced upon binding to heparin. These characteristics, coupled with the biocompatibility and commercial availability of heparin and protamine (Figure 1), have led to the use of particulate heparin–protamine complexes as carriers for proteins [28,29,38,39,40,41,42,43,44,45] and adhesives for cells [46,47,48]. Such studies demonstrate that heparin–protamine particles show great promise as versatile nanomaterials in biomedical engineering and medicine. In this review article, we summarize recent research progress on heparin–protamine particles for biomedical applications (Figure 2).
Figure 1.
Attractive characteristics of the heparin–protamine system.
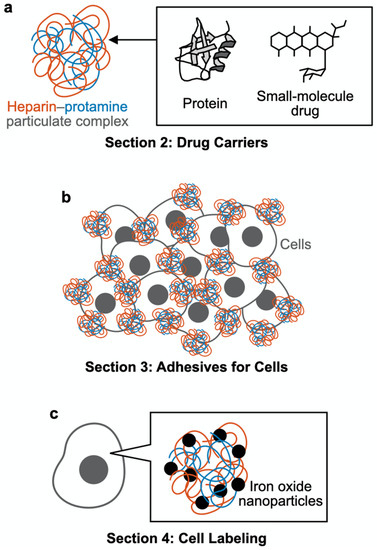
Figure 2.
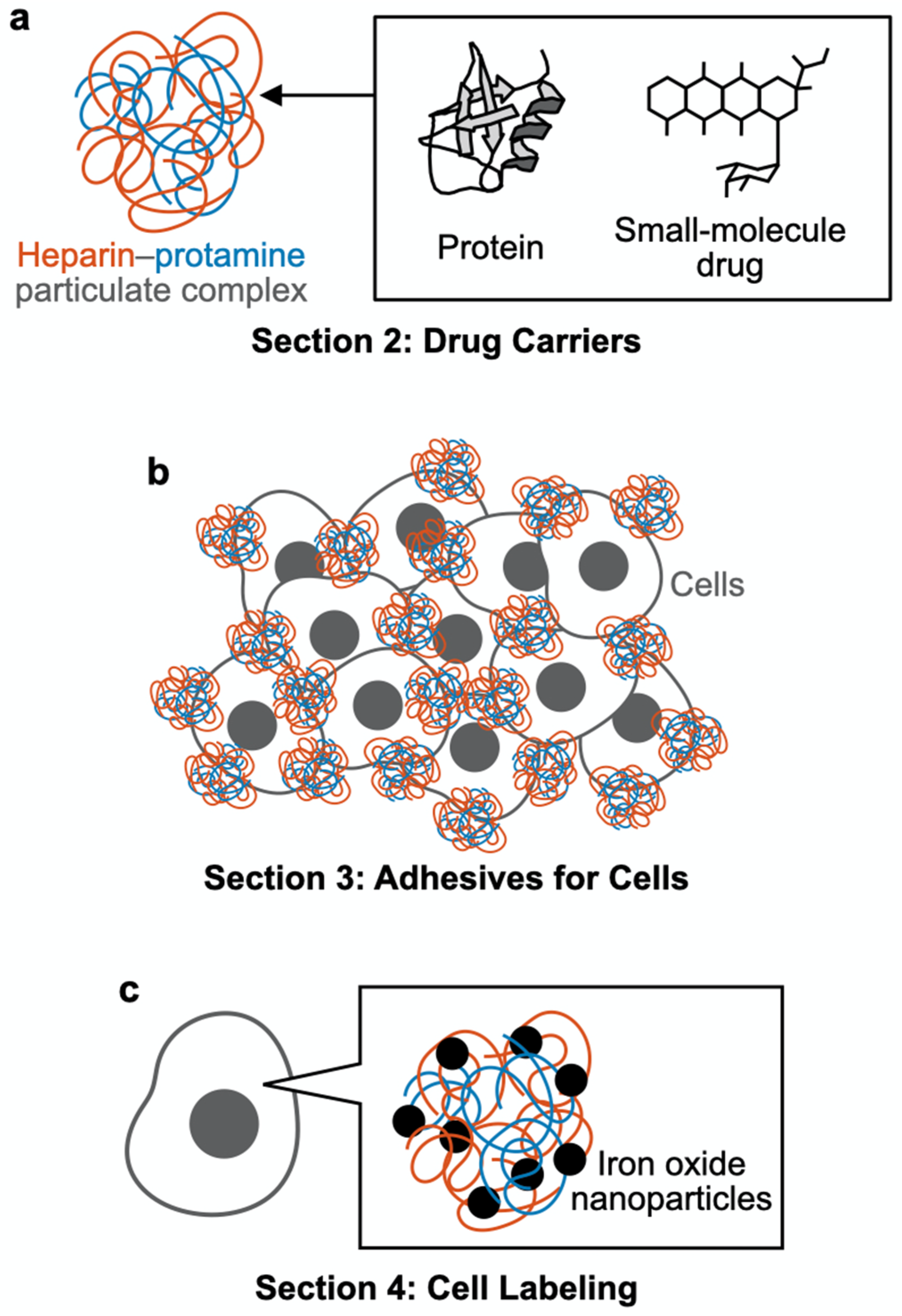
Schematic outline of this review article. (a) Section 2: Drug carriers. Heparin–protamine particles are useful as carriers for proteins and small-molecule drugs. (b) Section 3: Adhesives for cells. Heparin–protamine particles act as adhesives for cell aggregate formation. (c) Section 4: Cell labeling. Heparin–protamine complexes facilitate cell labeling with iron oxide nanoparticles.
2. Drug Carriers
As stated above, heparin–protamine particles are useful as carriers for proteins due to their ability to adsorb proteins via electrostatic interactions and other intermolecular interactions. The particles can preserve loaded proteins from degradation by protease and from heat inactivation [29]. Moreover, loaded proteins are released from the particles in a controlled manner, probably through enzymatic degradation of heparin and protamine. A study reported that the subcutaneously injected heparin–protamine particles disappeared visually after 14 d [28]. This section introduces recent studies that further investigated heparin–protamine particles as drug carriers for clinical application. Notably, heparin–protamine particles have sometimes been subjected to chemical and nonchemical modification to develop advanced drug carriers.
2.1. Intact (Nonmodified) Particles
The potential of heparin–protamine particles as drug carriers has been investigated, especially for fibroblast growth factor (FGF)-2, a protein that stimulates cell proliferation and is used clinically in wound care [49,50,51]. FGF-2 has the advantageous ability to strongly bind to heparin and heparin-like molecules and, moreover, is activated by its binding to heparin [20,21,52,53]. These characteristics have prompted us to use heparin–protamine particles as carriers of FGF-2 for various applications.
FGF-2-containing heparin–protamine nanoparticles were used for the treatment of crush syndrome [54]. A rat model of crush syndrome was prepared by compressing the hind limbs of anesthetized rats using a device, followed by the local administration of FGF-2-containing heparin–protamine nanoparticles. The treated rats exhibited a higher score in motor function, better blood flow, a higher number of blood vessels, and faster recovery of muscle tissue than rats administered FGF-2 alone (i.e., without heparin and protamine). Another study investigated the potential of heparin–protamine carriers for wound care associated with radiation therapy [55]; although radiation therapy is effective for cancer treatment, radiation exposure tends to cause a delay in wound healing as a side effect. Cutaneous full-thickness defect wounds in the backs of rats were made with a punch and a sharp blade. Although X-ray irradiation delayed wound healing, FGF-2-containing heparin–protamine nanoparticle administration prior to irradiation led to a significantly shorter delay accompanied by vascularization, fibrous tissue formation, and fewer apoptotic dermal fibroblasts. Studies on crush syndrome and irradiated wounds demonstrated that FGF-2-containing heparin–protamine particles can promote the healing of various kinds of injury.
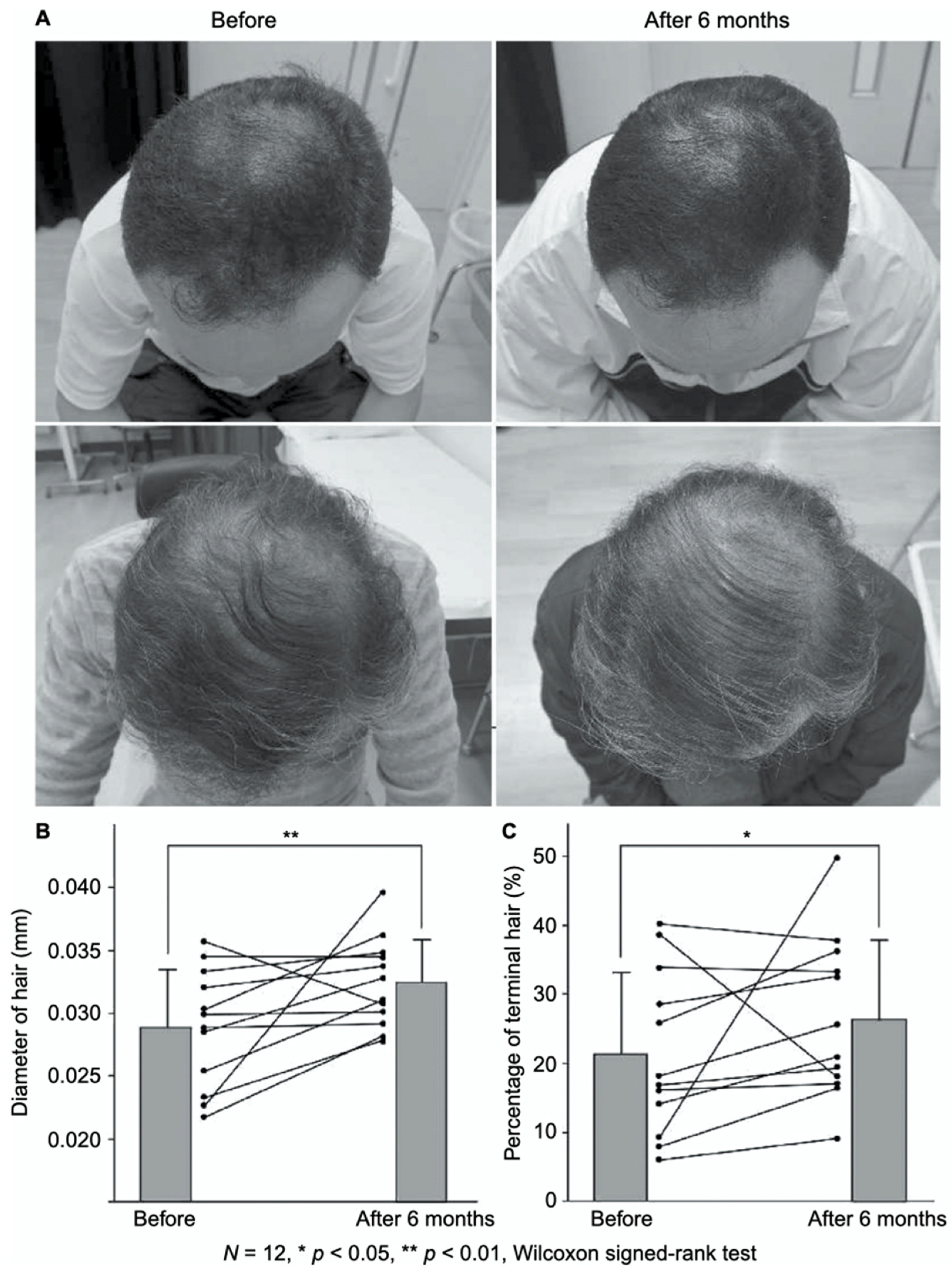
FGF-2-containing heparin–protamine nanoparticles were shown to promote hair growth in a clinical study (Figure 3) [56]. Twelve participants with thin hair transdermally applied FGF-2-containing nanoparticle dispersions to the skin of their scalps twice a day for 6 months, resulting in an increase in the mean diameter of their hairs. Objective improvements in thin hair were observed in two cases. Additionally, nine participants experienced greater bounce and hair resilience. Thus, the transdermal application of FGF-2-containing heparin–protamine nanoparticles to the scalp has potential as a new treatment for alopecia.
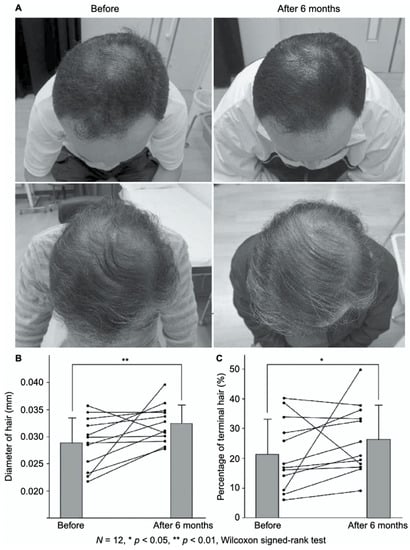
Figure 3.
Promotion of hair growth by FGF-2-containing heparin–protamine nanoparticles. (A) Representative photographs of improved cases after 6 months of treatment. Increases in (B) the hair diameter and (C) the percentage of terminal hair. Adapted from Ref. [56].
Heparin–protamine particles can carry not only FGF-2 but also other proteins. In fact, various growth factors contained in platelet-rich plasma were loaded into heparin–protamine particles [38]. Notably, many growth factors in platelet-rich plasma exhibit heparin-binding ability and are activated upon binding, similar to FGF-2. The resultant complexes containing growth factors from platelet-rich plasma were administered to split-thickness skin graft donor site wounds in male rats [57]. The treatment effectively promoted epithelialization and new vessel formation, suggesting that platelet-rich plasma-containing heparin–protamine particles are useful in healing split-thickness skin wounds.
2.2. Nonchemically Modified Particles
The nonchemical modification of heparin–protamine particles has been demonstrated to be a promising strategy for creating advanced drug carriers. Nonchemical modification strategies are generally advantageous in terms of simplicity compared with the chemical modification strategies described in the next subsection. Additionally, the use of US Food and Drug Administration (FDA)-approved and clinically used drugs (i.e., intact heparin and protamine) may contribute to shortening the time required for safety evaluation, even for off-label use.
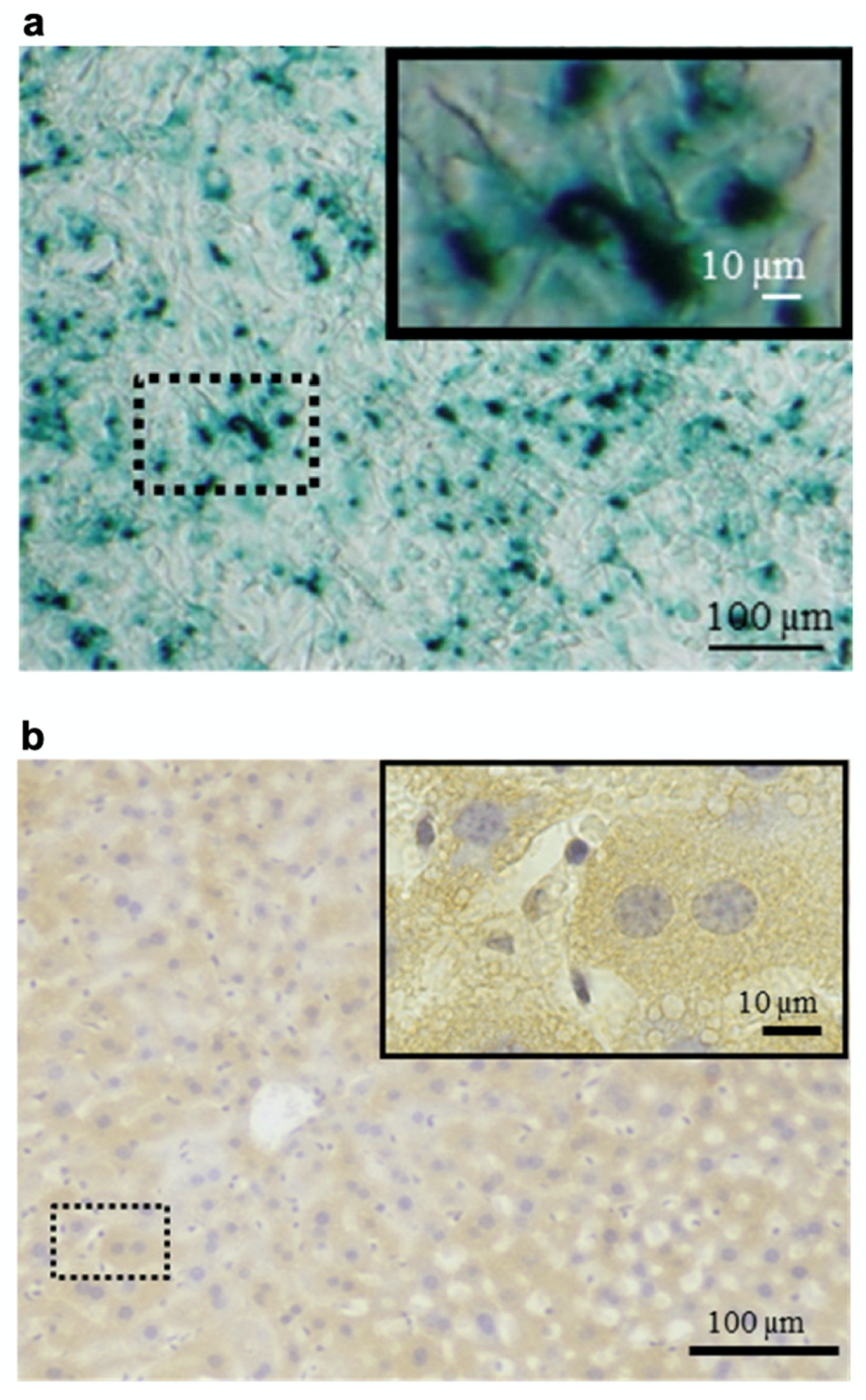
Heparin–protamine complexes tend to have a net negative charge because anionic heparin is the major component, as stated above. The net negative charge was exploited for the nonchemical modification of particles through electrostatic interaction with a cationic peptide, GRKKRRQRRRPPQ (Figure 4) [36]. This sequence is derived from the human immunodeficiency virus-1 (HIV-1) viral protein TAT (trans-activator of transcription) and is known as a cell-penetrating peptide [58,59]. Thus, the cationic peptide was used to endow heparin–protamine nanoparticles with transmembrane transport ability. The cationic peptide was adsorbed onto heparin–protamine nanoparticles, making the net charge of the particles less negative. The resultant peptide-decorated nanoparticles were loaded with model proteins, namely, β-galactosidase and RNase T1. It is noted that both of the loaded proteins have net negative charges, suggesting that those proteins interacted with the polyelectrolyte complexes through local charges in proteins. In vitro experiments revealed that the peptide-decorated nanoparticles transported proteins into cells due to the cell-penetrating ability derived from the attached peptides. Furthermore, targeted protein delivery to mouse hepatocytes was achieved in vivo with peptide-decorated nanoparticles through a hydrodynamics-based injection method.

Figure 4.
Protein delivery using heparin–protamine nanoparticles decorated with a cell-penetrating peptide. (a) In vitro delivery of β-galactosidase into cells. Blue cytoplasmic deposits indicate successful delivery. (b) In vivo delivery of β-galactosidase to mouse hepatocytes through hydrodynamics-based injection. Brown cytoplasmic deposits observed throughout the liver specimens indicate successful delivery. Adapted from Ref. [36].
While proteins can be loaded easily into heparin–protamine particles as described above, small-molecule drug-loading efficiency tends to be relatively low. To improve the efficiency, calcium carbonate (CaCO3) was incorporated into heparin–protamine complexes [60]. The organic–inorganic hybrid drug carriers were prepared by mixing the solution containing protamine and CO32− and the solution containing heparin and Ca2+ under particular conditions, resulting in particles with a vesicular morphology. The presence of CaCO3 increased the loading efficiency of a small-molecule drug, doxorubicin, possibly due to the presence of nanopores in the inorganic–polymer hybrid assemblies and decreased drug permeability by CaCO3. Another small-molecule drug, tariquidar, was also loaded at a low content into the hybrid nanovesicles. In addition to increased drug loading capacity, CaCO3, which has a relatively high water solubility at a low pH, endowed the system with pH sensitivity; the loaded antitumor drugs were preferentially released at lower pH. This pH sensitivity was favorable for drug delivery to tumor sites with a relatively low pH. In vitro experiments with nonresistant cells (HeLa and MCF-7) and drug-resistant cancer cells (MCF-7/ADR) showed that the dual drug-loaded nanovesicles exhibited improved tumor cell inhibitory efficiency, especially for drug-resistant cells. In a later study, a tumor-targeting ligand, biotin, was additionally introduced into hybrid nanovesicles to enhance cell uptake through biotin receptor-mediated endocytosis [61].
2.3. Chemically Modified Particles
Chemical modification is a powerful strategy to generate various functional drug carriers from heparin and protamine. To date, controlled release of small-molecule drugs, oral delivery, and improved anticancer efficacy have been achieved by using chemically modified heparin–protamine particles, as shown below.
Heparin–protamine nanocapsules were chemically crosslinked to serve as carriers of small-molecule drugs [62]. The nanocapsules were prepared by the layer-by-layer assembly of heparin and protamine on a silica template, followed by loading of the anticancer drug doxorubicin and chemical crosslinking. Chemical crosslinking prevented the premature release of loaded doxorubicin, possibly due to decreased permeability of the nanocapsule walls. In vitro experiments using MCF-7 breast cancer cells showed that the nanocapsules were readily internalized and degraded inside the cells, releasing the loaded doxorubicin and causing cancer cell death.
Bile acid-conjugated heparin–protamine nanoparticles were found to be orally available [63]; oral availability is a challenging characteristic for biomolecular nanoparticles due to biological barriers in the body [64,65,66]. After chemical conjugation with bile acid, low-molecular-weight heparin was mixed with protamine for nanoparticulate complex formation [63]. The bile acid-conjugated nanoparticles successfully attached to the enterocyte surface and were then internalized by the cells through interaction between the bile acid on the nanoparticles and the bile acid transporters of the cells. Animal experiments using nude mice revealed that orally administered nanoparticles interacted with bile acid transporters in the ileum and were taken up by epithelial cells.
For antiangiogenic therapy, a low-molecular-weight heparin–taurocholate conjugate—LHT7—which contains ~7 taurocholate groups in a heparin chain, has been developed and shown to act as an angiogenesis inhibitor [67,68,69]. Nevertheless, LHT7 showed toxicological effects including liver functional disturbances and limited anticancer effects [69]. To increase therapeutic duration while decreasing liver toxicity, PEGylated LHT7 was assembled with protamine to form nanoparticulate complexes [70]. The LHT7-containing nanoparticles exhibited improved antiangiogenic effects through the extended circulation and tumor accumulation of nanoparticles and the continued slow release of PEGylated LHT7. Notably, the nanoparticles diffused through leaky tumor blood vessels and extravasated through the blood vessels surrounding the collagen layer. A later study performed PEGylation on protamine, rather than heparin derivatives, to prevent undesirable structural changes to the heparin derivatives by the PEGylation process [71].
More recently, PEGylated LHT7–protamine nanoparticles were used as carriers for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [72], which exhibits selective cytotoxicity to cancer cells for cancer therapeutics but has low stability and a short half-life [73,74,75]. It was found that the loading of TRAIL into the nanoparticles improved both the pharmacokinetic properties and the tumor accumulation rate while maintaining the tumor-selective cytotoxicity of TRAIL [72]. Histological analysis revealed both antiangiogenic efficacy and the homogeneous induction of cancer cell apoptosis by the PEGylated LHT7–TRAIL–protamine nanocomplexes, suggesting synergistic antitumor effects of accumulated TRAIL and LHT7 in tumor tissue.
3. Adhesives for Cells
Heparin–protamine particles have attractive interactions with cells as well as proteins and, consequently, have been investigated as adhesives for cells. Recent studies demonstrated the application of heparin–protamine adhesives to cell culture, cell transplantation, and skin grafting.
3.1. Cell Culture
Heparin–protamine nanoparticles were used as coating materials for cell culture plastic plates to enhance the adhesion and growth of cells [76]. When the nanoparticle dispersions were applied to cell culture plates, the nanoparticles were adsorbed onto the plastic surfaces to form a stable coating layer. Adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells adhered well to the coated plates due to the adhesive properties of the heparin–protamine layer. Moreover, the heparin–protamine coating layers seemed to adsorb various heparin-binding substances from platelet-rich plasma supplemented with FGF-2, stimulating cell proliferation. Importantly, these cells maintained their multilineage potential for differentiation into adipocytes or osteoblasts. A later study demonstrated the three-dimensional culture of various human cells by using human plasma–medium gels containing heparin–protamine microparticles [77].
3.2. Cell Transplantation
Cell transplantation is a promising therapeutic strategy for tissue regeneration [78,79,80]. Nevertheless, there are still challenges, including poor survival and integration of transplanted cells in the targeted tissues. For cell transplantation, heparin–protamine microparticles were used as adhesives for the production of cell aggregates [81]. Human synovial mesenchymal stem cells formed aggregates upon mixing with the adhesive microparticles while maintaining cell viability. When injected into a cartilage defect model in the pig femoral trochlea, cell aggregates with heparin–protamine microparticles were prevented from leaking from the transplanted site. Additionally, further experiments using an osteoarthritic rabbit model suggested that the cell aggregates regenerated cartilage defects even in patients with advanced osteoarthritis, although the mechanisms mediating regeneration of cartilage and cardiomyocytes have yet to be elucidated.
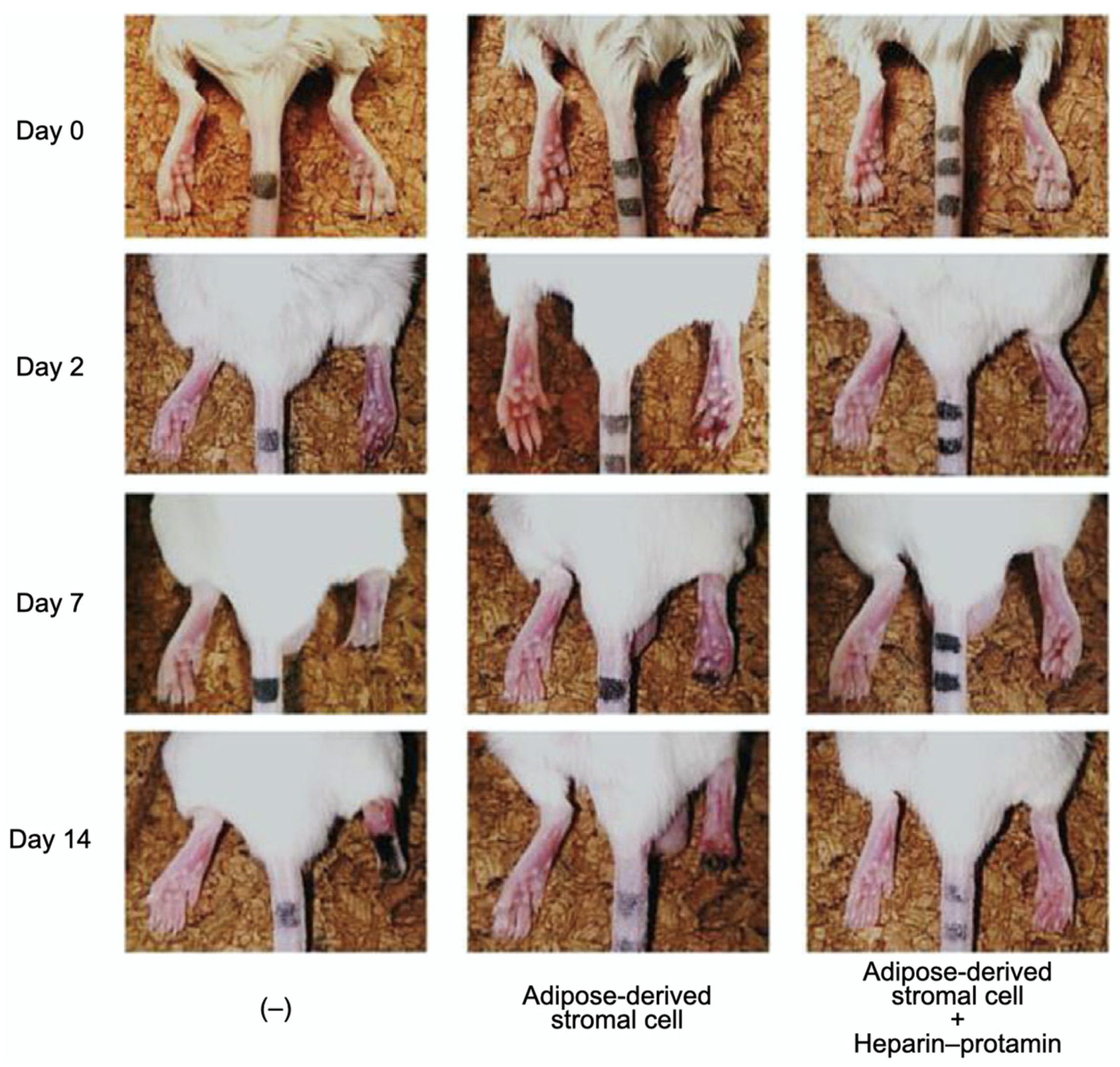
Adipose-derived stromal cell aggregates with heparin–protamine particles were shown to ameliorate limb ischemia in a mouse model (Figure 5) [82]. The cell aggregates were allotransplanted into unilateral hindlimb ischemic muscles induced in adult mice by ligation of the iliac artery and hindlimb vein. Cell transplantation promoted neovascularization and prevented ischemic limb loss. Heparin–protamine particles seemed not only to induce cell aggregate formation but also to immobilize, retain, and gradually release various heparin-binding growth factors from adipose-derived stromal cells, leading to sustained vascularization.
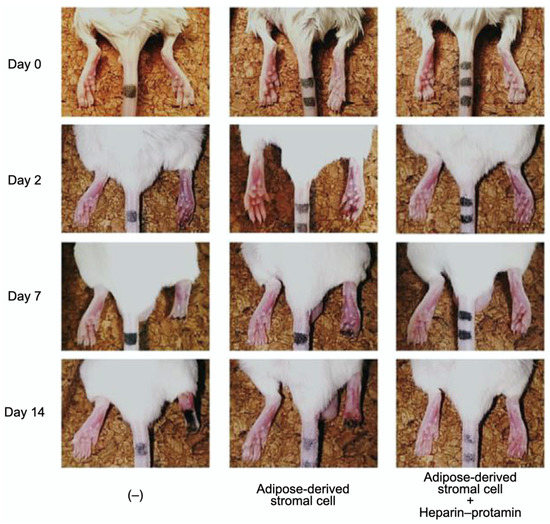
Figure 5.
Amelioration of limb ischemia in a mouse model by the transplantation of adipose-derived stromal cell aggregates with heparin–protamine particles. Adapted from Ref. [82].
3.3. Skin Grafting
Skin grafting is a common technique for treating burns, chronic ulcers, and skin defects after cutaneous surgical procedures [83,84,85]. Nevertheless, skin grafts tend to suffer from stagnated revascularization, which leads to poor outcomes. It was reported that heparin–protamine particles were useful to increase the survival rate of full-thickness skin grafts [86]. Heparin–protamine particles and various growth factors from platelet-rich plasma were injected into full-thickness skin wounds created on the dorsal skin of rats, followed by full-thickness skin grafting. This therapeutic approach effectively promoted the survival rate of full-thickness skin grafts with increased blood flow and new vessel formation at the grafting site.
4. Cell Labeling
Heparin–protamine complexes were reported to facilitate cell labeling with iron oxide nanoparticles as described below. This cell labeling method found applications in the magnetic resonance imaging (MRI) and magnetic targeting of transplanted cells.
4.1. MRI
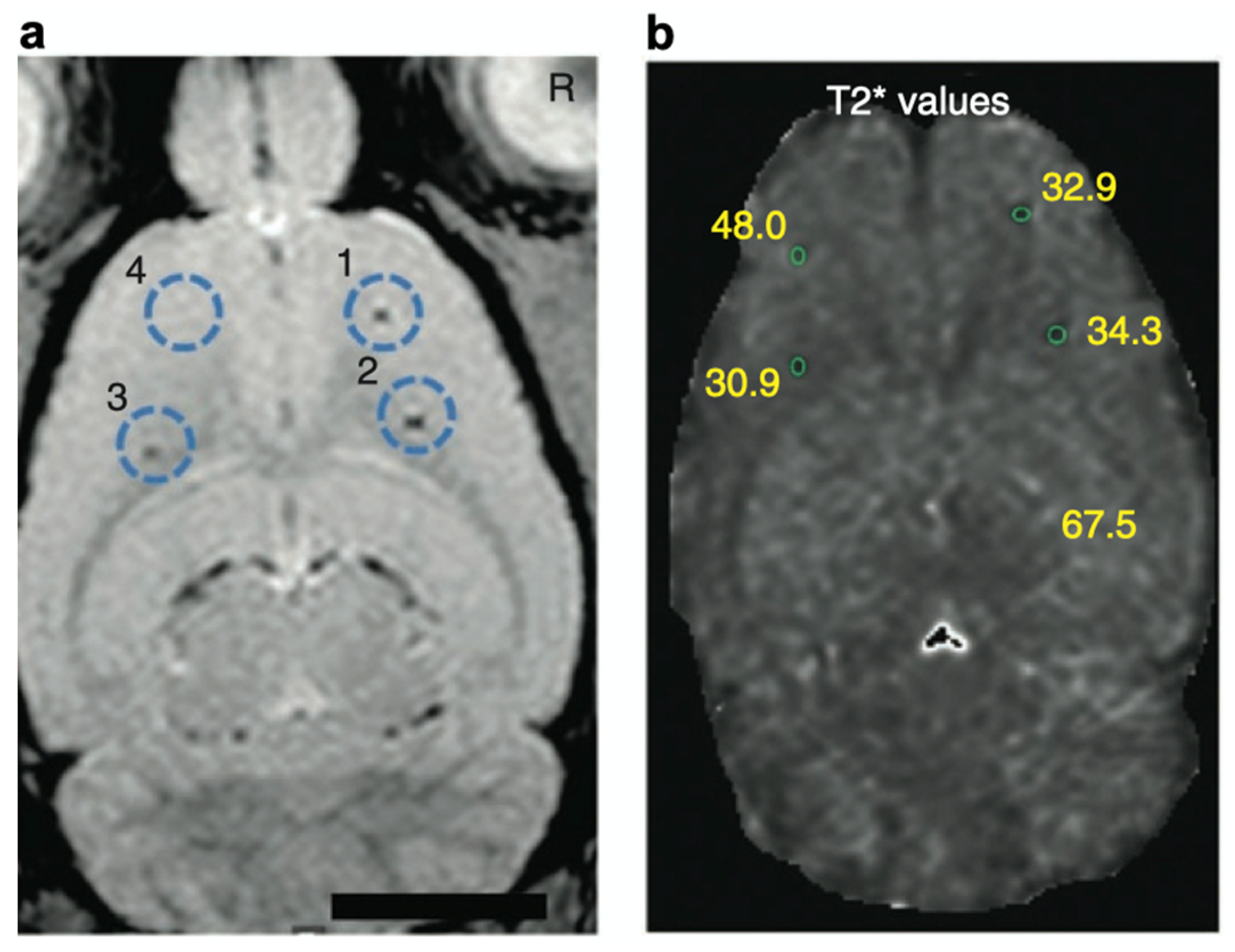
Cell-based therapies have attracted considerable attention in regenerative medicine [87,88]. To understand the therapeutic effects, noninvasive imaging approaches have been developed for monitoring the migration of cell products [89,90]. In this context, heparin and protamine were employed to label cells with ferumoxytol, a superparamagnetic iron oxide nanoparticle, for in vivo MRI (Figure 6) [91]. It should be highlighted that all of the components—namely, heparin, protamine, and ferumoxytol—are FDA-approved drugs, although this use is off label. As a cell labeling experiment, heparin, protamine and ferumoxytol were added to hematopoietic stem cells, neural stem cells, bone marrow stromal cells, and T cells. As a result, ternary nanocomplexes composed of heparin, protamine, and ferumoxytol were internalized into the endosomes of those cells. No long-term effect or toxicity on cellular physiology or function was observed for the cells labeled with the ternary nanocomplexes. In vivo MRI successfully visualized labeled human bone marrow stromal cells that had been intracranially implanted in rat brains.
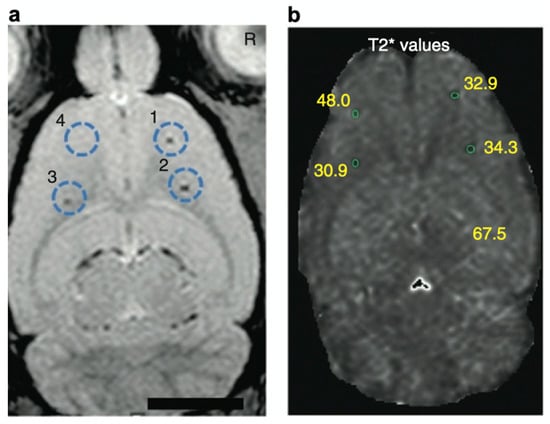
Figure 6.
In vivo magnetic resonance visualization of intracranially implanted cells labeled with heparin–protamine–ferumoxytol complexes. (a) A magnetic resonance image. Circles indicate injection sites of the labeled cells. (b) Calculated T2* map with T2* values at each injection site. Adapted by permission from Nature Publishing Group: Springer Nature Ref. [91], Copyright 2012.
After pioneering work, fundamental and preclinical studies have been conducted on the clinical use of the heparin–protamine–ferumoxytol cell labeling system for MRI [92,93,94,95]. A preclinical study optimized the labeling protocol and investigated the efficacy for MRI and the safety/toxicity of this cell labeling system [92]. Neural stem cells labeled through the optimized protocol were viable and proliferative and retained their tumor tropism in vitro. MRI revealed the dynamic in vivo distribution of the labeled cells after intracerebral or intravenous injection into glioma-bearing mice. Preclinical studies of the labeled cells intracerebrally administered to mice showed no significant clinical or behavioral changes, no neuronal or systemic toxicities, and no abnormal accumulation of iron in the liver or spleen. This report has led to a clinical trial of the heparin–protamine–ferumoxytol cell labeling system for posttransplant MRI visualization and tracking.
4.2. Magnetic Targeting
Stem cell transplantation is a promising therapeutic strategy for acute or chronic ischemic cardiomyopathy [96]. Nevertheless, its efficacy tends to suffer from the low efficiency of cell retention and engraftment, partly due to the “wash-out” of cells by coronary blood flow and heart contraction [97]. To overcome this issue, the magnetically targeted delivery of cells labeled with heparin–protamine–ferumoxytol complexes was investigated [98]. Rat cardiosphere-derived stem cells were labeled with ternary complexes and intracoronarily infused into syngeneic rats. Magnetic targeting successfully increased the cardiac retention of the transplanted cells without cardiac inflammation and iron overload, leading to attenuated left ventricular remodeling and therapeutic benefit.
5. Summary and Outlook
This review summarizes recent research progress on heparin–protamine particles for drug carriers, cell adhesives, and cell labels (Table 1). One of the most important characteristics of the biomolecular polyelectrolyte complex is the adhesive property, which is manifested via electrostatic interactions and other intermolecular interactions. This characteristic allows the loading of various substances, such as proteins and nanoparticles, and the adhesion of cells. Consequently, heparin–protamine particles are potentially versatile in various biomedical fields from drug delivery and regenerative medicine to plastic surgery. The fact that both components are commercially available as pharmaceuticals and are clinically used in surgery suggests that this multicomponent biomolecular system shows great promise for practical applications. In fact, some applications introduced in this review are under clinical or preclinical investigation.

Table 1.
Recent studies on the biomedical application of heparin–protamine particles.
Despite recent progress, there is still plenty of room for further research into heparin–protamine particles. Their fundamental characteristics, including their internal structure, interaction with proteins and other substances, physicochemical and biological stability, and pharmacokinetics, have yet to be fully revealed. Furthermore, the modification of heparin–protamine particles is still in the early stages of research. We are especially interested in nonchemical modification with poly- and oligoelectrolytes, which offer a large variety of functionalities and can be readily incorporated into polyelectrolyte complexes via electrostatic adsorption. Attractive candidates include synthetic aptamers [99] and peptide growth factors [100], which will endow heparin–protamine complexes with excellent biofunctionalities. The resulting functionalized particles will find novel biomedical applications. Further safety testing is necessary for heparin–protamine particles; although heparin and protamine are FDA-approved drugs in clinical use, the biomedical applications described in this review are off-label uses. Nevertheless, the fact that both components have been FDA-approved for some purposes will contribute to shortening the time required for safety evaluation of the particles.
It appears that heparin–protamine particles have been developed mostly in biomedical engineering and medicine. Nevertheless, further investigations from the perspective of molecular self-assembly may lead to innovations in assembled heparin–protamine materials. It is hoped that this review will inspire polymer and materials scientists to contribute to developing advanced biomedical materials via heparin–protamine coassembly.
Author Contributions
Conceptualization, H.M., M.I. and S.N.; writing—original draft preparation, Y.H.; writing—review and editing, H.M., M.I. and S.N.; funding acquisition, Y.H. and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly supported by JSPS KAKENHI Grant Numbers JP21K14688 for Y.H. and JP20K21912 for H.M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kunitake, T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew. Chem. Int. Ed. 1992, 31, 709–726. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef]
- Tørring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of complex structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef]
- Hata, Y.; Serizawa, T. Self-assembly of cellulose for creating green materials with tailor-made nanostructures. J. Mater. Chem. B 2021, 9, 3944–3966. [Google Scholar] [CrossRef]
- Hata, Y.; Serizawa, T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull. Chem. Soc. Jpn. 2021, 94, 2279–2289. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- McMillan, J.R.; Hayes, O.G.; Winegar, P.H.; Mirkin, C.A. Protein materials engineering with DNA. Acc. Chem. Res. 2019, 52, 1939–1948. [Google Scholar] [CrossRef]
- Stephanopoulos, N. Hybrid nanostructures from the self-assembly of proteins and DNA. Chem 2020, 6, 364–405. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef]
- Ishihara, M.; Shaklee, P.N.; Yang, Z.; Liang, W.; Wei, Z.; Stack, R.J.; Holme, K. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology 1994, 4, 451–458. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K. Structure and function of heparin and heparan sulfate; heparinoid library and modification of FGF-activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef]
- Park, K.W. Protamine and protamine reactions. Int. Anesthesiol. Clin. 2004, 42, 135–145. [Google Scholar] [CrossRef]
- Maurer, J.; Haselbach, S.; Klein, O.; Baykut, D.; Vogel, V.; Mäntele, W. Analysis of the complex formation of heparin with protamine by light scattering and analytical ultracentrifugation: Implications for blood coagulation management. J. Am. Chem. Soc. 2011, 133, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Hogwood, J.; Mulloy, B.; Gray, E. Precipitation and neutralization of heparin from different sources by protamine sulfate. Pharmaceuticals 2017, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, P.; Matoušovic, K.; Horáček, V. Protamine-heparin aggregates—Their fine structure, histochemistry, and renal deposition. Virchows Arch. B Cell Pathol. 1982, 40, 81–98. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nakamura, S.; Ohno, C.; Horio, T.; Masanori, F.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. Part A 2009, 91, 814–823. [Google Scholar] [CrossRef]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Mori, Y.; Hattori, H.; Fujita, M.; Nakamura, S. Novel experimental and clinical therapeutic uses of low-molecular-weight heparin/protamine microparticles. Pharmaceutics 2012, 4, 42–57. [Google Scholar] [CrossRef]
- Nemeno, J.G.E.; Lee, S.; Yang, W.; Lee, K.M.; Lee, J.I. Applications and implications of heparin and protamine in tissue engineering and regenerative medicine. Biomed Res. Int. 2014, 2014, 936196. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Nakamura, S.; Shimizu, M. Biomedical application of low molecular weight heparin/protamine nano/micro particles as cell- and growth factor-carriers and coating matrix. Int. J. Mol. Sci. 2015, 16, 11785–11803. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid complex-based heparin-binding cytokines and cell delivery carriers. Molecules 2019, 24, 4630. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost. 2000, 84, 1052–1056. [Google Scholar] [PubMed]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Magnus Ohman, E.; Dalen, J.E. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef]
- Nakamura, S.; Ando, N.; Ishihara, M.; Sato, M. Development of novel heparin/protamine nanoparticles useful for delivery of exogenous proteins in vitro and in vivo. Nanomaterials 2020, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–polyelectrolyte complexes and micellar assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Takikawa, M.; Nakamura, S.I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 373–380. [Google Scholar] [CrossRef]
- Takikawa, M.; Sumi, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J. Surg. Res. 2011, 170, e189–e196. [Google Scholar]
- Takikawa, M.; Nakamura, S.; Nakamura, S.; Ishirara, M.; Kishimoto, S.; Sasaki, K.; Yanagibayashi, S.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol. Surg. 2011, 37, 1721–1729. [Google Scholar] [CrossRef]
- Nakamura, S.; Takikawa, M.; Ishihara, M.; Nakayama, T.; Kishimoto, S.; Isoda, S.; Ozeki, Y.; Sato, M.; Maehara, T. Delivery system for autologous growth factors fabricated with low-molecular-weight heparin and protamine to attenuate ischemic hind-limb loss in a mouse model. J. Artif. Organs 2012, 15, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ishihara, M.; Takikawa, M.; Kishimoto, S.; Isoda, S.; Fujita, M.; Sato, M.; Maehara, T. Attenuation of limb loss in an experimentally induced hindlimb ischemic model by fibroblast growth factor-2/fragmin/protamine microparticles as a delivery system. Tissue Eng. Part A 2012, 18, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Ishihara, M.; Nakamura, S.; Fujita, M.; Takikawa, M.; Sumi, Y.; Kiyosawa, T.; Sato, T.; Kanatani, Y. Fragmin/protamine microparticles to adsorb and protect HGF and to function as local HGF carriers in vivo. Acta Biomater. 2013, 9, 4763–4770. [Google Scholar] [CrossRef]
- Sumi, Y.; Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Takikawa, M.; Azuma, R.; Nakamura, S.; Fujita, M.; Kiyosawa, T. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell transplantation in plasma-gel containing fragmin/protamine microparticles. Ann. Plast. Surg. 2014, 72, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Ishihara, M.; Takabayashi, Y.; Sumi, Y.; Takikawa, M.; Yoshida, R.; Nakamura, S.; Hattori, H.; Yanagibayashi, S.; Yamamoto, N.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with PRP-containing fragmin/protamine microparticles (PRP&F/P MPs). J. Plast. Surg. Hand Surg. 2015, 49, 268–274. [Google Scholar] [PubMed]
- Kishimoto, S.; Hattori, H.; Nakamura, S.; Amano, Y.; Kanatani, Y.; Tanaka, Y.; Mori, Y.; Harada, Y.; Tagawa, M.; Ishihara, M. Expansion and characterization of human bone marrow-derived mesenchymal stem cells cultured on fragmin/protamine microparticle-coated matrix with fibroblast growth factor-2 in low serum medium. Tissue Eng. Part C Methods 2009, 15, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kishimoto, S.; Nakamura, S.; Nambu, M.; Fujita, M.; Tanaka, Y.; Mori, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J. Biomed. Mater. Res. Part A 2010, 92, 1614–1622. [Google Scholar] [CrossRef]
- Kumano, I.; Kishimoto, S.; Nakamura, S.; Hattori, H.; Tanaka, Y.; Nakata, M.; Sato, T.; Fujita, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles (F/P MPs) as cell carriers enhance the formation and growth of tumors in vivo. Cell. Mol. Bioeng. 2011, 4, 476–483. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Fibroblast growth factor and its involvement in developmental processes. Curr. Top. Dev. Biol. 1990, 24, 57–93. [Google Scholar]
- Akita, S.; Akino, K.; Hirano, A. Basic fibroblast growth factor in scarless wound healing. Adv. Wound Care 2013, 2, 44–49. [Google Scholar] [CrossRef]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trends Glycosci. Glycotechnol. 1993, 5, 343–354. [Google Scholar] [CrossRef]
- Ishihara, M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology 1994, 4, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Nakamura, S.; Ishihara, M.; Takabayashi, Y.; Fujita, M.; Hattori, H.; Kushibiki, T.; Ishihara, M. Improved angiogenesis and healing in crush syndrome by fibroblast growth factor-2-containing low-molecular-weight heparin (Fragmin)/protamine nanoparticles. J. Surg. Res. 2015, 196, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kinoda, J.; Ishihara, M.; Nakamura, S.; Fujita, M.; Fukuda, K.; Sato, Y.; Yokoe, H. Protective effect of FGF-2 and low-molecular-weight heparin/protamine nanoparticles on radiation-induced healing-impaired wound repair in rats. J. Radiat. Res. 2018, 59, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, Y.; Nambu, M.; Ishihara, M.; Kuwabara, M.; Fukuda, K.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin. Cosmet. Investig. Dermatol. 2016, 9, 127–134. [Google Scholar] [CrossRef]
- Takabayashi, Y.; Ishihara, M.; Sumi, Y.; Takikawa, M.; Nakamura, S.; Kiyosawa, T. Platelet-rich plasma-containing fragmin-protamine micro-nanoparticles promote epithelialization and angiogenesis in split-thickness skin graft donor sites. J. Surg. Res. 2015, 193, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.; Lebleu, B.; Vivès, E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef]
- Torchilin, V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558. [Google Scholar] [CrossRef]
- Gong, M.-Q.; Wu, J.-L.; Chen, B.; Zhuo, R.-X.; Cheng, S.-X. Self-assembled polymer/inorganic hybrid nanovesicles for multiple drug delivery to overcome drug resistance in cancer chemotherapy. Langmuir 2015, 31, 5115–5122. [Google Scholar] [CrossRef]
- Gong, M.-Q.; Wu, C.; He, X.-Y.; Zong, J.-Y.; Wu, J.-L.; Zhuo, R.-X.; Cheng, S.-X. Tumor targeting synergistic drug delivery by self-assembled hybrid nanovesicles to overcome drug resistance. Pharm. Res. 2017, 34, 148–160. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Thomas, M.B.; Pulakkat, S.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Stimuli-responsive protamine-based biodegradable nanocapsules for enhanced bioavailability and intracellular delivery of anticancer agents. J. Nanopart. Res. 2015, 17, 341. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.U.; Kim, K.; Byun, Y. Bile acid transporter mediated endocytosis of oral bile acid conjugated nanocomplex. Biomaterials 2017, 147, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, Y.-S.; Bae, S.M.; Kim, S.K.; Jin, S.; Chung, S.W.; Lee, M.; Moon, H.T.; Jeon, O.-C.; Park, R.W.; et al. Polyproline-type helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int. J. Cancer 2009, 124, 2755–2765. [Google Scholar] [CrossRef]
- Chung, S.W.; Lee, M.; Bae, S.M.; Park, J.; Jeon, O.C.; Lee, H.S.; Choe, H.; Kim, H.S.; Lee, B.S.; Park, R.-W.; et al. Potentiation of anti-angiogenic activity of heparin by blocking the ATIII-interacting pentasaccharide unit and increasing net anionic charge. Biomaterials 2012, 33, 9070–9079. [Google Scholar] [CrossRef]
- Alam, F.; Chung, S.W.; Hwang, S.R.; Kim, J.; Park, J.; Moon, H.T.; Byun, Y. Preliminary safety evaluation of a taurocholate-conjugated low-molecular-weight heparin derivative (LHT7): A potent angiogenesis inhibitor. J. Appl. Toxicol. 2015, 35, 104–115. [Google Scholar] [CrossRef]
- Alam, F.; Al-Hilal, T.A.; Chung, S.W.; Park, J.; Mahmud, F.; Seo, D.; Kim, H.S.; Lee, D.S.; Byun, Y. Functionalized heparin-protamine based self-assembled nanocomplex for efficient anti-angiogenic therapy. J. Control. Release 2015, 197, 180–189. [Google Scholar] [CrossRef]
- Park, J.; Hwang, S.R.; Choi, J.U.; Alam, F.; Byun, Y. Self-assembled nanocomplex of PEGylated protamine and heparin–suramin conjugate for accumulation at the tumor site. Int. J. Pharm. 2018, 535, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Kim, J.; Chung, S.W.; Lee, N.K.; Park, J.; Kweon, S.; Cho, Y.S.; Kim, H.R.; Lim, S.M.; Park, J.W.; et al. Dual mechanistic TRAIL nanocarrier based on PEGylated heparin taurocholate and protamine which exerts both pro-apoptotic and anti-angiogenic effects. J. Control. Release 2021, 336, 181–191. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Herbst, R.S. To kill a tumor cell: The potential of proapoptotic receptor agonists. J. Clin. Investig. 2008, 118, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Kruyt, F.A.E. TRAIL and cancer therapy. Cancer Lett. 2008, 263, 14–25. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Mori, Y.; Takikawa, M.; Hattori, H.; Nakamura, S.; Sato, T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J. Tissue Eng. Regen. Med. 2013, 7, 955–964. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Takikawa, M.; Sumi, Y.; Nakamura, S.; Fujita, M.; Sato, T.; Kiyosawa, T. Three-dimensional culture using human plasma-medium gel with fragmin/protamine microparticles for proliferation of various human cells. Cytotechnology 2014, 66, 791–802. [Google Scholar] [CrossRef]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Yeo, J.E.; Nam, B.M.; Yang, W.; Jo, Y.H.; Lee, S.; Nemeno, J.G.; Kiml, B.Y.; Koh, Y.G.; Lee, J.I. Fragmin/protamine microparticle carriers as a drug repositioning strategy for cell transplantation. Transplant. Proc. 2013, 45, 3122–3126. [Google Scholar] [CrossRef]
- Kishimoto, S.; Inoue, K.; Nakamura, S.; Hattori, H.; Ishihara, M.; Sakuma, M.; Toyoda, S.; Iwaguro, H.; Taguchi, I.; Inoue, T.; et al. Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia. Atherosclerosis 2016, 249, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Ratner, D.; Nelson, B.R. Soft tissue reconstruction with skin grafting. J. Am. Acad. Dermatol. 1992, 27, 151–165. [Google Scholar] [CrossRef]
- Valencia, I.C.; Falabella, A.F.; Eaglstein, W.H. Skin grafting. Dermatol. Clin. 2000, 18, 521–532. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, Y.; Ishihara, M.; Kuwabara, M.; Takikawa, M.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Improved survival of full-thickness skin graft with low-molecular weight heparin-protamine micro/nanoparticles including platelet-rich plasma. Ann. Plast. Surg. 2017, 78, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef]
- Motaln, H.; Schichor, C.; Lah, T.T. Human mesenchymal stem cells and their use in cell-based therapies. Cancer 2010, 116, 2519–2530. [Google Scholar] [CrossRef]
- Bengel, F.M.; Schachinger, V.; Dimmeler, S. Cell-based therapies and imaging in cardiology. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, S404–S416. [Google Scholar] [CrossRef]
- Arbab, A.S.; Janic, B.; Haller, J.; Pawelczyk, E.; Liu, W.; Frank, J.A. In vivo cellular imaging for translational medical research. Curr. Med. Imaging Rev. 2009, 5, 19–38. [Google Scholar] [CrossRef]
- Thu, M.S.; Bryant, L.H.; Coppola, T.; Jordan, E.K.; Budde, M.D.; Lewis, B.K.; Chaudhry, A.; Ren, J.; Varma, N.R.S.; Arbab, A.S.; et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012, 18, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Gutova, M.; Frank, J.A.; D’Apuzzo, M.; Khankaldyyan, V.; Gilchrist, M.M.; Annala, A.J.; Metz, M.Z.; Abramyants, Y.; Herrmann, K.A.; Ghoda, L.Y.; et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: Studies leading to clinical use. Stem Cells Transl. Med. 2013, 2, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhou, X.; Guan, X.; Liu, Y.; Jiang, C.; Liu, J. In vivo tracking of human adipose-derived stem cells labeled with ferumoxytol in rats with middle cerebral artery occlusion by magnetic resonance imaging. Neural Regen. Res. 2015, 10, 909–915. [Google Scholar]
- Bryant, L.H.; Kim, S.J.; Hobson, M.; Milo, B.; Kovacs, Z.I.; Jikaria, N.; Lewis, B.K.; Aronova, M.A.; Sousa, A.A.; Zhang, G.; et al. Physicochemical characterization of ferumoxytol, heparin and protamine nanocomplexes for improved magnetic labeling of stem cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 503–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, J.; Guan, X.; Liu, Y.; Piao, H.; Liu, R.; Zhou, X.; Sun, B.; Du, Y.; Liu, J. Potential role of tracing stem cell transplantation and effects on the immune cell function of ferumoxytol combining with heparin and protamine in vivo/in vitro. Cell Biol. Int. 2017, 41, 423–432. [Google Scholar] [CrossRef]
- Wollert, K.C.; Drexler, H. Clinical applications of stem cells for the heart. Circ. Res. 2005, 96, 151–163. [Google Scholar] [CrossRef]
- Al Kindi, A.; Ge, Y.; Shum-Tim, D.; Chiu, R.C.-J. Cellular cardiomyoplasty: Routes of cell delivery and retention. Front. Biosci. 2008, 13, 2421–2434. [Google Scholar] [CrossRef]
- Vandergriff, A.C.; Hensley, T.M.; Henry, E.T.; Shen, D.; Anthony, S.; Zhang, J.; Cheng, K. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Lin, X.; Takahashi, K.; Campion, S.L.; Liu, Y.; Gustavsen, G.G.; Peña, L.A.; Zamora, P.O. Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo. Int. J. Mol. Med. 2006, 17, 833–839. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).