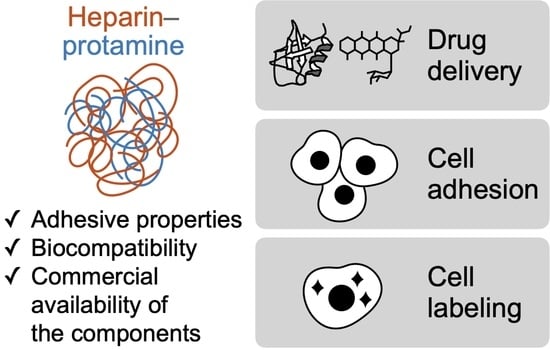
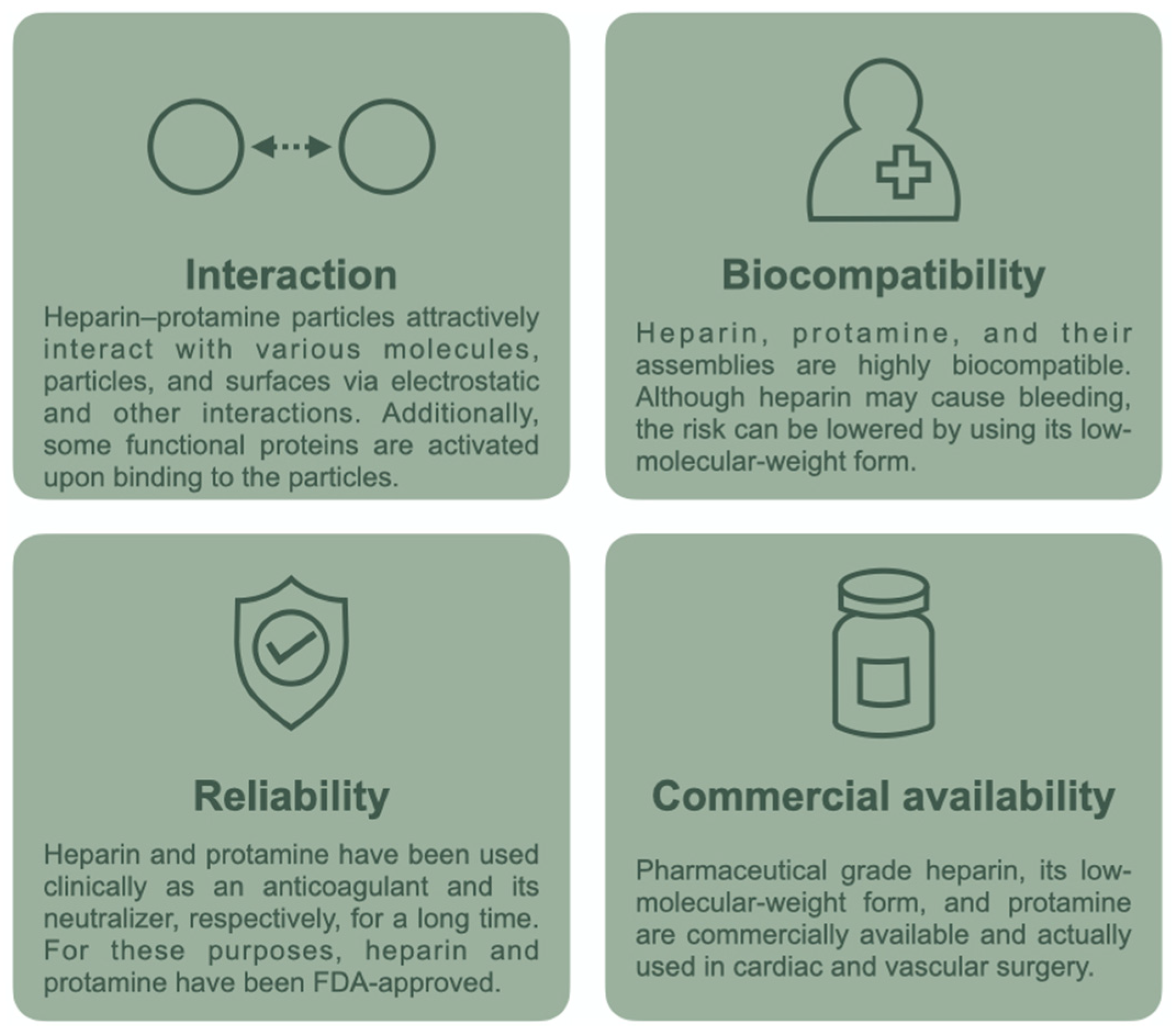
Recent Progress on Heparin–Protamine Particles for Biomedical Application
Abstract
:1. Introduction
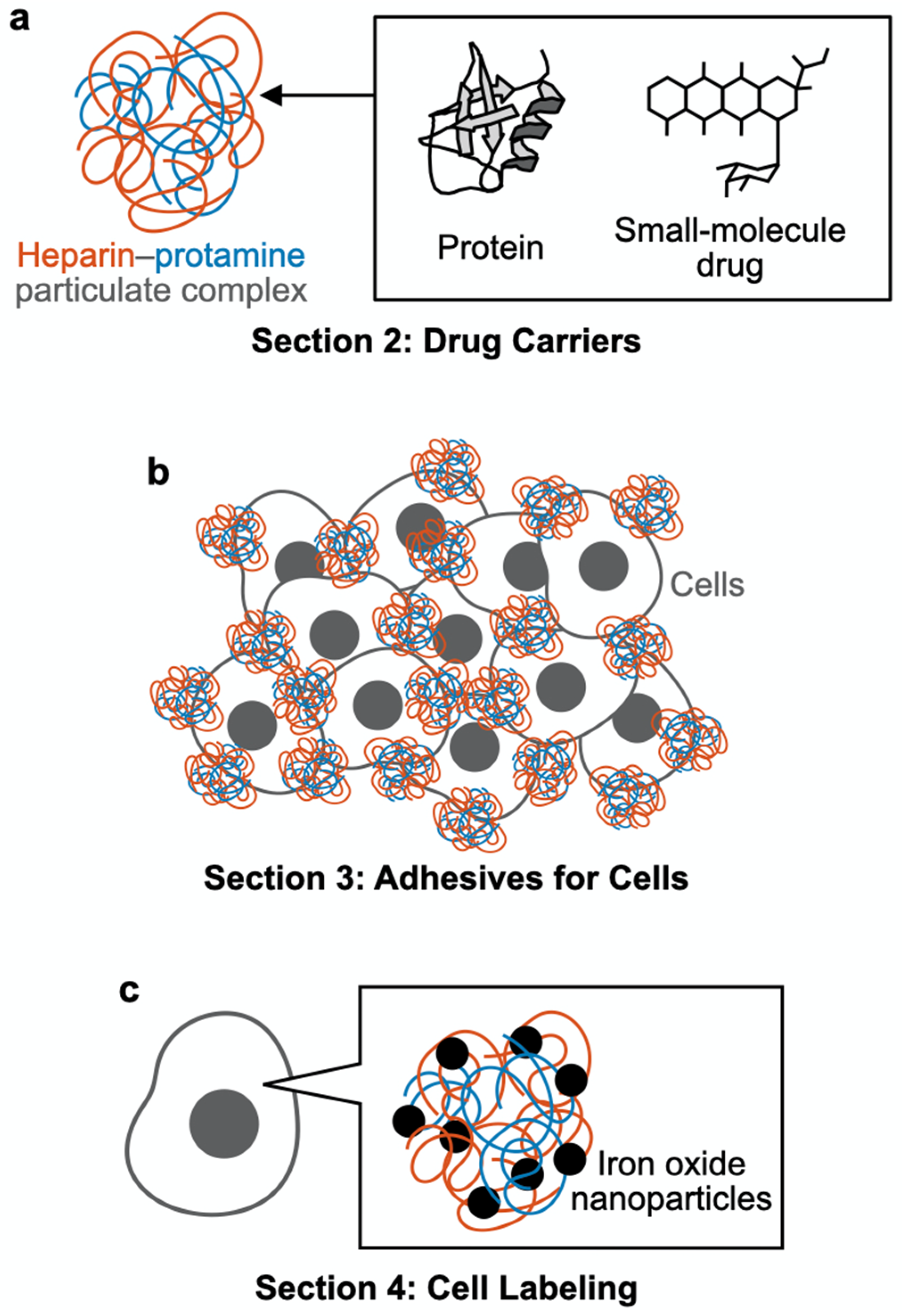
2. Drug Carriers
2.1. Intact (Nonmodified) Particles
2.2. Nonchemically Modified Particles
2.3. Chemically Modified Particles
3. Adhesives for Cells
3.1. Cell Culture
3.2. Cell Transplantation
3.3. Skin Grafting
4. Cell Labeling
4.1. MRI
4.2. Magnetic Targeting
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Kunitake, T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew. Chem. Int. Ed. 1992, 31, 709–726. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonietti, M.; Förster, S. Vesicles and liposomes: A self-assembly principle beyond lipids. Adv. Mater. 2003, 15, 1323–1333. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Pal, S.; Nangreave, J.; Deng, Z.; Liu, Y.; Yan, H. DNA origami with complex curvatures in three-dimensional space. Science 2011, 332, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Tørring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of complex structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef]
- Hata, Y.; Serizawa, T. Self-assembly of cellulose for creating green materials with tailor-made nanostructures. J. Mater. Chem. B 2021, 9, 3944–3966. [Google Scholar] [CrossRef]
- Hata, Y.; Serizawa, T. Robust gels composed of self-assembled cello-oligosaccharide networks. Bull. Chem. Soc. Jpn. 2021, 94, 2279–2289. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- McMillan, J.R.; Hayes, O.G.; Winegar, P.H.; Mirkin, C.A. Protein materials engineering with DNA. Acc. Chem. Res. 2019, 52, 1939–1948. [Google Scholar] [CrossRef]
- Stephanopoulos, N. Hybrid nanostructures from the self-assembly of proteins and DNA. Chem 2020, 6, 364–405. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Shaklee, P.N.; Yang, Z.; Liang, W.; Wei, Z.; Stack, R.J.; Holme, K. Structural features in heparin which modulate specific biological activities mediated by basic fibroblast growth factor. Glycobiology 1994, 4, 451–458. [Google Scholar] [CrossRef]
- Ishihara, M.; Ono, K. Structure and function of heparin and heparan sulfate; heparinoid library and modification of FGF-activities. Trends Glycosci. Glycotechnol. 1998, 10, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and side-effects of protamine in cardiac surgery: A narrative review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef] [Green Version]
- Park, K.W. Protamine and protamine reactions. Int. Anesthesiol. Clin. 2004, 42, 135–145. [Google Scholar] [CrossRef]
- Maurer, J.; Haselbach, S.; Klein, O.; Baykut, D.; Vogel, V.; Mäntele, W. Analysis of the complex formation of heparin with protamine by light scattering and analytical ultracentrifugation: Implications for blood coagulation management. J. Am. Chem. Soc. 2011, 133, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Hogwood, J.; Mulloy, B.; Gray, E. Precipitation and neutralization of heparin from different sources by protamine sulfate. Pharmaceuticals 2017, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmann, P.; Matoušovic, K.; Horáček, V. Protamine-heparin aggregates—Their fine structure, histochemistry, and renal deposition. Virchows Arch. B Cell Pathol. 1982, 40, 81–98. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanatani, Y.; Kishimoto, S.; Nakamura, S.; Ohno, C.; Horio, T.; Masanori, F.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J. Biomed. Mater. Res. Part A 2009, 91, 814–823. [Google Scholar] [CrossRef]
- Mori, Y.; Nakamura, S.; Kishimoto, S.; Kawakami, M.; Suzuki, S.; Matsui, T.; Ishihara, M. Preparation and characterization of low-molecular-weight heparin/protamine nanoparticles (LMW-H/P NPs) as FGF-2 carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Mori, Y.; Hattori, H.; Fujita, M.; Nakamura, S. Novel experimental and clinical therapeutic uses of low-molecular-weight heparin/protamine microparticles. Pharmaceutics 2012, 4, 42–57. [Google Scholar] [CrossRef]
- Nemeno, J.G.E.; Lee, S.; Yang, W.; Lee, K.M.; Lee, J.I. Applications and implications of heparin and protamine in tissue engineering and regenerative medicine. Biomed Res. Int. 2014, 2014, 936196. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Nakamura, S.; Shimizu, M. Biomedical application of low molecular weight heparin/protamine nano/micro particles as cell- and growth factor-carriers and coating matrix. Int. J. Mol. Sci. 2015, 16, 11785–11803. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Nakamura, S.; Sato, Y.; Takayama, T.; Fukuda, K.; Fujita, M.; Murakami, K.; Yokoe, H. Heparinoid complex-based heparin-binding cytokines and cell delivery carriers. Molecules 2019, 24, 4630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost. 2000, 84, 1052–1056. [Google Scholar] [PubMed]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Magnus Ohman, E.; Dalen, J.E. Heparin and low-molecular-weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Ando, N.; Ishihara, M.; Sato, M. Development of novel heparin/protamine nanoparticles useful for delivery of exogenous proteins in vitro and in vivo. Nanomaterials 2020, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–polyelectrolyte complexes and micellar assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Takikawa, M.; Nakamura, S.I.; Nakamura, S.; Nambu, M.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Doumoto, T.; Yanagibayashi, S.; Azuma, R.; et al. Enhancement of vascularization and granulation tissue formation by growth factors in human platelet-rich plasma-containing fragmin/protamine microparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 373–380. [Google Scholar] [CrossRef]
- Takikawa, M.; Sumi, Y.; Ishihara, M.; Kishimoto, S.; Nakamura, S.; Yanagibayashi, S.; Hattori, H.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. PRP&F/P MPs improved survival of dorsal paired pedicle skin flaps in rats. J. Surg. Res. 2011, 170, e189–e196. [Google Scholar]
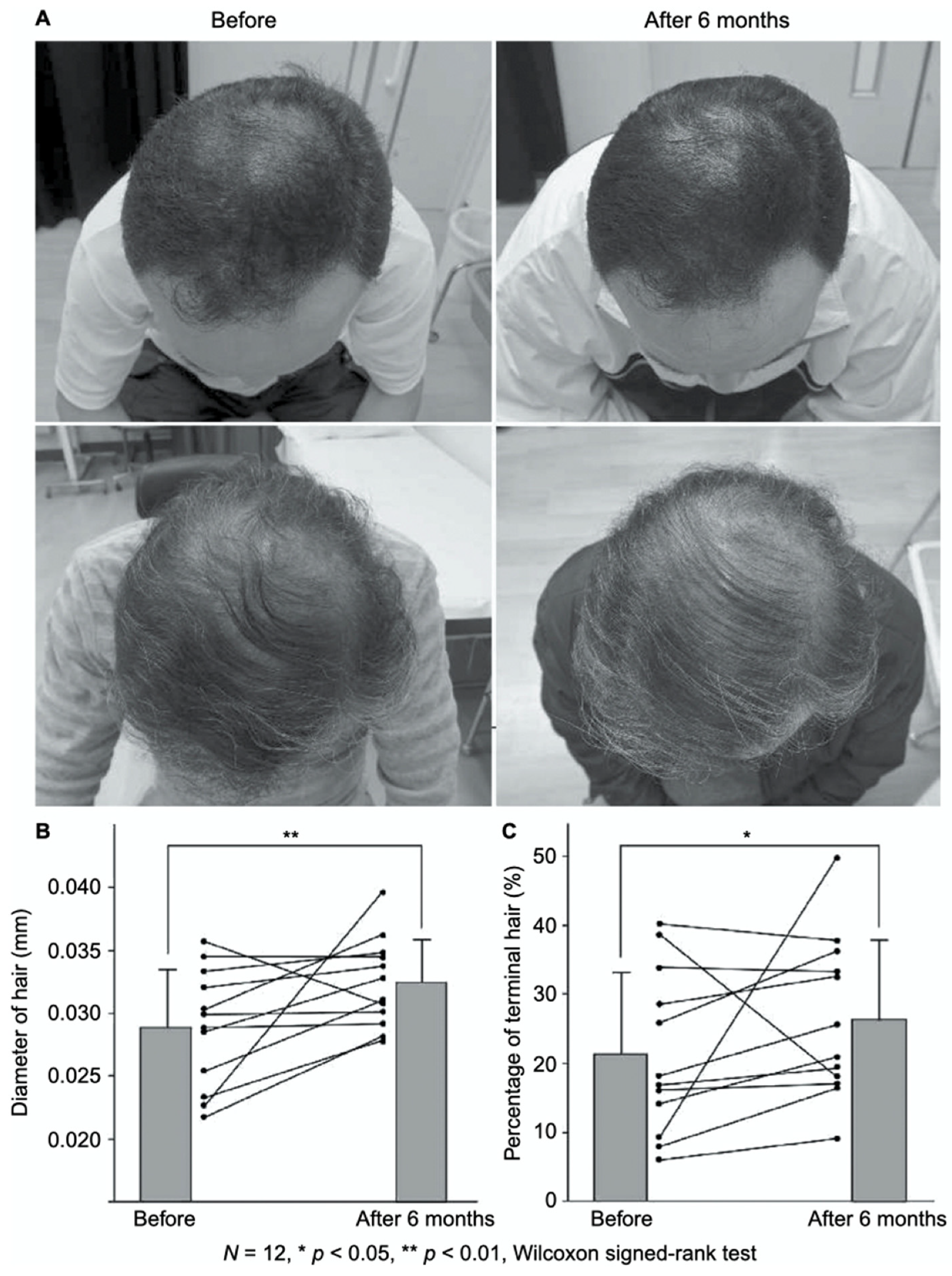
- Takikawa, M.; Nakamura, S.; Nakamura, S.; Ishirara, M.; Kishimoto, S.; Sasaki, K.; Yanagibayashi, S.; Azuma, R.; Yamamoto, N.; Kiyosawa, T. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol. Surg. 2011, 37, 1721–1729. [Google Scholar] [CrossRef]
- Nakamura, S.; Takikawa, M.; Ishihara, M.; Nakayama, T.; Kishimoto, S.; Isoda, S.; Ozeki, Y.; Sato, M.; Maehara, T. Delivery system for autologous growth factors fabricated with low-molecular-weight heparin and protamine to attenuate ischemic hind-limb loss in a mouse model. J. Artif. Organs 2012, 15, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ishihara, M.; Takikawa, M.; Kishimoto, S.; Isoda, S.; Fujita, M.; Sato, M.; Maehara, T. Attenuation of limb loss in an experimentally induced hindlimb ischemic model by fibroblast growth factor-2/fragmin/protamine microparticles as a delivery system. Tissue Eng. Part A 2012, 18, 2239–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, S.; Ishihara, M.; Nakamura, S.; Fujita, M.; Takikawa, M.; Sumi, Y.; Kiyosawa, T.; Sato, T.; Kanatani, Y. Fragmin/protamine microparticles to adsorb and protect HGF and to function as local HGF carriers in vivo. Acta Biomater. 2013, 9, 4763–4770. [Google Scholar] [CrossRef]
- Sumi, Y.; Ishihara, M.; Kishimoto, S.; Takikawa, M.; Hattori, H.; Takikawa, M.; Azuma, R.; Nakamura, S.; Fujita, M.; Kiyosawa, T. Effective wound healing in streptozotocin-induced diabetic rats by adipose-derived stromal cell transplantation in plasma-gel containing fragmin/protamine microparticles. Ann. Plast. Surg. 2014, 72, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Ishihara, M.; Takabayashi, Y.; Sumi, Y.; Takikawa, M.; Yoshida, R.; Nakamura, S.; Hattori, H.; Yanagibayashi, S.; Yamamoto, N.; et al. Enhanced healing of mitomycin C-treated healing-impaired wounds in rats with PRP-containing fragmin/protamine microparticles (PRP&F/P MPs). J. Plast. Surg. Hand Surg. 2015, 49, 268–274. [Google Scholar] [PubMed]
- Kishimoto, S.; Hattori, H.; Nakamura, S.; Amano, Y.; Kanatani, Y.; Tanaka, Y.; Mori, Y.; Harada, Y.; Tagawa, M.; Ishihara, M. Expansion and characterization of human bone marrow-derived mesenchymal stem cells cultured on fragmin/protamine microparticle-coated matrix with fibroblast growth factor-2 in low serum medium. Tissue Eng. Part C Methods 2009, 15, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kishimoto, S.; Nakamura, S.; Nambu, M.; Fujita, M.; Tanaka, Y.; Mori, Y.; Tagawa, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles as cell carriers to enhance viability of adipose-derived stromal cells and their subsequent effect on in vivo neovascularization. J. Biomed. Mater. Res. Part A 2010, 92, 1614–1622. [Google Scholar] [CrossRef]
- Kumano, I.; Kishimoto, S.; Nakamura, S.; Hattori, H.; Tanaka, Y.; Nakata, M.; Sato, T.; Fujita, M.; Maehara, T.; Ishihara, M. Fragmin/protamine microparticles (F/P MPs) as cell carriers enhance the formation and growth of tumors in vivo. Cell. Mol. Bioeng. 2011, 4, 476–483. [Google Scholar] [CrossRef]
- Gospodarowicz, D. Fibroblast growth factor and its involvement in developmental processes. Curr. Top. Dev. Biol. 1990, 24, 57–93. [Google Scholar]
- Akita, S.; Akino, K.; Hirano, A. Basic fibroblast growth factor in scarless wound healing. Adv. Wound Care 2013, 2, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trends Glycosci. Glycotechnol. 1993, 5, 343–354. [Google Scholar] [CrossRef]
- Ishihara, M. Structural requirements in heparin for binding and activation of FGF-1 and FGF-4 are different from that for FGF-2. Glycobiology 1994, 4, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Nakamura, S.; Ishihara, M.; Takabayashi, Y.; Fujita, M.; Hattori, H.; Kushibiki, T.; Ishihara, M. Improved angiogenesis and healing in crush syndrome by fibroblast growth factor-2-containing low-molecular-weight heparin (Fragmin)/protamine nanoparticles. J. Surg. Res. 2015, 196, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kinoda, J.; Ishihara, M.; Nakamura, S.; Fujita, M.; Fukuda, K.; Sato, Y.; Yokoe, H. Protective effect of FGF-2 and low-molecular-weight heparin/protamine nanoparticles on radiation-induced healing-impaired wound repair in rats. J. Radiat. Res. 2018, 59, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takabayashi, Y.; Nambu, M.; Ishihara, M.; Kuwabara, M.; Fukuda, K.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Enhanced effect of fibroblast growth factor-2-containing dalteparin/protamine nanoparticles on hair growth. Clin. Cosmet. Investig. Dermatol. 2016, 9, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Takabayashi, Y.; Ishihara, M.; Sumi, Y.; Takikawa, M.; Nakamura, S.; Kiyosawa, T. Platelet-rich plasma-containing fragmin-protamine micro-nanoparticles promote epithelialization and angiogenesis in split-thickness skin graft donor sites. J. Surg. Res. 2015, 193, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.; Lebleu, B.; Vivès, E. Tat peptide-mediated cellular delivery: Back to basics. Adv. Drug Deliv. Rev. 2005, 57, 559–577. [Google Scholar] [CrossRef]
- Torchilin, V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558. [Google Scholar] [CrossRef]
- Gong, M.-Q.; Wu, J.-L.; Chen, B.; Zhuo, R.-X.; Cheng, S.-X. Self-assembled polymer/inorganic hybrid nanovesicles for multiple drug delivery to overcome drug resistance in cancer chemotherapy. Langmuir 2015, 31, 5115–5122. [Google Scholar] [CrossRef]
- Gong, M.-Q.; Wu, C.; He, X.-Y.; Zong, J.-Y.; Wu, J.-L.; Zhuo, R.-X.; Cheng, S.-X. Tumor targeting synergistic drug delivery by self-assembled hybrid nanovesicles to overcome drug resistance. Pharm. Res. 2017, 34, 148–160. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Thomas, M.B.; Pulakkat, S.; Gnanadhas, D.P.; Chakravortty, D.; Raichur, A.M. Stimuli-responsive protamine-based biodegradable nanocapsules for enhanced bioavailability and intracellular delivery of anticancer agents. J. Nanopart. Res. 2015, 17, 341. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.U.; Kim, K.; Byun, Y. Bile acid transporter mediated endocytosis of oral bile acid conjugated nanocomplex. Biomaterials 2017, 147, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelikin, A.N.; Ehrhardt, C.; Healy, A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016, 8, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, Y.-S.; Bae, S.M.; Kim, S.K.; Jin, S.; Chung, S.W.; Lee, M.; Moon, H.T.; Jeon, O.-C.; Park, R.W.; et al. Polyproline-type helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int. J. Cancer 2009, 124, 2755–2765. [Google Scholar] [CrossRef]
- Chung, S.W.; Lee, M.; Bae, S.M.; Park, J.; Jeon, O.C.; Lee, H.S.; Choe, H.; Kim, H.S.; Lee, B.S.; Park, R.-W.; et al. Potentiation of anti-angiogenic activity of heparin by blocking the ATIII-interacting pentasaccharide unit and increasing net anionic charge. Biomaterials 2012, 33, 9070–9079. [Google Scholar] [CrossRef]
- Alam, F.; Chung, S.W.; Hwang, S.R.; Kim, J.; Park, J.; Moon, H.T.; Byun, Y. Preliminary safety evaluation of a taurocholate-conjugated low-molecular-weight heparin derivative (LHT7): A potent angiogenesis inhibitor. J. Appl. Toxicol. 2015, 35, 104–115. [Google Scholar] [CrossRef]
- Alam, F.; Al-Hilal, T.A.; Chung, S.W.; Park, J.; Mahmud, F.; Seo, D.; Kim, H.S.; Lee, D.S.; Byun, Y. Functionalized heparin-protamine based self-assembled nanocomplex for efficient anti-angiogenic therapy. J. Control. Release 2015, 197, 180–189. [Google Scholar] [CrossRef]
- Park, J.; Hwang, S.R.; Choi, J.U.; Alam, F.; Byun, Y. Self-assembled nanocomplex of PEGylated protamine and heparin–suramin conjugate for accumulation at the tumor site. Int. J. Pharm. 2018, 535, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Kim, J.; Chung, S.W.; Lee, N.K.; Park, J.; Kweon, S.; Cho, Y.S.; Kim, H.R.; Lim, S.M.; Park, J.W.; et al. Dual mechanistic TRAIL nanocarrier based on PEGylated heparin taurocholate and protamine which exerts both pro-apoptotic and anti-angiogenic effects. J. Control. Release 2021, 336, 181–191. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Herbst, R.S. To kill a tumor cell: The potential of proapoptotic receptor agonists. J. Clin. Investig. 2008, 118, 1979–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruyt, F.A.E. TRAIL and cancer therapy. Cancer Lett. 2008, 263, 14–25. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Mori, Y.; Takikawa, M.; Hattori, H.; Nakamura, S.; Sato, T. Effective expansion of human adipose-derived stromal cells and bone marrow-derived mesenchymal stem cells cultured on a fragmin/protamine nanoparticles-coated substratum with human platelet-rich plasma. J. Tissue Eng. Regen. Med. 2013, 7, 955–964. [Google Scholar] [CrossRef]
- Kishimoto, S.; Ishihara, M.; Takikawa, M.; Takikawa, M.; Sumi, Y.; Nakamura, S.; Fujita, M.; Sato, T.; Kiyosawa, T. Three-dimensional culture using human plasma-medium gel with fragmin/protamine microparticles for proliferation of various human cells. Cytotechnology 2014, 66, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Rafii, S.; Lyden, D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003, 9, 702–712. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Mitrousis, N.; Fokina, A.; Shoichet, M.S. Biomaterials for cell transplantation. Nat. Rev. Mater. 2018, 3, 441–456. [Google Scholar] [CrossRef]
- Yeo, J.E.; Nam, B.M.; Yang, W.; Jo, Y.H.; Lee, S.; Nemeno, J.G.; Kiml, B.Y.; Koh, Y.G.; Lee, J.I. Fragmin/protamine microparticle carriers as a drug repositioning strategy for cell transplantation. Transplant. Proc. 2013, 45, 3122–3126. [Google Scholar] [CrossRef]
- Kishimoto, S.; Inoue, K.; Nakamura, S.; Hattori, H.; Ishihara, M.; Sakuma, M.; Toyoda, S.; Iwaguro, H.; Taguchi, I.; Inoue, T.; et al. Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia. Atherosclerosis 2016, 249, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.M.; Ratner, D.; Nelson, B.R. Soft tissue reconstruction with skin grafting. J. Am. Acad. Dermatol. 1992, 27, 151–165. [Google Scholar] [CrossRef]
- Valencia, I.C.; Falabella, A.F.; Eaglstein, W.H. Skin grafting. Dermatol. Clin. 2000, 18, 521–532. [Google Scholar] [CrossRef]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, Y.; Ishihara, M.; Kuwabara, M.; Takikawa, M.; Nakamura, S.; Hattori, H.; Kiyosawa, T. Improved survival of full-thickness skin graft with low-molecular weight heparin-protamine micro/nanoparticles including platelet-rich plasma. Ann. Plast. Surg. 2017, 78, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Motaln, H.; Schichor, C.; Lah, T.T. Human mesenchymal stem cells and their use in cell-based therapies. Cancer 2010, 116, 2519–2530. [Google Scholar] [CrossRef]
- Bengel, F.M.; Schachinger, V.; Dimmeler, S. Cell-based therapies and imaging in cardiology. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, S404–S416. [Google Scholar] [CrossRef] [Green Version]
- Arbab, A.S.; Janic, B.; Haller, J.; Pawelczyk, E.; Liu, W.; Frank, J.A. In vivo cellular imaging for translational medical research. Curr. Med. Imaging Rev. 2009, 5, 19–38. [Google Scholar] [CrossRef] [Green Version]
- Thu, M.S.; Bryant, L.H.; Coppola, T.; Jordan, E.K.; Budde, M.D.; Lewis, B.K.; Chaudhry, A.; Ren, J.; Varma, N.R.S.; Arbab, A.S.; et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012, 18, 463–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutova, M.; Frank, J.A.; D’Apuzzo, M.; Khankaldyyan, V.; Gilchrist, M.M.; Annala, A.J.; Metz, M.Z.; Abramyants, Y.; Herrmann, K.A.; Ghoda, L.Y.; et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: Studies leading to clinical use. Stem Cells Transl. Med. 2013, 2, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhou, X.; Guan, X.; Liu, Y.; Jiang, C.; Liu, J. In vivo tracking of human adipose-derived stem cells labeled with ferumoxytol in rats with middle cerebral artery occlusion by magnetic resonance imaging. Neural Regen. Res. 2015, 10, 909–915. [Google Scholar]
- Bryant, L.H.; Kim, S.J.; Hobson, M.; Milo, B.; Kovacs, Z.I.; Jikaria, N.; Lewis, B.K.; Aronova, M.A.; Sousa, A.A.; Zhang, G.; et al. Physicochemical characterization of ferumoxytol, heparin and protamine nanocomplexes for improved magnetic labeling of stem cells. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Guan, X.; Liu, Y.; Piao, H.; Liu, R.; Zhou, X.; Sun, B.; Du, Y.; Liu, J. Potential role of tracing stem cell transplantation and effects on the immune cell function of ferumoxytol combining with heparin and protamine in vivo/in vitro. Cell Biol. Int. 2017, 41, 423–432. [Google Scholar] [CrossRef]
- Wollert, K.C.; Drexler, H. Clinical applications of stem cells for the heart. Circ. Res. 2005, 96, 151–163. [Google Scholar] [CrossRef]
- Al Kindi, A.; Ge, Y.; Shum-Tim, D.; Chiu, R.C.-J. Cellular cardiomyoplasty: Routes of cell delivery and retention. Front. Biosci. 2008, 13, 2421–2434. [Google Scholar] [CrossRef]
- Vandergriff, A.C.; Hensley, T.M.; Henry, E.T.; Shen, D.; Anthony, S.; Zhang, J.; Cheng, K. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Lin, X.; Takahashi, K.; Campion, S.L.; Liu, Y.; Gustavsen, G.G.; Peña, L.A.; Zamora, P.O. Synthetic peptide F2A4-K-NS mimics fibroblast growth factor-2 in vitro and is angiogenic in vivo. Int. J. Mol. Med. 2006, 17, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Section | Application | Additive/Modification for Particles | Ref. |
|---|---|---|---|
| 2. Drug carriers | Healing of crush syndrome with FGF-2 | - | [54] |
| Healing of irradiated wounds with FGF-2 | - | [55] | |
| Healing of skin graft donor sites with platelet-rich plasma | - | [57] | |
| Hair growth with FGF-2 | - | [56] | |
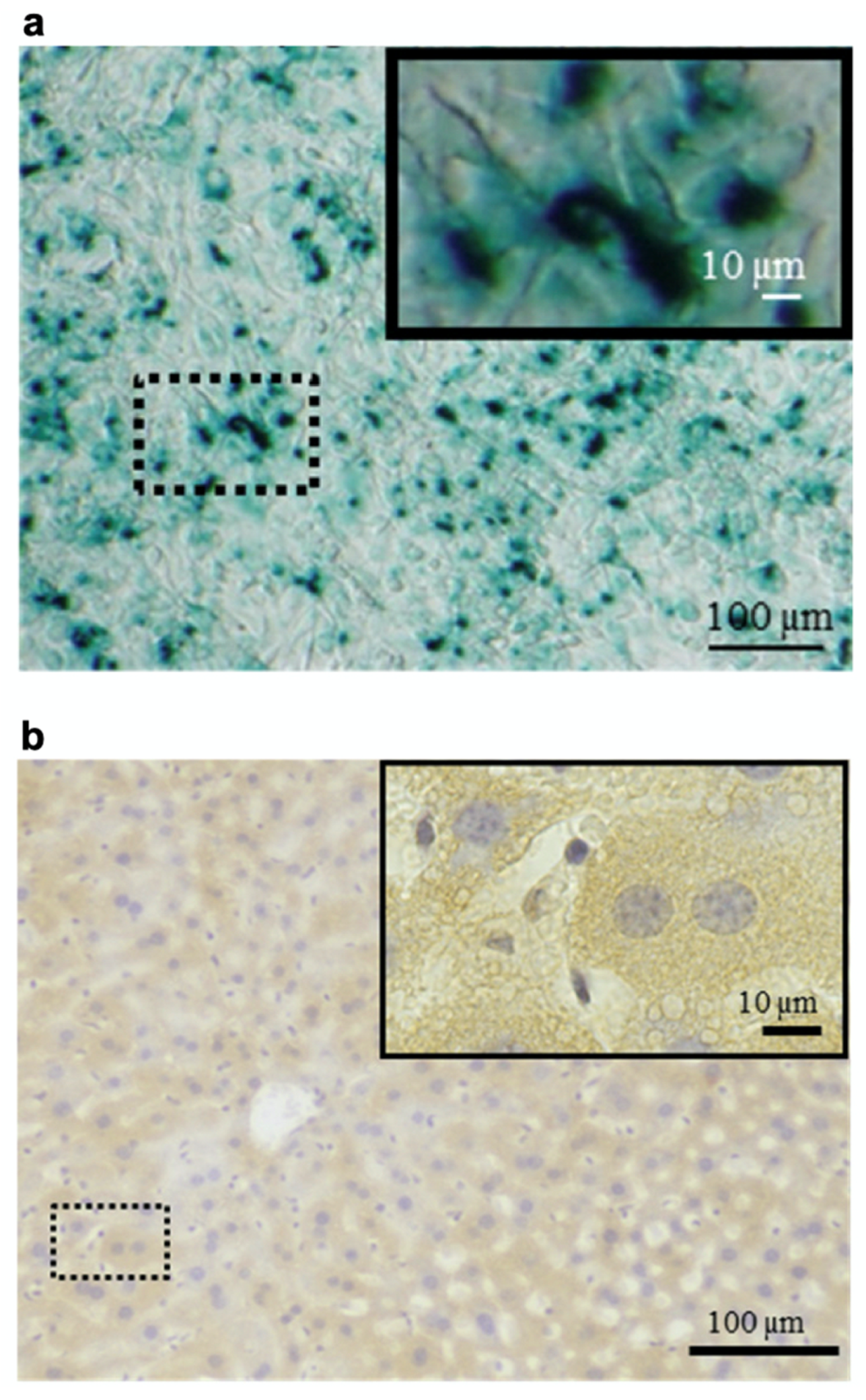
| Targeted protein delivery to mouse hepatocytes | Cell-penetrating peptide | [36] | |
| Antitumor drug delivery in vitro | CaCO3 | [60] | |
| Targeted antitumor drug delivery in vitro | CaCO3, conjugation of biotin | [61] | |
| Antitumor drug delivery in vitro | Chemical crosslinking | [62] | |
| Oral delivery | Conjugation of bile acid | [63] | |
| Antiangiogenic therapy of tumors | Conjugation of taurocholate, PEGylation | [70] | |
| Antiangiogenic therapy of tumors | Conjugation of suramin, PEGylation | [71] | |
| Proapoptotic and antiangiogenic therapy of tumors with TRAIL | Conjugation of taurocholate, PEGylation | [72] | |
| 3. Adhesives for cells | Two-dimensional cell culture | - | [76] |
| Three-dimensional cell culture | - | [77] | |
| Cell transplantation for cartilage regeneration | - | [81] | |
| Cell transplantation for ameliorating limb ischemia | - | [82] | |
| Improving the survival of full-thickness skin grafts with platelet-rich plasma | - | [86] | |
| 4. Cell labeling | Cell tracking by MRI | Ferumoxytol (iron oxide nanoparticles) | [91,92,93,94,95] |
| Magnetically targeted delivery of cells | Ferumoxytol (iron oxide nanoparticles) | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, Y.; Miyazaki, H.; Ishihara, M.; Nakamura, S. Recent Progress on Heparin–Protamine Particles for Biomedical Application. Polymers 2022, 14, 932. https://doi.org/10.3390/polym14050932
Hata Y, Miyazaki H, Ishihara M, Nakamura S. Recent Progress on Heparin–Protamine Particles for Biomedical Application. Polymers. 2022; 14(5):932. https://doi.org/10.3390/polym14050932
Chicago/Turabian StyleHata, Yuuki, Hiromi Miyazaki, Masayuki Ishihara, and Shingo Nakamura. 2022. "Recent Progress on Heparin–Protamine Particles for Biomedical Application" Polymers 14, no. 5: 932. https://doi.org/10.3390/polym14050932
APA StyleHata, Y., Miyazaki, H., Ishihara, M., & Nakamura, S. (2022). Recent Progress on Heparin–Protamine Particles for Biomedical Application. Polymers, 14(5), 932. https://doi.org/10.3390/polym14050932