Vertical Alignment of Liquid Crystals on Phenylphenoxymethyl-Substituted Polystyrene—PS Derivatives Structurally Similar to LC Molecules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
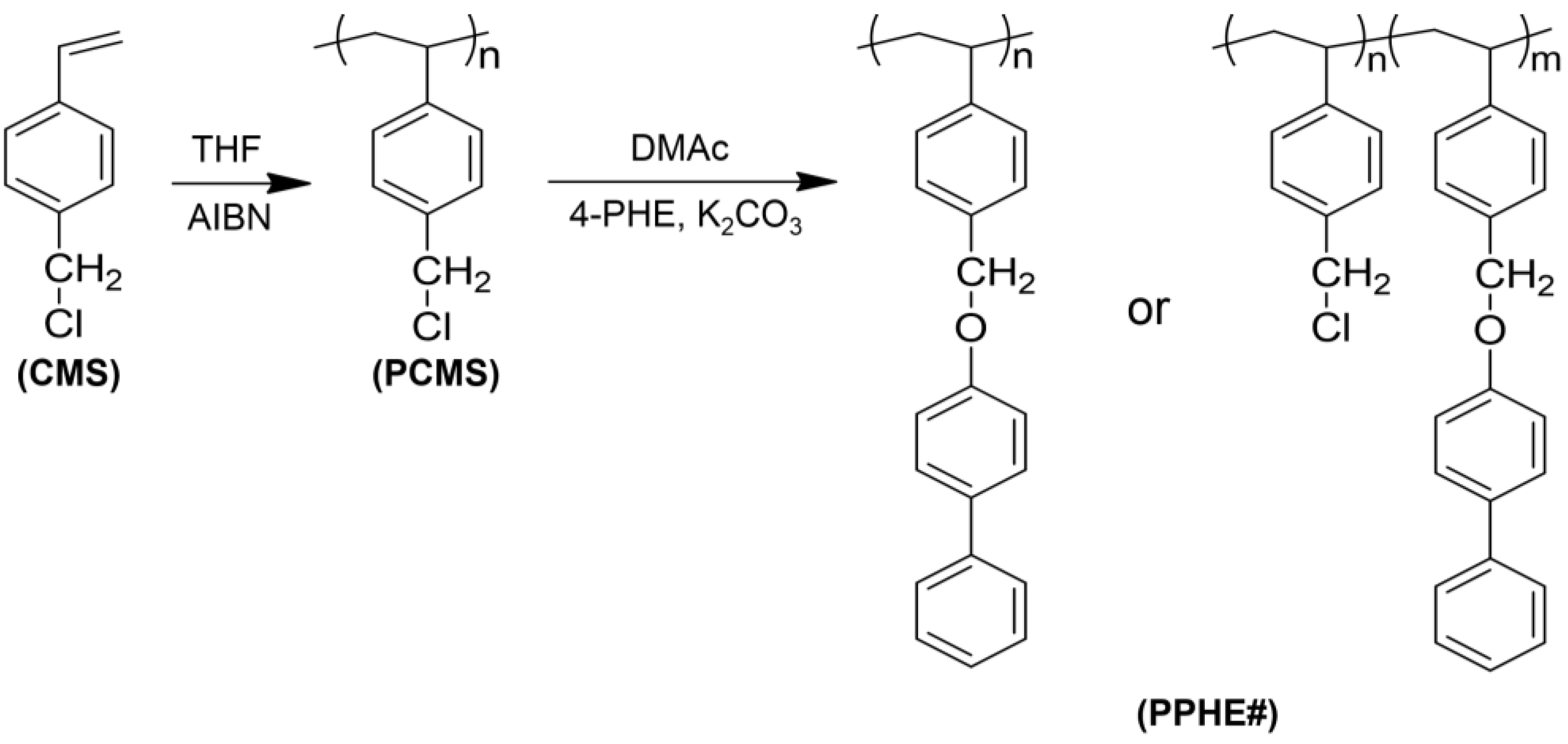
2.2. Synthesis of Phenylphenoxymethyl-Substituted Polystyrene
2.3. Film Preparation and LC Cell Assembly
2.4. Instrumentation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIBN | 2,2′-Azobisisobutyronitrile |
| 5CB | 4′-Pentyl-4-biphenylcarbonitrile |
| DMAc | N,N′-Dimethylacetamide |
| DSC | Differential scanning calorimetry |
| 1H NMR | Proton nuclear magnetic resonance |
| Tg | Glass transition temperature |
| THF | Tetrahydrofuran |
| LC | Liquid crystal |
| PCMS | Poly(4-chloromethylstyrene) |
| PPHE# | Phenylphenoxymethyl-substituted polystyrene |
| POM | Polarized optical microscopy |
| PS | Polystyrene |
References
- Kurabayashi, K. Anisotropic thermal properties of solid polymers. Int. J. Thermophys. 2001, 22, 277–288. [Google Scholar] [CrossRef]
- Kurabayashi, K.; Asheghi, M.; Touzelbaev, M.; Goodson, K.E. Measurement of the thermal conductivity anisotropy in polyimide films. J. Microelectromech. Syst. 1999, 8, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Zhao, T.; Wang, M.; Deng, L.; Lin, B.; Zhang, X.; Sun, Y.; Yang, H.; Chen, E. A homeotropic main-chain tolane-type liquid crystal elastomer film exhibiting high anisotropic thermal conductivity. Soft Matter 2017, 13, 5463–5468. [Google Scholar] [CrossRef]
- Ryu, M.; Takezoe, H.; Haba, O.; Yonetake, K.; Morikawa, J. Photo-controllable thermal diffusivity and thermal conductivity driven by the orientation change of nematic liquid crystal with azo-dendrimers. Appl. Phys. Lett. 2015, 107, 221901. [Google Scholar] [CrossRef]
- Gupta, M.K.; Srivastava, R.K. Mechanical properties of hybrid fibers-reinforced polymer composite: A review. Polym.-Plast. Technol. Eng. 2016, 55, 626–642. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Liu, Y.; Cao, A.; Qian, W.; Lu, Y.; Wei, F. Energy-absorbing hybrid composites based on alternate carbon nanotube and inorganic layers. Adv. Mater. 2009, 21, 2876–2880. [Google Scholar] [CrossRef]
- Shambina, S.L.; Virchenko, G.A. Special features of design and calculation for structures made of anisotropic fiberglass. IOP Conf. Ser. Mater. Sci. Eng. 2017, 222, 012011. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Choy, C.L.; Wong, S.P.; Young, K. Model calculation of the thermal conductivity of polymer crystals. J. Polym. Sci. B Polym. Phys. 1985, 23, 1495–1504. [Google Scholar] [CrossRef]
- Hong, S.M.; Kim, S.H.; Kim, J.H.; Hwang, H.I. Hydrophilic surface modification of PDMS using atmospheric RF plasma. J. Phys. Conf. Ser. 2006, 34, 656–661. [Google Scholar] [CrossRef]
- Meincken, M.; Berhane, T.A.; Mallon, P.E. Tracking the hydrophobicity recovery of PDMS compounds using the adhesive force determined by AFM force distance measurements. Polymer 2005, 46, 203–208. [Google Scholar] [CrossRef]
- Boxshall, K.; Wu, M.; Cui, Z.; Cui, Z.; Watts, J.F.; Baker, M.A. Simple surface treatments to modify protein adsorption and cell attachment properties within a poly(dimethylsiloxane) micro-bioreactor. Surf. Interface Anal. 2006, 38, 198–201. [Google Scholar] [CrossRef]
- Paguirigan, A.L.; Beebe, D.J. From the cellular perspective: Exploring differences in the cellular baseline in macroscale and microfluidic cultures. Integr. Biol. 2009, 1, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Abbasi, F.; Mirzadeh, H.; Katbab, A. Modification of polysiloxane polymers for biomedical applications: A review. Polym. Int. 2001, 50, 1279–1287. [Google Scholar] [CrossRef]
- Demus, D.; Goodby, J.; Gray, G.W.; Spiess, H.-W.; Vill, V. Handbook of Liquid Crystals, 1st ed.; Wiley-VCH: Weinheim, Germany, 1998; pp. 44–63. ISBN 978-3-527-62076-0. [Google Scholar]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mat. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Collings, P.J.; Goodby, J.W. Introduction to Liquid Crystals: Chemistry and Physics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 29–84. ISBN 978-1-138-29876-7. [Google Scholar]
- Ye, L.; Zhao, C.; Feng, Y.; Gu, B.; Cui, Y.; Lu, Y. Study on the polarization of random lasers from dye-doped nematic liquid crystals. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stöhr, J.; Samant, M.G.; Cossy-Favre, A.; Diaz, J.; Momoi, Y.; Odahara, S.; Nagata, T. Microscopic origin of liquid crystal alignment on rubbed polymer surfaces. Macromolecules 1998, 31, 1942–1946. [Google Scholar] [CrossRef]
- Ishihara, S.; Mizusaki, M. Alignment control technology of liquid crystal molecules. J. Soc. Inf. Disp. 2020, 28, 44–74. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Matsuyoshi, K.; Hayashi, M.; Takatsuka, H.; Yamamoto, T. Photoreaction of photo-cross-linkable methacrylate polymer films comprising 2-cinnamoyloxyethoxybiphenyl side group by linearly polarized ultraviolet light and liquid crystal alignment on the resultant films. Chem. Mater. 2000, 12, 1549–1555. [Google Scholar] [CrossRef]
- Rempel, T.D.; Gandy, R.F.; Wootton, A.J. Density fluctuation effects on electron cyclotron emission correlation measurements in optically gray plasmas. Rev. Sci. Instrum. 1994, 65, 2044–2048. [Google Scholar] [CrossRef]
- Van Aerle, N.; Tol, A. Molecular orientation in rubbed polyimide alignment layers used for liquid-crystal displays. Macromolecules 1994, 27, 6520–6526. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.; Dong, K.; Oh, B.; Kim, Y.; Jeong, H.; Ju, B.; Seo, D. Homeotropic alignment of liquid crystals on a nano-patterned polyimide surface using nanoimprint lithography. Soft Matter 2011, 7, 5610–5614. [Google Scholar] [CrossRef]
- Kang, D.; Kim, S.; Kim, B.; Kim, J.; Ok, C.; Kim, Y.; Han, J.; Kim, J.; Hwang, J.; Oh, B. Liquid crystal alignment effects for nematic liquid crystal on homeotropic polyimide surface using new ion-beam source. Jpn. J. Appl. Phys. 2007, 46, 6601–6603. [Google Scholar] [CrossRef]
- Chae, B.; Lee, S.W.; Ree, M.; Jung, Y.M.; Kim, S.B. Photoreaction and molecular reorientation in a nanoscaled film of poly(methyl 4-(methacryloyloxy)cinnamate) studied by two-dimensional FTIR and UV correlation spectroscopy. Langmuir 2003, 19, 687–695. [Google Scholar] [CrossRef]
- Kim, J.B.; Kim, K.C.; Ahn, H.J.; Hwang, B.H.; Hyun, D.C.; Baik, H.K. Variable liquid crystal pretilt angles on various compositions of alignment layers. Appl. Phys. Lett. 2007, 90, 043515. [Google Scholar] [CrossRef]
- Ishihara, S.; Wakemoto, H.; Nakazima, K.; Matsuo, Y. The effect of rubbed polymer films on the liquid crystal alignment. Liq. Cryst. 1989, 4, 669–675. [Google Scholar] [CrossRef]
- Stöhr, J.; Samant, M.G. Liquid crystal alignment by rubbed polymer surfaces: A microscopic bond orientation model. J. Electron Spectrosc. Relat. Phenom. 1999, 98, 189–207. [Google Scholar] [CrossRef]
- Liaw, D.; Wang, K.; Huang, Y.; Lee, K.; Lai, J.; Ha, C. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Pattison, L.R.; Hexemer, A.; Kramer, E.J.; Krishnan, S.; Petroff, P.M.; Fischer, D.A. Probing the ordering of semiconducting fluorenethiophene copolymer surfaces on rubbed polyimide substrates by near-edge x-ray absorption fine structure. Macromolecules 2006, 39, 2225–2231. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Fuh, A.Y. Controlling pre-tilt angles of liquid crystal using mixed polyimide alignment layer. Opt. Express 2008, 16, 17131–17137. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Wang, K.; Fan, L.; Yang, S. Synthesis and characterization of novel fluorinated polyimides derived from 4,4´-[2,2,2-trifluoro-1-(3,5-ditrifluoromethylphenyl)ethylidene]diphthalic anhydride and aromatic diamines. Polymer 2006, 47, 1443–1450. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.Y.; Park, I.C.; Rho, B.G.; Park, J.S.; Park, H.S.; Lee, C.H. Rubbing-free, vertically aligned nematic liquid crystal display controlled by in-plane field. Appl. Phys. Lett. 1997, 71, 2851–2853. [Google Scholar] [CrossRef]
- Bechtold, I.H.; De Santo, M.P.; Bonvent, J.; Oliveira, E.A.; Barberi, R.; Rasing, T. Rubbing-induced charge domains observed by electrostatic force microscopy: Effect on liquid crystal alignment. Liq. Cryst. 2003, 30, 591–598. [Google Scholar] [CrossRef]
- Kim, J.; Acharya, B.R.; Kumar, S.; Ha, K.R. A method for liquid crystal alignment using in situ ultraviolet exposure during imidization of polyimide. Appl. Phys. Lett. 1998, 73, 3372–3374. [Google Scholar] [CrossRef]
- Chigrinov, V.G.; Kozenkov, V.M.; Kwok, H. Photoalignment of Liquid Crystalline Materials: Physics and Applications, 1st ed.; John Wiley & Sons: West Sussex, UK, 2008; pp. 69–93. ISBN 978-0-470-06539-6. [Google Scholar]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.; Kelly, S.M. Photoinduced surface alignment for liquid crystal displays. J. Phys. D Appl. Phys. 2000, 33, 67–84. [Google Scholar] [CrossRef]
- Worzakowska, M. Thermal and mechanical properties of polystyrene modified with esters derivatives of 3-phenylprop-2-en-1-ol. J. Therm. Anal. Calorim. 2015, 121, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Slaný, M.; Zhang, J.; Liu, Y.; Zang, Y.; Li, Y.; Chen, G. Acetylation modification of waste polystyrene and its use as a crude oil flow improver. Polymer 2021, 13, 2505. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Slaný, M.; Yang, Y.; Li, S.; Qin, F.; Zhao, Y.; Zhang, Z.; Zhang, L. Highly active Mg-Al hydrotalcite for efficient O-methylation of phenol with DMC based on soft colloidal templates. J. Chem. Technol. Biotechnol. 2022, 97, 79–86. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; Garcia-Ramos, J.V. Adsorption and chemical modification of phenols on a silver surface. J. Colloid Interface Sci. 2000, 231, 98–106. [Google Scholar] [CrossRef]
- Baravkar, M.; Bhagavatula, P. Selective electro-oxidation of phenol to 1,4-hydroquinone employing carbonaceous electrodes: Surface modification is the key. New J. Chem. 2022, 46, 2518–2525. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.W.; Ha, J.D.; Oh, J.M.; Yi, M.H. Synthesis and characterization of novel polyimides with 1-octadecyl side chains for liquid crystal alignment layers. Polym. Adv. Technol. 2007, 18, 226–234. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.I.; Park, Y.H.; Reea, M.; Rim, Y.N.; Yoon, H.J.; Kim, H.C.; Kim, Y.B. Liquid-crystal alignment on the rubbed film surface of semi-flexible copolyimides containing n-alkyl side groups. Mol. Cryst. Liq. Cryst. 2000, 349, 279–282. [Google Scholar] [CrossRef]
- Lee, S.W.; Chae, B.; Lee, B.; Choi, W.; Kim, S.B.; Kim, S.I.; Park, S.; Jung, J.C.; Lee, K.H.; Ree, M. Rubbing-induced surface morphology and polymer segmental reorientations of a model brush polyimide and interactions with liquid crystals at the surface. Chem. Mater. 2003, 15, 3105–3112. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, G.J.; Chi, J.H.; Zin, W.; Jung, J.C.; Hahm, S.G.; Ree, M.; Chang, T. Synthesis, characterization and liquid-crystal-aligning properties of novel aromatic polypyromellitimides bearing (n-alkyloxy)biphenyloxy side chains. Polymer 2006, 47, 6606–6621. [Google Scholar] [CrossRef]
- Ju, C.; Kim, T.; Kang, H. Liquid crystal alignment behaviors on capsaicin substituted polystyrene films. RSC Adv. 2017, 7, 41376–41383. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.; Kim, T.; Kang, H. Renewable, eugenol-modified polystyrene layer for liquid crystal orientation. Polymer 2018, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.; Park, C.; Kim, T.; Kang, H. Vertical alignment of liquid crystals on plant-based vanillin derivative-substituted polystyrene films. RSC Adv. 2019, 9, 14188–14193. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.; Kang, H. Vertical orientation of liquid crystal on comb-like 4-(trans-4-alkylcyclohexyl) phenoxymethyl-substituted polystyrene containing liquid crystal precursor. Polymer 2021, 13, 1404. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Kang, H. Vertical orientation of liquid crystal on polystyrene substituted with n-alkylbenzoate-p-oxymethyl pendant group as a liquid crystal precursor. Polymer 2021, 13, 2058. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Kang, H. Vertical orientation of liquid crystal on 4-n-alkyloxyphenoxymethyl-substituted polystyrene containing liquid crystal precursor. Polymer 2021, 13, 736. [Google Scholar] [CrossRef]
- Lee, K.; Paek, S.; Lien, A.; Durning, C.; Fukuro, H. Microscopic molecular reorientation of alignment layer polymer surfaces induced by rubbing and its effects on LC pretilt angles. Macromolecules 1996, 29, 8894–8899. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J.-C. Liquid crystal alignment property of n-alkylthiomethyl- or n-alkylsulfonylmethylsubstituted polystyrenes. Polym. Adv. Technol. 2009, 20, 878–886. [Google Scholar] [CrossRef]
- Hanemann, T.; Haase, W.; Svoboda, I.; Fuess, H. Crystal structure of 4´-pentyl-4-cyanobiphenyl (5CB). Liq. Cryst. 1995, 19, 699–702. [Google Scholar] [CrossRef]
- Bogi, A.; Faetti, S. Elastic, dielectric and optical constants of 4´-pentyl-4-cyanobiphenyl. Liq. Cryst. 2001, 28, 729–739. [Google Scholar] [CrossRef]
- Maze, C. Determination of nematic liquid crystal elastic and dielectric properties from the shape of a capacitance-voltage curve. Mol. Cryst. Liq. Cryst. 1978, 48, 273–287. [Google Scholar] [CrossRef]
- Schell, K.T.; Porter, R.S. Dielectric studies of highly polar nematic liquid crystals and their mixtures. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1990, 188, 97–103. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kang, H.; Park, J.S.; Kang, D.; Lee, J.-C. 2-Naphthoxymethyl-substituted polystyrenes for homeotropic liquid-crystal alignment layers. Macromol. Chem. Phys. 2008, 209, 1900–1908. [Google Scholar] [CrossRef]
- Kang, H.; Kang, D.; Lee, J.-C. Liquid crystal alignment property of polystyrene derivatives containing dual photoreactive side groups. Polymer 2009, 50, 2104–2112. [Google Scholar] [CrossRef]
- Zhang, T.; Lang, Q.; Zeng, L.; Li, T.; Wei, M.; Liu, A. Substituent effect on the oxidation peak potentials of phenol derivatives at ordered mesoporous carbons modified electrode and its application in determination of acidity coefficients (pKa). Electrochim. Acta 2014, 115, 283–289. [Google Scholar] [CrossRef]
- Hayes, N.V.; Branch, G.E.K. The acidic dissociation constants of phenoxyacetic acid and its derivatives. J. Am. Chem. Soc. 1943, 65, 1555–1564. [Google Scholar] [CrossRef]
- Rempp, P.; Lutz, P.; Masson, P.; Franta, E. Macromonomers-a new class of polymeric intermediates in macromolecular synthesis. I-synthesis and characterization. Die Makromol. Chem. 1984, 8, 3–15. [Google Scholar] [CrossRef]
- Fowles, J.; Boatman, R.; Bootman, J.; Lewis, C.; Morgott, D.; Rushton, E.; Van Rooij, J.; Banton, M. A review of the toxicological and environmental hazards and risks of tetrahydrofuran. Crit. Rev. Toxicol. 2013, 43, 811–828. [Google Scholar] [CrossRef]
- Privalko, V.P.; Lipatov, Y.S. Glass transition and chain flexibility of linear polymers. J. Macromol. Sci. B Phys. 1974, 9, 551–564. [Google Scholar] [CrossRef]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 5, 318–321. [Google Scholar] [CrossRef]
- Wesslen, B.; Lenz, R.W.; MacKnight, W.J.; Karasz, F.E. Glass transition temperatures of poly(ethyl α-chloroacrylates). Macromolecules 1971, 4, 24–26. [Google Scholar] [CrossRef]
- Lee, J.-C.; Litt, M.H.; Rogers, C.E. Oxyalkylene polymers with alkylsulfonylmethyl side chains: Gas barrier properties. J. Polym. Sci. B Polym. Phys. 1998, 36, 75–83. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009; pp. 129–187. ISBN 978-0-080-54819-7. [Google Scholar]
- Senta, R.; Leo, M. Glass transitions of the poly-(n-alkyl methacrylates). J. Phys. Chem. 1957, 61, 985–991. [Google Scholar] [CrossRef]
- Kahn, F.J.; Taylor, G.N.; Schonhorn, H. Surface-produced alignment of liquid crystals. Proc. IEEE 1973, 61, 823–828. [Google Scholar] [CrossRef]
- Kim, S.I.; Ree, M.; Shin, T.J.; Jung, J.C. Synthesis of new aromatic polyimides with various side chains containing a biphenyl mesogen unit and their abilities to control liquid-crystal alignments on the rubbed surface. J. Polym. Sci. A Polym. Chem. 1999, 37, 2909–2921. [Google Scholar] [CrossRef]
- Schwartz, J.J.; Mendoza, A.; Wattanatorn, N.; Zhao, Y.; Nguyen, V.T.; Spokoyny, A.M.; Mirkin, C.A.; Baše, T.; Weiss, P. Surface dipole control of liquid crystal alignment. J. Am. Chem. Soc. 2016, 138, 5957–5967. [Google Scholar] [CrossRef] [Green Version]
- Bouchiat, M.; Langevin-Cruchon, D. Molecular order at the free surface of a nematic liquid crystal from light reflectivity measurements. Phys. Lett. A 1971, 34, 331–332. [Google Scholar] [CrossRef]
- Haller, I. Alignment and wetting properties of nematic liquids. Appl. Phys. Lett. 1974, 24, 349–351. [Google Scholar] [CrossRef]
- Shafrin, E.G.; Zisman, W.A. Constitutive relations in the wetting of low energy surfaces and the theory of the retraction method of preparing monolayers1. J. Phys. Chem. 1960, 64, 519–524. [Google Scholar] [CrossRef]
- Paek, S.H.; Durning, C.J.; Lee, K.W.; Lien, A. A mechanistic picture of the effects of rubbing on polyimide surfaces and liquid crystal pretilt angles. J. Appl. Phys. 1998, 83, 1270–1280. [Google Scholar] [CrossRef]
- Ban, B.S.; Kim, Y.B. Surface free energy and pretilt angle on rubbed polyimide surfaces. J. Appl. Polym. Sci. 1999, 74, 267–271. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, C.Y.; Lin, C.J.; Pan, R.P.; Lin, S.S.; Lee, C.D.; Kou, C.S. Mechanism in determining pretilt angle of liquid crystals aligned on fluorinated copolymer films. J. Phys. D Appl. Phys. 2009, 42, 155303. [Google Scholar] [CrossRef]
- Hussain, M.; Jull, E.I.; Mandle, R.J.; Raistrick, T.; Hine, P.J.; Gleeson, H.F. Liquid crystal elastomers for biological applications. Nanomaterials 2021, 11, 813. [Google Scholar] [CrossRef]
- Mysliwiec, J.; Szukalska, A.; Szukalski, A.; Sznitko, L. Liquid crystal lasers: The last decade and the future. Nanophotonics 2021, 10, 2309–2346. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, D.P.; Singh, G. Recent progress and future perspectives on carbon-nanomaterial-dispersed liquid crystal composites. J. Phys. D Appl. Phys. 2021, 55, 083002. [Google Scholar] [CrossRef]
- Del Pozo, M.; Sol, J.A.; Schenning, A.P.; Debije, M.G. 4D Printing of liquid crystals: What’s right for me? Adv. Mater. 2022, 34, 2104390. [Google Scholar] [CrossRef]





| Polymer Designation | Contact Angle a (°) | Surface Energy b (mJ/m2) | Vertical LC Aligning Ability | |||
|---|---|---|---|---|---|---|
| Water | Diiodomethane | Polar | Dispersion | Total | ||
| PPHE5 | 73.8 (2.4) c | 46.4 (2.8) c | 8.9 (0.5)c | 31.5 (1.6) c | 40.4 (1.0) c | No |
| PPHE15 | 76.1 (3.1) | 44.8 (1.9) | 7.5 (0.3) | 32.8 (1.1) | 40.3 (0.8) | No |
| PPHE25 | 82.6 (3.0) | 43.4 (0.9) | 4.2 (0.1) | 35.2 (0.6) | 39.4 (0.5) | Yes |
| PPHE50 | 89.0 (2.4) | 42.2 (0.5) | 1.8 (0.3) | 36.0 (0.1) | 37.8 (0.4) | Yes |
| PPHE75 | 93.3 (5.1) | 42.1 (0.3) | 0.9 (0.4) | 37.3 (0.3) | 38.2 (0.1) | Yes |
| PPHE100 | 95.5 (2.6) | 38.3 (0.8) | 0.3 (0.2) | 40.2 (0.2) | 40.5 (0.1) | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Kang, C.; Kang, H. Vertical Alignment of Liquid Crystals on Phenylphenoxymethyl-Substituted Polystyrene—PS Derivatives Structurally Similar to LC Molecules. Polymers 2022, 14, 934. https://doi.org/10.3390/polym14050934
Moon J, Kang C, Kang H. Vertical Alignment of Liquid Crystals on Phenylphenoxymethyl-Substituted Polystyrene—PS Derivatives Structurally Similar to LC Molecules. Polymers. 2022; 14(5):934. https://doi.org/10.3390/polym14050934
Chicago/Turabian StyleMoon, Jihyeon, Chaewon Kang, and Hyo Kang. 2022. "Vertical Alignment of Liquid Crystals on Phenylphenoxymethyl-Substituted Polystyrene—PS Derivatives Structurally Similar to LC Molecules" Polymers 14, no. 5: 934. https://doi.org/10.3390/polym14050934





