Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Printable Filaments for Material Extrusion Printing
2.3. Rheological Measurement
3. Results and Discussion
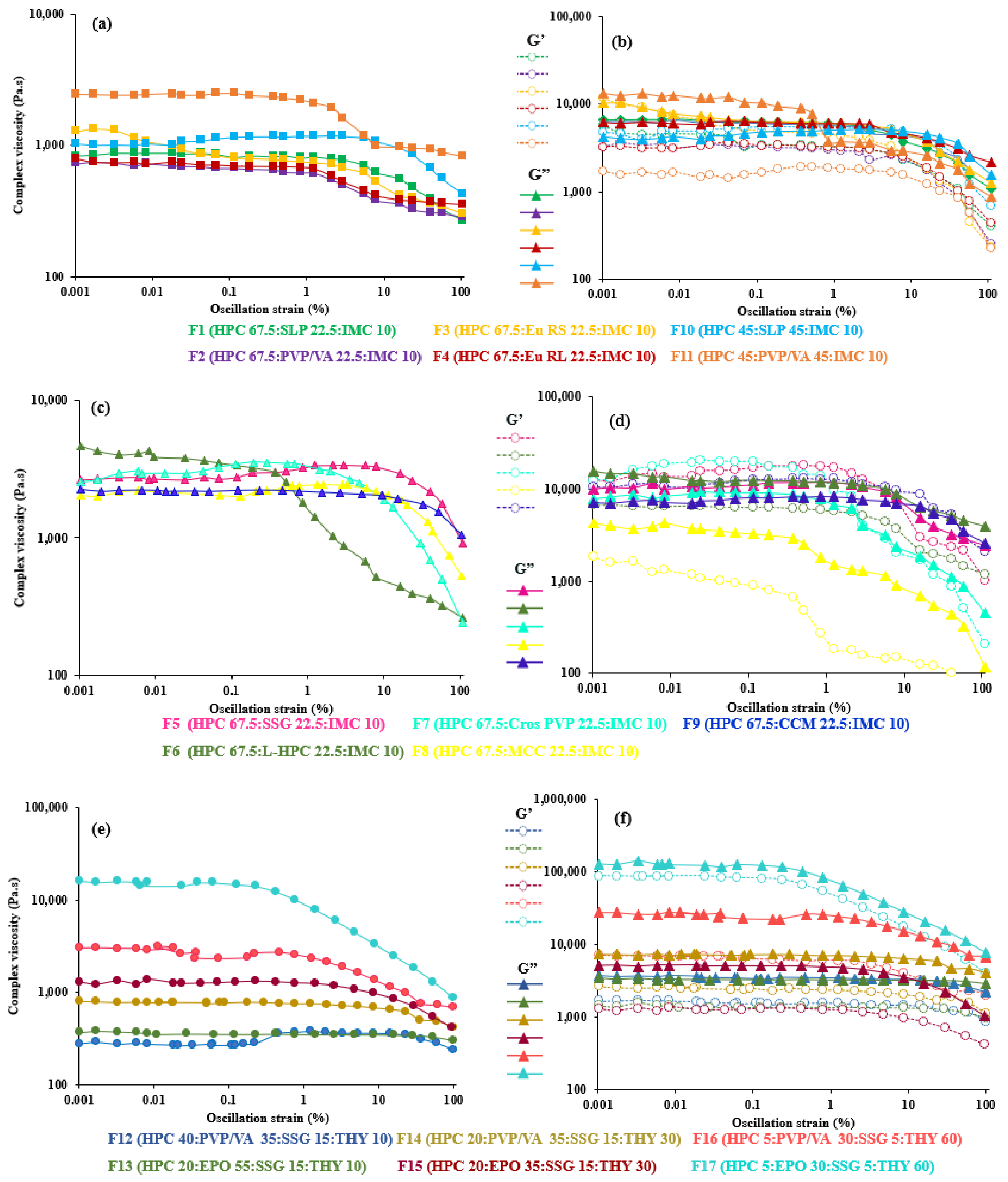
3.1. Oscillatory Shear Analysis
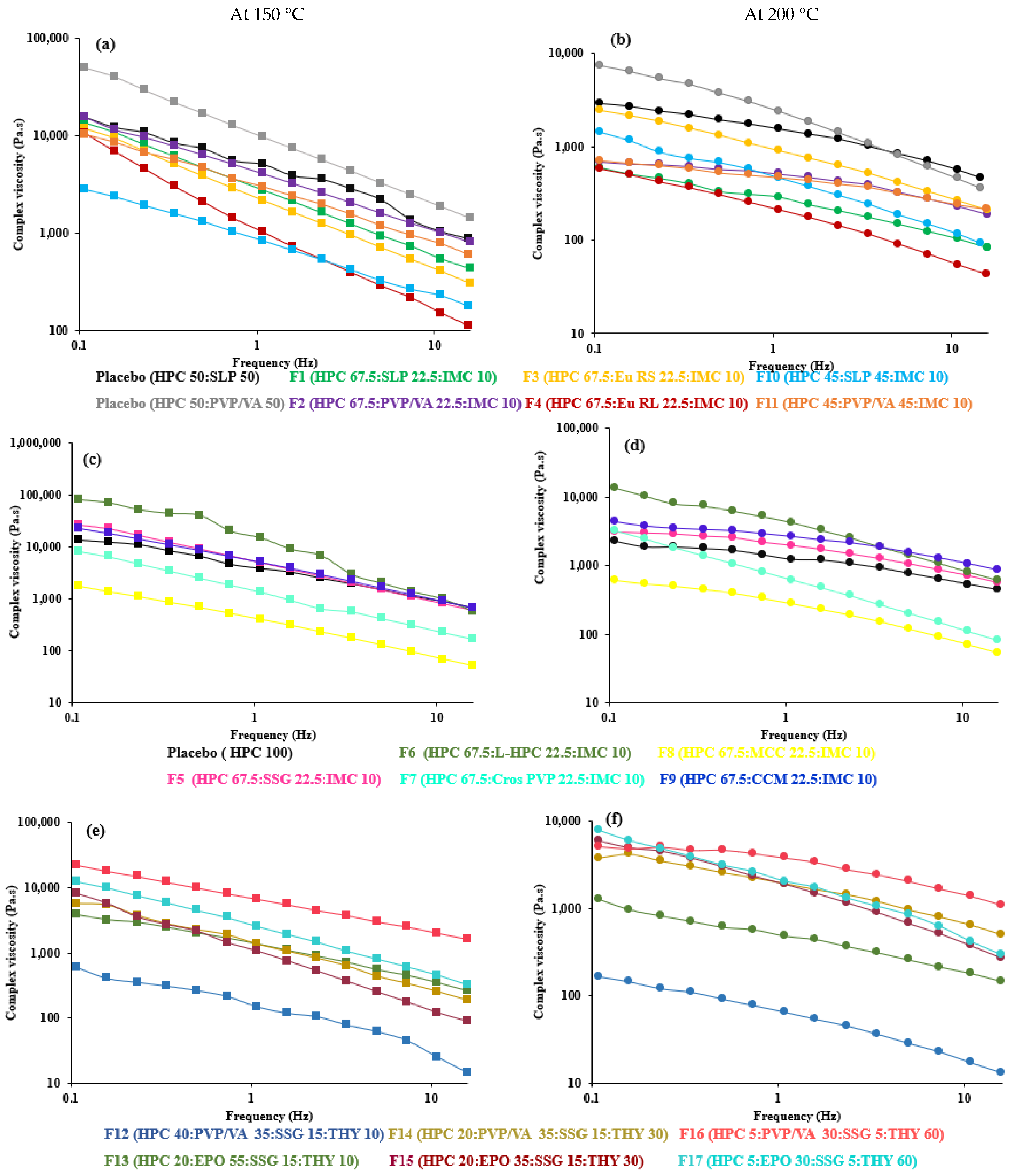
3.2. Viscosity
3.3. Shear Thinning
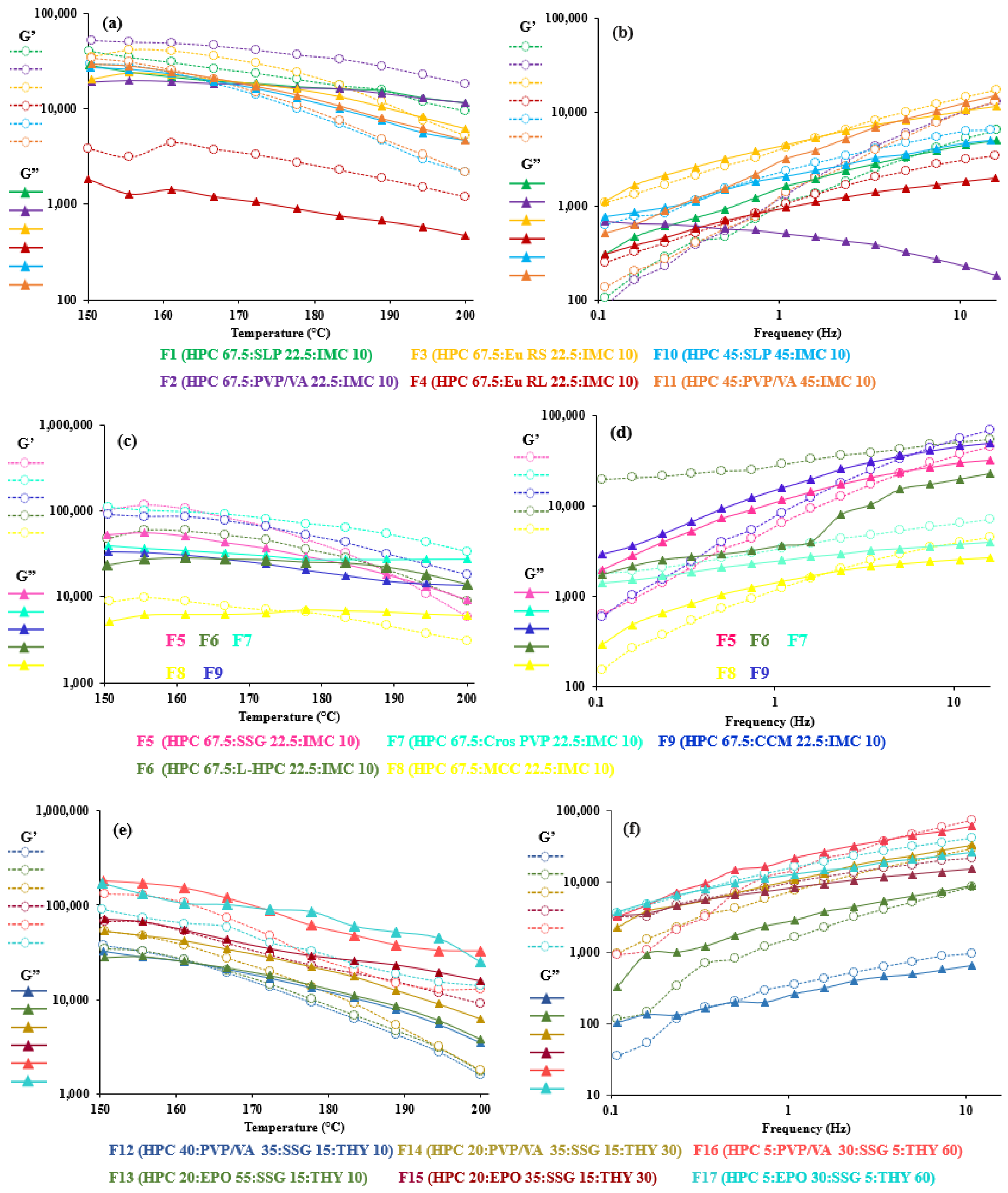
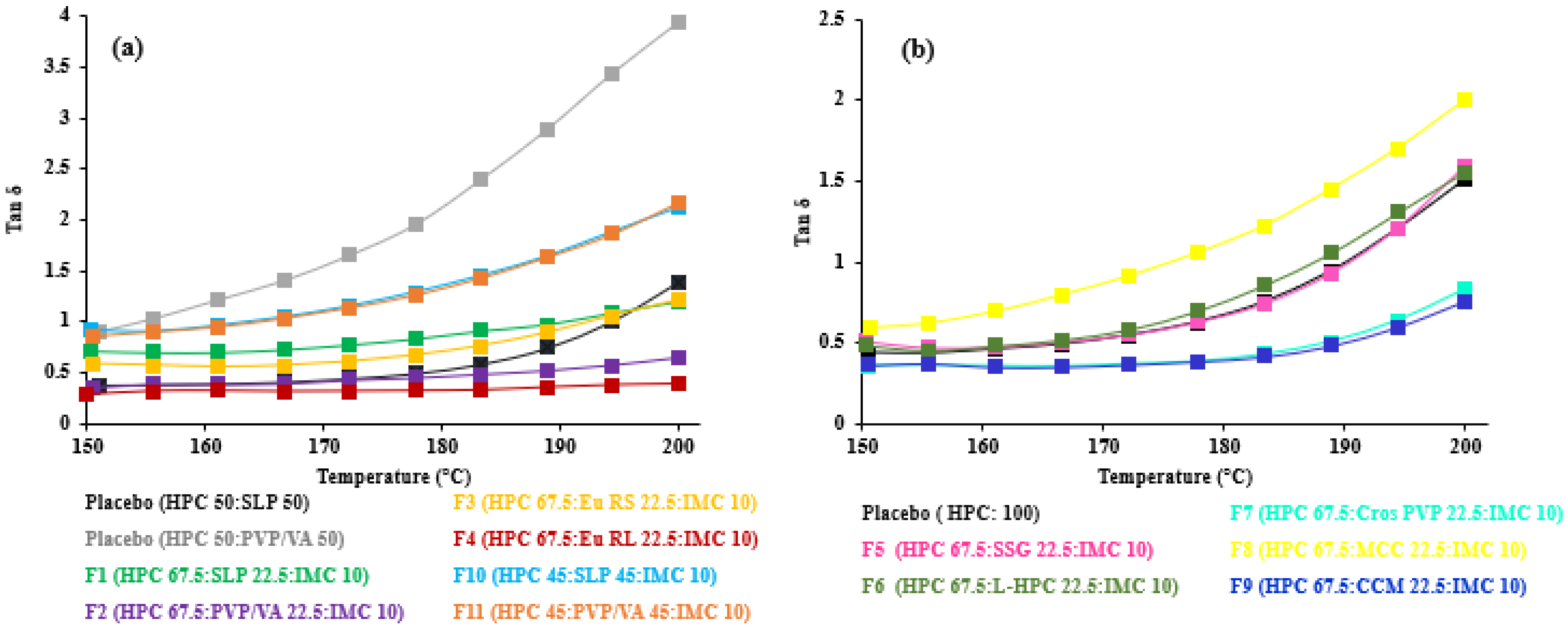
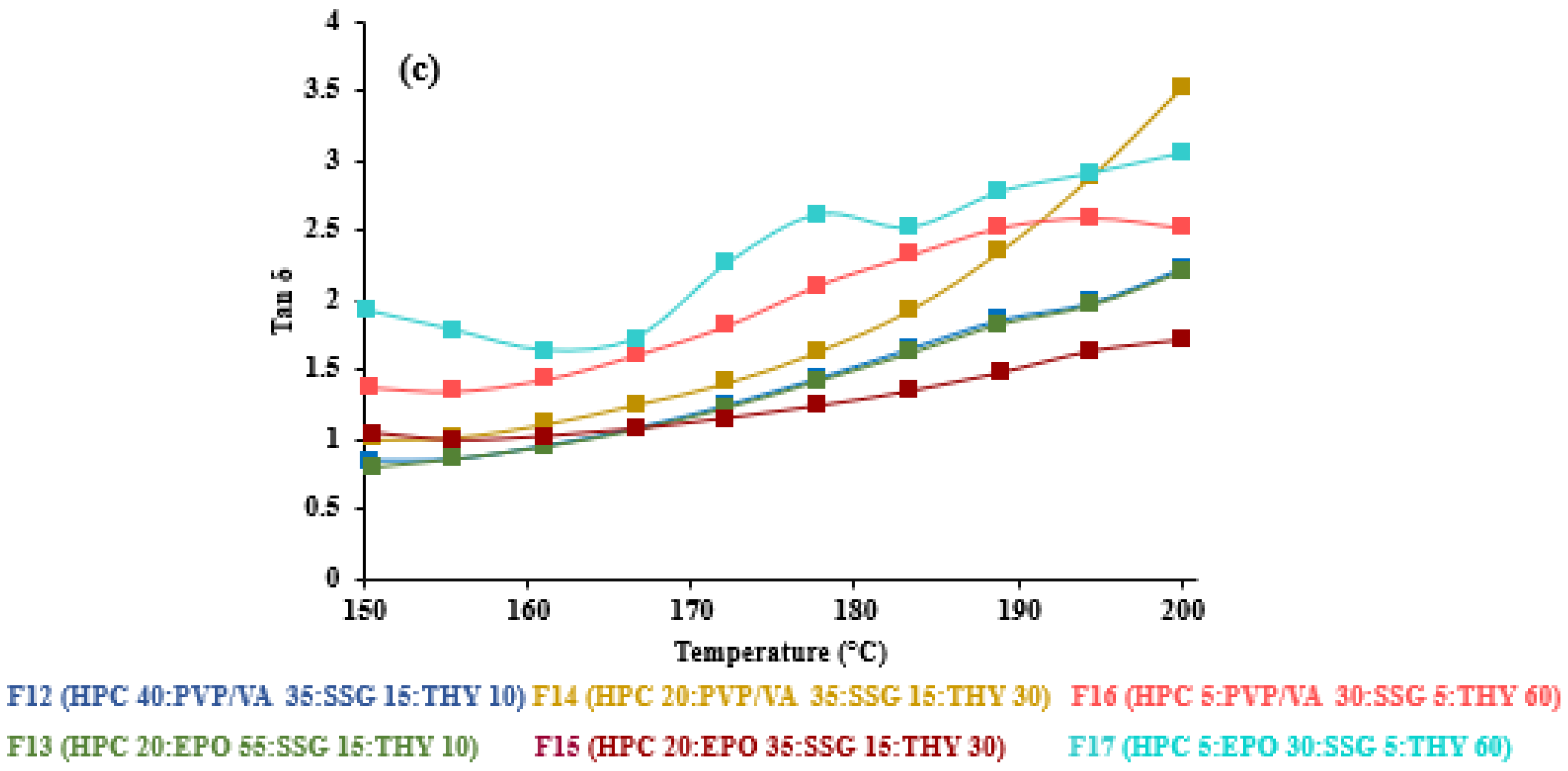
3.4. Viscoelastic Properties of HME-and FDM-Printable Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Murugan, S.; Vinodh, S. Parametric optimization of fused deposition modelling process using Grey based Taguchi and TOPSIS methods for an automotive component. Rapid Prototyp. J. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- Lalegani, M.; Mohd Ariffin, M.K.A.; Hatami, S. An overview of fused deposition modelling (FDM): Research, development and process optimisation. Rapid Prototyp. J. 2021, 27, 562–582. [Google Scholar] [CrossRef]
- Gilmer, E.L.; Miller, D.; Chatham, C.A.; Zawaski, C.; Fallon, J.J.; Pekkanen, A.; Long, T.E.; Williams, C.B.; Bortner, M.J. Model analysis of feedstock behavior in fused filament fabrication: Enabling rapid materials screening. Polymer 2018, 152, 51–61. [Google Scholar] [CrossRef]
- Awad, A.; Gaisford, S.; Basit, A.W. Fused Deposition Modelling: Advances in Engineering and Medicine. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 107–132. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Rolfe, B.; Gibson, I.J.R.P.J. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2021, 28, 87–100. [Google Scholar] [CrossRef]
- Ranjan, N.; Singh, R.; Ahuja, I. Mechanical, Rheological and Thermal Investigations of Biocompatible Feedstock Filament Comprising of PVC, PP and HAp. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2020, 91, 159–168. [Google Scholar] [CrossRef]
- Ching-hao, L.; Mohammad Padzil, F.N.; Lee, S.H.; Mohamed, A.Z.; Luqman Chuah, A. Potential for Natural Fiber Reinforcement in PLA Polymer Filaments for Fused Deposition Modeling (FDM) Additive Manufacturing: A Review. Polymers 2021, 13, 1407. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Coburn, J.; Asfari, S.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Extrudability analysis of drug loaded pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 554, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced Via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Hempenstall, J.; Tucker, I.; Rades, T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int. J. Pharm. 2001, 226, 147–161. [Google Scholar] [CrossRef]
- Liu, H.; Wang, P.; Zhang, X.; Shen, F.; Gogos, C.G. Effects of extrusion process parameters on the dissolution behavior of indomethacin in Eudragit® E PO solid dispersions. Int. J. Pharm. 2010, 383, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Suwardie, H.; Wang, P.; Todd, D.B.; Panchal, V.; Yang, M.; Gogos, C.G. Rheological study of the mixture of acetaminophen and polyethylene oxide for hot-melt extrusion application. Eur. J. Pharm. Biopharm. 2011, 78, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Kalivoda, A.; Fischbach, M.; Kleinebudde, P. Application of mixtures of polymeric carriers for dissolution enhancement of oxeglitazar using hot-melt extrusion. Int. J. Pharm. 2012, 439, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sathigari, S.K.; Radhakrishnan, V.K.; Davis, V.A.; Parsons, D.L.; Babu, R.J. Amorphous-State Characterization of Efavirenz—polymer Hot-Melt Extrusion Systems for Dissolution Enhancement. J. Pharm. Sci. 2012, 101, 3456. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F.J.L. Use of viscoelastic measurements in studying interactions in concentrated dispersions. Langmuir 1990, 6, 28–35. [Google Scholar] [CrossRef]
- Aho, J.; Edinger, M.; Botker, J.; Baldursdottir, S.; Rantanen, J. Oscillatory Shear Rheology in Examining the Drug-Polymer Interactions Relevant in Hot Melt Extrusion. J. Pharm. Sci. 2016, 105, 160–167. [Google Scholar] [CrossRef] [PubMed]
- De Brabander, C.; Van Den Mooter, G.; Vervaet, C.; Remon, J.P. Characterization of ibuprofen as a nontraditional plasticizer of ethyl cellulose. J. Pharm. Sci. 2002, 91, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M. Rheological and Mechanical Investigation into the Effect of Different Molecular Weight Poly(ethylene glycol)s on Polycaprolactone-Ciprofloxacin Filaments. ACS Omega 2019, 4, 5412–5423. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.; Gold, S. A review of melt extrusion additive manufacturing processes: II. Materials, dimensional accuracy, and surface roughness. Rapid Prototyp. J. 2015, 21, 250–261. [Google Scholar] [CrossRef]
- Greeff, P.; Schilling, M. Closed Loop Control of Slippage during Filament Transport in Molten Material Extrusion. Addit. Manuf. 2016, 14. [Google Scholar] [CrossRef]
- Bellini, A.; Güçeri, S.u.; Bertoldi, M. Liquefier Dynamics in Fused Deposition. J. Manuf. Sci. Eng. 2004, 126, 237–246. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D Printing with Hot-Melt Extrusion to Produce Controlled-Release Tablets. Int. J. Pharm. 2017, 519, 186. [Google Scholar] [CrossRef] [PubMed]
- Than, Y.M.; Titapiwatanakun, V. Tailoring immediate release FDM 3D printed tablets using a quality by design (QbD) approach. Int. J. Pharm. 2021, 599, 120402. [Google Scholar] [CrossRef] [PubMed]
- Than, Y.M.; Titapiwatanakun, V. Statistical design of experiment-based formulation development and optimization of 3D printed oral controlled release drug delivery with multi target product profile. J. Pharm. Investig. 2021, 51, 715–734. [Google Scholar] [CrossRef]
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a Tool for Evaluation of Melt Processability of Innovative Dosage Forms. Int. J. Pharm. 2015, 494, 623. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yu, W.; Zhou, C. Agglomeration of Crystals during Crystallization of Semicrystalline Polymers: A Suspension-Based Rheological Study. Macromolecules 2019, 52, 1042–1054. [Google Scholar] [CrossRef]
- Defeng, w.; Wu, L.; Wu, L.; Zhang, M. Rheology and thermal stability of polylactide/clay nanocomposites. Polym. Degrad. Stab. 2006, 91, 3149–3155. [Google Scholar] [CrossRef]
- Calafel, I.; Aguirresarobe, R.H.; Peñas, M.I.; Santamaria, A.; Tierno, M.; Conde, J.I.; Pascual, B. Searching for Rheological Conditions for FFF 3D Printing with PVC Based Flexible Compounds. Materials 2020, 13, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbadawi, M. Polymeric Additive Manufacturing: The Necessity and Utility of Rheology. In Polymer Rheology; IntechOpen: London, UK, 2018. [Google Scholar]
- Isreb, A.; Baj, K.; Wojsz, M.; Isreb, M.; Peak, M.; Alhnan, M.A. 3D printed oral theophylline doses with innovative ‘radiator-like’ design: Impact of polyethylene oxide (PEO) molecular weight. Int. J. Pharm. 2019, 564, 98–105. [Google Scholar] [CrossRef]
- Henry, S.; Samaro, A.; Marchesini, F.H.; Shaqour, B.; Macedo, J.; Vanhoorne, V.; Vervaet, C. Extrusion-based 3D printing of oral solid dosage forms: Material requirements and equipment dependencies. Int. J. Pharm. 2021, 598, 120361. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef] [PubMed]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Kennedy, J.E.; Higginbotham, C.L. Mechanical properties and thermal behaviour of PEGDMA hydrogels for potential bone regeneration application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.K.; Lee, J.M.; Chao, J.R.; Lee, Y.J.; Seo, Y.B.; Sultan, M.T.; Lee, O.J.; Lee, J.S.; Yoon, S.-I.; et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 2018, 9, 1620. [Google Scholar] [CrossRef] [PubMed]
- Calvet, D.; Wong, J.; Giasson, S. Rheological Monitoring of Polyacrylamide Gelation: Importance of Cross-Link Density and Temperature. Macromolecules 2004, 37, 7762–7771. [Google Scholar] [CrossRef]
- Erwin, B.; Cloitre, M.; Gauthier, M.; Vlassopoulos, D. Dynamics and rheology of colloidal star polymers. Soft Matter 2010, 6, 2825–2833. [Google Scholar] [CrossRef]
- Coogan, T.; Kazmer, D. In-line rheological monitoring of fused deposition modeling. J. Rheol. 2019, 63, 141–155. [Google Scholar] [CrossRef]
- Ramos, L. Principles of Polymer Processing; Wiley: Hoboken, NJ, USA, 2013; pp. 451–461. [Google Scholar]
- Fan, B.; Kazmer, D.O.; Nageri, R. An Analytical Non-Newtonian and Nonisothermal Viscous Flow Simulation. Polym.-Plast. Technol. Eng. 2006, 45, 429–438. [Google Scholar] [CrossRef]
- Ramanath, H.S.; Chua, C.; Leong, K.; Shah, K.D. Melt flow behaviour of poly0µ-caprolactone in fused deposition modelling. J. Mater. Sci. Mater. Med. 2008, 19, 2541–2550. [Google Scholar] [CrossRef]
- Cerda-Avila, S.; Medellín-Castillo, H.; Lim, T. Analytical models to estimate the structural behaviour of fused deposition modelling components. Rapid Prototyp. J. 2021. ahead-of-print. [Google Scholar] [CrossRef]
- Bakır, A.; Atik, R.; Özerinç, S. Mechanical properties of thermoplastic parts produced by fused deposition modeling: A review. Rapid Prototyp. J. 2021. ahead-of-print. [Google Scholar] [CrossRef]
- Vanaei, H.; Shirinbayan, M.; Vanaei, S.; Fitoussi, J.; Khelladi, S.; Tcharkhtchi, A. Multi-scale Damage Analysis and Fatigue Behavior of PLA Manufactured By Fused Deposition Modeling (FDM). Rapid Prototyp. J. 2020. ahead-of-print. [Google Scholar] [CrossRef]
- Fuenmayor, E.; Forde, M.; Healy, A.; Devine, D.; Lyons, J.; McConville, C.; Major, I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Sadia, M.; Isreb, A.; Abbadi, I.; Isreb, M.; Aziz, D.; Selo, A.; Timmins, P.; Alhnan, M.A. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. Eur. J. Pharm. Sci. 2018, 123, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Genina, N.; Edinger, M.; Botker, J.P.; Baldursdottir, S.; Rantanen, J. Drug-loaded poly (ε-caprolactone) for 3D printing of personalized medicine: A rheological study. In Proceedings of the 25th Nordic Rheology Conference, Helsinki, Finland, 30 May–1 June 2016; pp. 97–100. [Google Scholar]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. Eur. J. Pharm. Sci. 2016, 90, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Zhang, J.; Nyavanandi, D.; Bandari, S.; Repka, M.A. Hot melt extrusion paired fused deposition modeling 3D printing to develop hydroxypropyl cellulose based floating tablets of cinnarizine. Carbohydr. Polym. 2020, 246, 116519. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Chai, H.; Wang, X.; Yang, J.; Li, J.; Zhao, Y.; Cai, W.; Tao, T.; Xiang, X.J.S.R. Fused Deposition Modeling (FDM) 3D Printed Tablets for Intragastric Floating Delivery of Domperidone. Sci. Rep. 2017, 7, 2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorkem Buyukgoz, G.; Soffer, D.; Defendre, J.; Pizzano, G.M.; Davé, R.N. Exploring tablet design options for tailoring drug release and dose via fused deposition modeling (FDM) 3D printing. Int. J. Pharm. 2020, 591, 119987. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech 2019, 20, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline Caplets (PrintCap) via FDM 3D Printing. Polymers 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Samaro, A.; Janssens, P.; Vanhoorne, V.; Van Renterghem, J.; Eeckhout, M.; Cardon, L.; De Beer, T.; Vervaet, C. Screening of pharmaceutical polymers for extrusion-Based Additive Manufacturing of patient-tailored tablets. Int. J. Pharm. 2020, 586, 119591. [Google Scholar] [CrossRef]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Meena, A.K.; Parikh, T.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2014, 5, 32–45. [Google Scholar]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Diab, A.; You, Z. Small and large strain rheological characterizations of polymer- and crumb rubber-modified asphalt binders. Constr. Build. Mater. 2017, 144, 168–177. [Google Scholar] [CrossRef]
- Mueller, S.; Llewellin, E.; Mader, H.; Mueller, B.; Mader, A. The rheology of suspensions of solid particles. Proc. R. Soc. A Proc. R. Soc. A 2009, 466, 1201–1228. [Google Scholar] [CrossRef] [Green Version]
- Yurekli, K.; Krishnamoorti, R.; Tse, M.; McElrath, K.; Tsou, A.; Wang, H. Structure and dynamics of carbon black-filled elastomers. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 256–275. [Google Scholar] [CrossRef]
- Gupta, S.S.; Parikh, T.; Meena, A.K.; Mahajan, N.; Vitez, I.; Serajuddin, A.T.M. Effect of carbamazepine on viscoelastic properties and hot melt extrudability of Soluplus®. Int. J. Pharm. 2015, 478, 232–239. [Google Scholar] [CrossRef]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: Affinisol™ HPMC HME Polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Lee, K.; Brandt, M.; Shanks, R.; Daver, F. Rheology and 3D Printability of Percolated Graphene-Polyamide-6 Composites. Polymers 2020, 12, 2014. [Google Scholar] [CrossRef] [PubMed]
- Hardung, H.; Djuric, D.; Ali, S. Combining HME & Solubilization: Soluplus®—The Solid Solution. Drug Deliv. Technol. 2010, 10, 20–27. [Google Scholar]
- Solanki, N.; Gupta, S.S.; Serajuddin, A.T.M. Rheological analysis of itraconazole-polymer mixtures to determine optimal melt extrusion temperature for development of amorphous solid dispersion. Eur. J. Pharm. Sci. 2018, 111, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Kaully, T.; Siegmann, A.; Shacham, D. Rheology of highly filled natural CaCO3 composites. II. Effects of solid loading and particle size distribution on rotational rheometry. Polym. Compos. 2007, 28, 524–533. [Google Scholar] [CrossRef]
- Gómez-Carracedo, A.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; Concheiro, A. Chemical structure and glass transition temperature of non-ionic cellulose ethers. J. Therm. Anal. Calorim. 2003, 73, 587–596. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidau, M.; Kwade, A.; Finke, H.J. Influence of High, Disperse API Load on Properties along the Fused-Layer Modeling Process Chain of Solid Dosage Forms. Pharmaceutics 2019, 11, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Renterghem, J.; Vervaet, C.; De Beer, T. Rheological Characterization of Molten Polymer-Drug Dispersions as a Predictive Tool for Pharmaceutical Hot-Melt Extrusion Processability. Pharm. Res. 2017, 34, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Su, Y.; Zhang, J.; DiNunzio, J.; Leone, A.; Huang, C.; Brown, C.D. Rheology Guided Rational Selection of Processing Temperature To Prepare Copovidone–Nifedipine Amorphous Solid Dispersions via Hot Melt Extrusion (HME). Mol. Pharm. 2016, 13, 3494–3505. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Bowland, C.C.; Naskar, A.K. A general method to improve 3D-printability and inter-layer adhesion in lignin-based composites. Appl. Mater. Today 2018, 12, 138–152. [Google Scholar] [CrossRef]
- Mackay, M.E. The importance of rheological behavior in the additive manufacturing technique material extrusion. J. Rheol. 2018, 62, 1549–1561. [Google Scholar] [CrossRef]
- Feuerbach, T.; Kock, S.; Thommes, M. Characterisation of fused deposition modeling 3D printers for pharmaceutical and medical applications. Pharm. Dev. Technol. 2018, 23, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, J.; Strutt, D. 6-Rheology of Molten Polymers. In Multilayer Flexible Packaging, 2nd ed.; Wagner, J.R., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 77–96. [Google Scholar] [CrossRef]
- Behzadfar, E.; Abdolrasouli, M.H.; Sharif, F.; Nazockdast, H. Effect of solid loading and aggregate size on the rheological behavior of PDMS/Calcium Carbonate suspensions. Braz. J. Chem. Eng. 2009, 26, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Pryamitsyn, V.; Ganesan, V. Mechanisms of steady-shear rheology in polymer-nanoparticle composites. J. Rheol. 2006, 50, 655–683. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, X.; Zhao, G.; Guo, Z. Microcellular Injection Molded Polylactic Acid/Poly (ε-Caprolactone) Blends with Supercritical CO2: Correlation between Rheological Properties and Their Foaming Behavior. Polym. Eng. Sci. 2016, 56, 939–946. [Google Scholar] [CrossRef]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) Filaments a Biobased Solution for Additive Manufacturing: Correlating Rheology and Thermomechanical Properties with Printing Quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion—II: Cellulosic polymers. J. Excip. Food Chem. 2014, 5, 46–55. [Google Scholar]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J. Control Release 2016, 234, 41. [Google Scholar] [CrossRef]
- Polamaplly, P.; Cheng, Y.; Shi, X.; Manikandan, K.; Kremer, G.E.; Qin, H. 3D Printing and Characterization of Hydroxypropyl Methylcellulose and Methylcellulose for Biodegradable Support Structures. Procedia Manuf. 2019, 34, 552–559. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Anton, N.; Vandamme, T.F. Oral pellets loaded with nanoemulsions. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 8; pp. 203–230. [Google Scholar] [CrossRef]
- Shah, R.D.; Kabadi, M.; Pope, D.G.; Augsburger, L.L. Physico-Mechanical Characterization of the Extrusion-Spheronization Process. Part II: Rheological Determinants for Successful Extrusion and Spheronization. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 496–507. [Google Scholar] [CrossRef]
- Rojas, J.; Guisao, S.; Ruge, V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scoutaris, N.; Ross, S.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zheng, Q. Estimation of the Agglomeration Structure for Conductive Particles and Fiber-Filled High-Density Polyethylene through Dynamic Rheological Measurements. J. Polym. Sci. Part B: Polym. Phys. 2004, 42, 1199–1205. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Ding, Y.; Abeykoon, C.; Perera, Y.S. The effects of extrusion parameters and blend composition on the mechanical, rheological and thermal properties of LDPE/PS/PMMA ternary polymer blends. Adv. Ind. Manuf. Eng. 2022, 4, 100067. [Google Scholar] [CrossRef]
| Code | HPC (%w/w) | Polymer (%w/w) | Disintegrant (%w/w) | IMC (%w/w) | THY (%w/w) | Extrusion Temperature (°C) | Screw Speed (rpm) |
|---|---|---|---|---|---|---|---|
| F1 | 67.5 | SLP, 22.5 | 10 | 150 | 35 | ||
| F2 | 67.5 | PVP/VA, 22.5 | 10 | 150 | 35 | ||
| F3 | 67.5 | Eu RS, 22.5 | 10 | 150 | 35 | ||
| F4 | 67.5 | Eu RL, 22.5 | 10 | 150 | 35 | ||
| F5 | 67.5 | SSG, 22.5 | 10 | 150 | 35 | ||
| F6 | 67.5 | L-HPC, 22.5 | 10 | 150 | 35 | ||
| F7 | 67.5 | CrosPVP, 22.5 | 10 | 150 | 35 | ||
| F8 | 67.5 | MCC, 22.5 | 10 | 150 | 35 | ||
| F9 | 67.5 | CCM, 22.5 | 10 | 150 | 35 | ||
| F10 | 45 | SLP, 45 | 10 | 150 | 35 | ||
| F11 | 45 | PVP/VA, 45 | 10 | 150 | 35 | ||
| F12 | 40 | PVP/VA, 35 | SSG, 15 | 10 | 135 | 45 | |
| F13 | 20 | EPO, 55 | SSG, 15 | 10 | 135 | 35 | |
| F14 | 20 | PVP/VA, 35 | SSG, 15 | 30 | 160 | 45 | |
| F15 | 20 | EPO, 35 | SSG, 15 | 30 | 160 | 45 | |
| F16 | 5 | PVP/VA, 30 | SSG, 5 | 60 | 160 | 45 | |
| F17 | 5 | EPO, 30 | SSG, 5 | 60 | 160 | 45 |
| Temperature | 150 °C | 160 °C | 200 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | Complex Viscosity (Pa·s) at 0.1 rad/s | Complex Viscosity (Pa·s) at 100 rad/s | Slope of Complex Viscosity | Complex Viscosity (Pa·s) at 0.1 rad/s | Complex Viscosity (Pa·s) at 100 rad/s | Slope of Complex Viscosity | Complex Viscosity (Pa·s) at 0.1 rad/s | Complex Viscosity (Pa·s) at 100 rad/s | Slope of Complex Viscosity |
| F1 | 31,346 | 429 | −0.703 | 17,897 | 372 | −0.636 | 968 | 82 | −0.395 |
| F2 | 33,669 | 799 | −0.579 | 18,781 | 617 | −0.546 | 856 | 185 | −0.299 |
| F3 | 32,346 | 304 | −0.745 | 21,881 | 308 | −0.693 | 3984 | 207 | −0.511 |
| F4 | 38,347 | 110 | −0.906 | 19,636 | 190 | −0.818 | 984 | 42 | −0.564 |
| F5 | 58,240 | 595 | −0.786 | 47,888 | 556 | −0.745 | 9334 | 555 | −0.363 |
| F6 | 188,783 | 567 | −0.992 | 168,311 | 1457 | −0.767 | 31,445 | 618 | −0.555 |
| F7 | 20,773 | 163 | −0.799 | 19,870 | 138 | −0.802 | 7380 | 82 | −0.736 |
| F8 | 5855 | 42 | −0.814 | 5445 | 35 | −0.721 | 1678 | 52 | −0.511 |
| F9 | 49,644 | 667 | −0.731 | 45,230 | 594 | −0.744 | 6684 | 855 | −0.320 |
| F10 | 4899 | 177 | −0.572 | 3286 | 120 | −0.580 | 2768 | 92 | −0.582 |
| F11 | 18,653 | 596 | −0.567 | 18,334 | 744 | −1.196 | 1200 | 218 | −0.517 |
| F12 | 1721 | 14 | −0.693 | 1043 | 15 | −0.680 | 295 | 13 | −0.490 |
| F13 | 5768 | 263 | −0.518 | 4309 | 184 | −0.524 | 3138 | 146 | −0.424 |
| F14 | 8923 | 186 | −0.677 | 7089 | 394 | −0.447 | 6522 | 498 | −0.378 |
| F15 | 23,865 | 89 | −0.909 | 17,776 | 118 | −0.708 | 9334 | 274 | −0.629 |
| F16 | 47,227 | 1630 | −0.521 | 30,560 | 1134 | −0.556 | 7522 | 1099 | −0.284 |
| F17 | 27,773 | 323 | −0.718 | 22,887 | 465 | −0.603 | 19,560 | 292 | −0.636 |
| Code | Crossover Temperature (°C) at Fixed Frequency (1 Hz) | Crossover Frequency (Hz) at 200 °C |
|---|---|---|
| F1 | NC | 5.09 |
| F2 | NC | 0.50 |
| F3 | 194 | 2.34 |
| F4 | NC | 0.74 |
| F5 | 190 | 5.03 |
| F6 | 189 | NC |
| F7 | 200 | NC |
| F8 | 178 | 1.59 |
| F9 | NC | 5.03 |
| F10 | 161 | 0.50 |
| F11 | 167 | NC |
| F12 | 161 | 0.23 |
| F13 | 161 | 7.38 |
| F14 | 150 | 10.8 |
| F15 | 150 | 0.34 |
| F16 | NC | 3.43 |
| F17 | NC | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Than, Y.M.; Suriyarak, S.; Titapiwatanakun, V. Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing. Polymers 2022, 14, 1108. https://doi.org/10.3390/polym14061108
Than YM, Suriyarak S, Titapiwatanakun V. Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing. Polymers. 2022; 14(6):1108. https://doi.org/10.3390/polym14061108
Chicago/Turabian StyleThan, Yee Mon, Sarisa Suriyarak, and Varin Titapiwatanakun. 2022. "Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing" Polymers 14, no. 6: 1108. https://doi.org/10.3390/polym14061108
APA StyleThan, Y. M., Suriyarak, S., & Titapiwatanakun, V. (2022). Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing. Polymers, 14(6), 1108. https://doi.org/10.3390/polym14061108