Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Separator
2.3. Electrode Preparation
2.4. Characterization
2.5. Electrolysis Tests
- GS: determining overpotential (η500) for HER/OER at ± 500 mA/cm2 after 5 h.
- Tafel plot: potential-current density behavior.
- Pretreatment CV: removing adsorbed and absorbed H-species.
- CV: determining Cdl at OCP after HER.
2.6. In Situ Cell Tests
3. Results
3.1. Characterization of Prepared Separator Membranes
Characterization of the Anode and Cathode Material
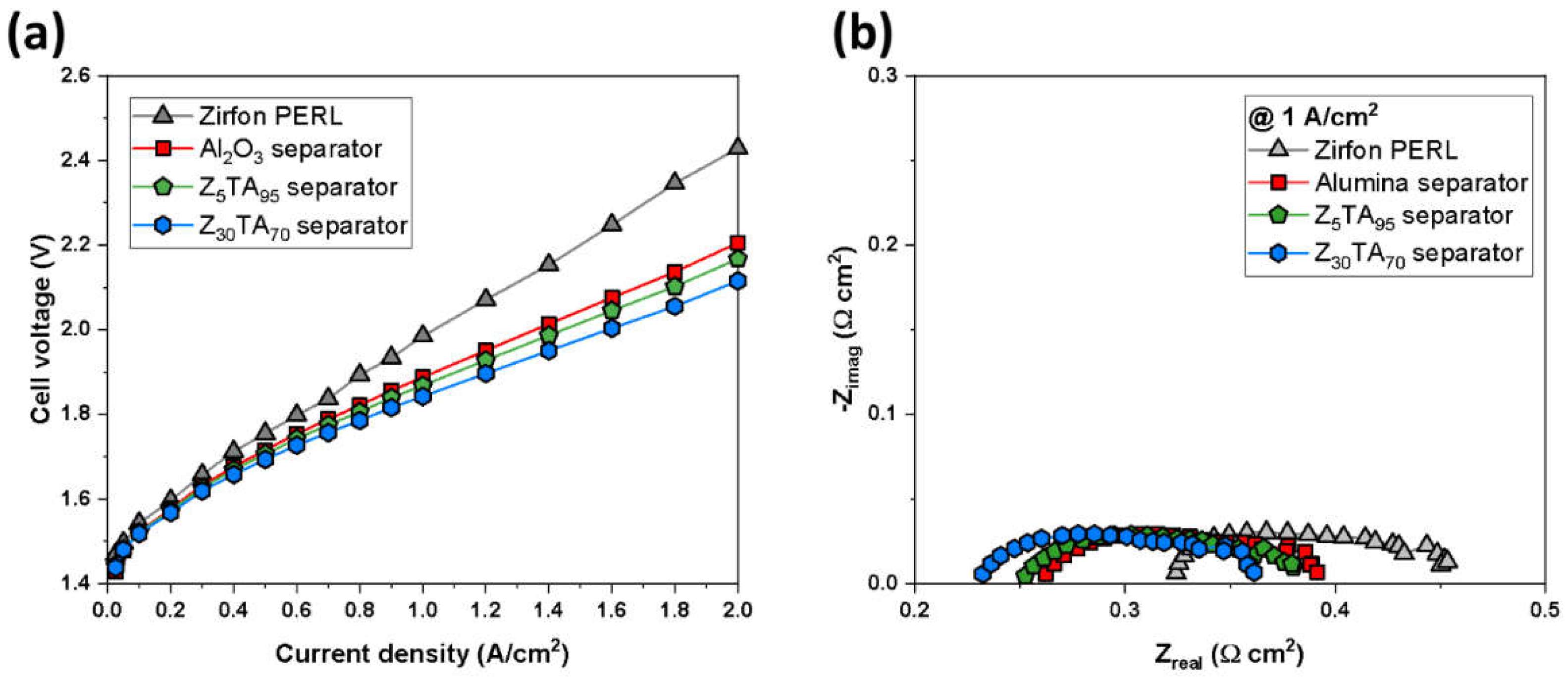
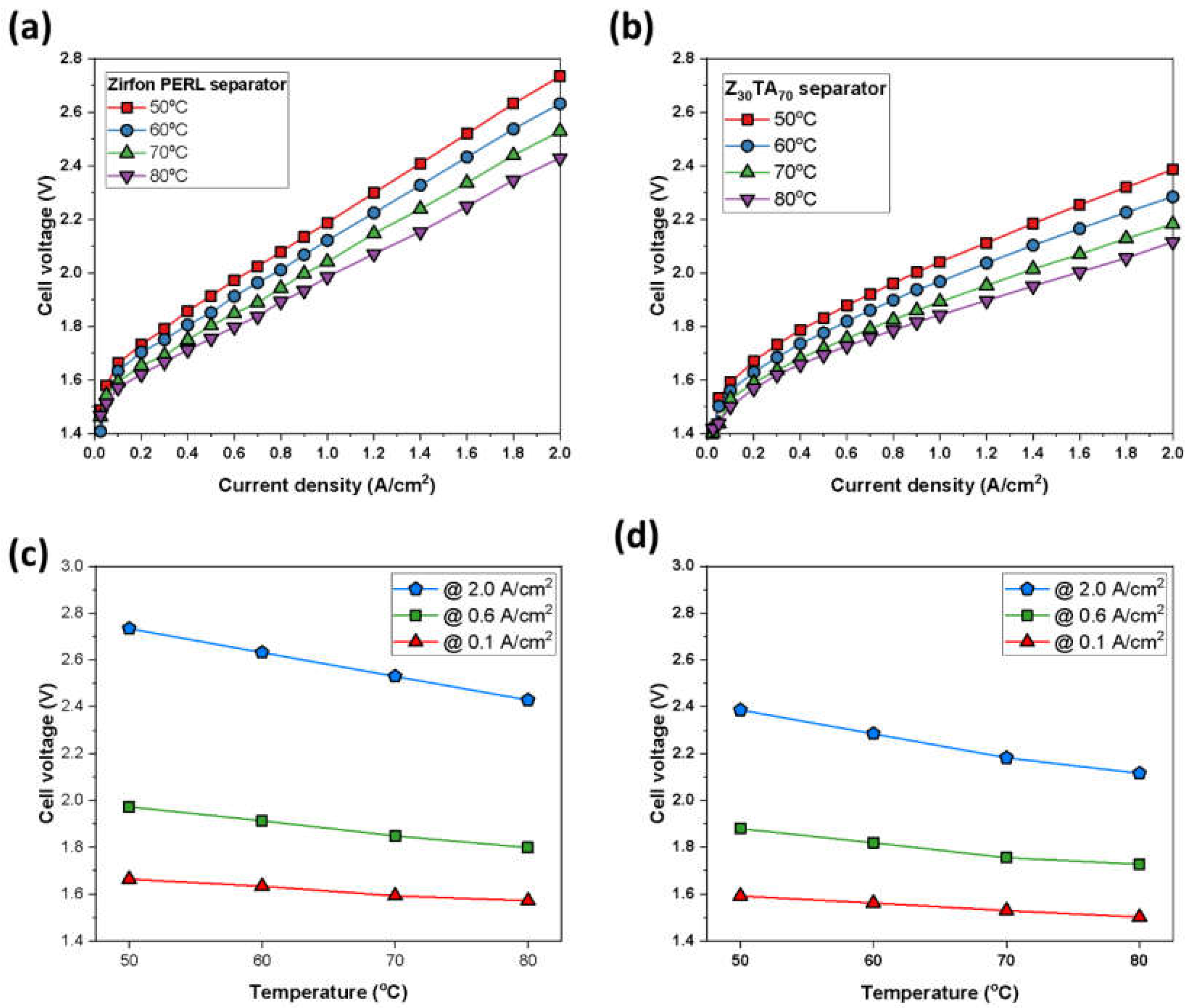
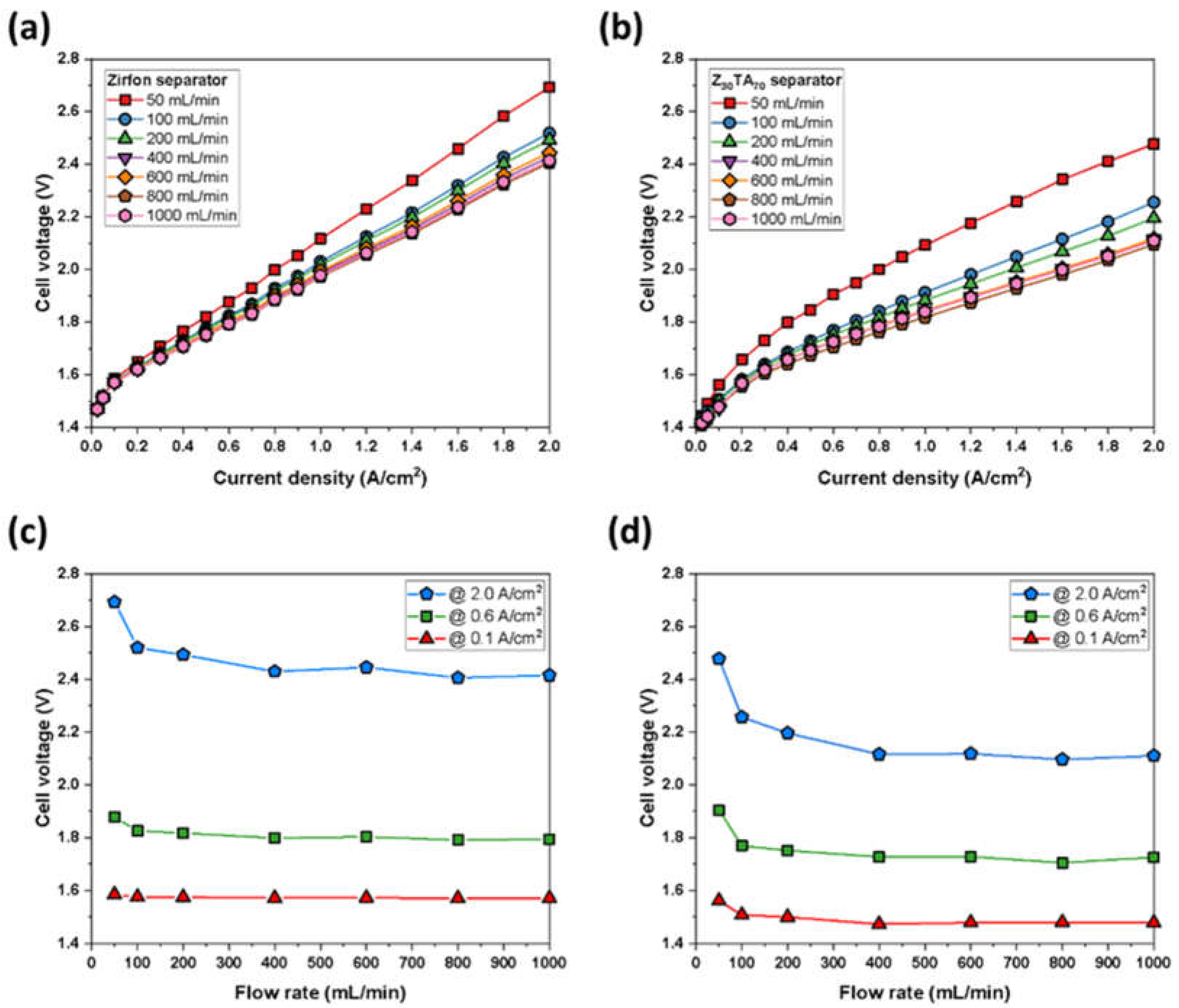
3.2. Cell Test of the Separator Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| K | Permeability of the electrolyte (cm2) |
| Hydrogen gas solubility in the electrolyte (mol m–3 bar–1) | |
| Electrolyte’s viscosity (bar s) | |
| Δp | Differential pressure (bar) |
| AWE | Alkaline water electrolysis |
| GEIS | Galvanostatic electrochemical impedance spectroscopy |
| PTL | Porous transport layer |
| ZTA | Zirconia-toughened alumina |
| RPM | Revolutions per minute |
References
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Tjarks, G.; Mergel, J.; Stolten, D. Dynamic Operation of Electrolyzers—Systems Design and Operating Strategies. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2016; pp. 309–330. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrog. production by alkaline water electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- Albonetti, S.; Perathoner, S.; Quadrelli, A.E. Horizons in Sustainable Industrial Chemistry and Catalysis; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Takach, M.; Sarajlić, M.; Peters, D.; Kroener, M.; Schuldt, F.; von Maydell, K. Review of Hydrog. Production Techniques from Water Using Renewable Energy Sources and Its Storage in Salt Caverns. Energies 2022, 15, 1415. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef] [Green Version]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef] [Green Version]
- Wickramaarachchi, W.A.M.K.P.; Minakshi, M.; Gao, X.; Dabare, R.; Wong, K.W. Hierarchical porous carbon from mango seed husk for electro-chemical energy storage. Chem. Eng. J. Adv. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Baur, C.; Chable, J.; Barlow, A.J.; Fichtner, M.; Banerjee, A.; Chakraborty, S.; Ahuja, R. Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage. Nanoscale Adv. 2019, 1, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Minakshi, M.; Mitchell, D.R.; Jones, R.T.; Pramanik, N.C.; Jean-Fulcrand, A.; Garnweitner, G. A hybrid electrochemical energy storage device using sustainable electrode materials. ChemistrySelect 2020, 5, 1597–1606. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the Anionic Counterpart: Molybdate vs. Tungstate in Energy Storage for Pseudo-Capacitor Applications. Nanomaterials 2021, 11, 580. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Stolten, D. Hydrogen and Fuel Cells: Fundamentals, Technologies, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gandía, L.M.; Oroz, R.; Ursúa, A.; Sanchis, P.; Diéguez, P.M. Renewable Hydrog. Production: Performance of an Alkaline Water Electrolyzer Working under Emulated Wind Conditions. Energy Fuels 2007, 21, 1699–1706. [Google Scholar] [CrossRef]
- Clarke, R.; Giddey, S.; Badwal, S. Stand-alone PEM water electrolysis system for fail safe operation with a renewable energy source. Int. J. Hydrog. Energy 2010, 35, 928–935. [Google Scholar] [CrossRef]
- Millet, P.; Andolfatto, F.; Durand, R. Design and performance of a solid polymer electrolyte water electrolyzer. Int. J. Hydrog. Energy 1996, 21, 87–93. [Google Scholar] [CrossRef]
- David, M.R.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. J. Energy Storage 2019, 23, 392–403. [Google Scholar] [CrossRef] [Green Version]
- Grigoriev, S.; Millet, P.; Korobtsev, S.; Porembskiy, V.; Pepic, M.; Etievant, C.; Puyenchet, C.; Fateev, V. Hydrogen safety aspects related to high-pressure polymer electrolyte membrane water electrolysis. Int. J. Hydrog. Energy 2009, 34, 5986–5991. [Google Scholar] [CrossRef]
- Lettenmeier, P.; Wang, R.; Abouatallah, R.; Helmly, S.; Morawietz, T.; Hiesgen, R.; Kolb, S.; Burggraf, F.; Kallo, J.; Gago, A.; et al. Durable Membrane Electrode Assemblies for Proton Exchange Membrane Electrolyzer Systems Operating at High Current Densities. Electrochim. Acta 2016, 210, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Reier, T.; Nong, H.N.; Teschner, D.; Schlögl, R.; Strasser, P. Electrocatalytic oxygen evolution reaction in acidic environments–reaction mechanisms and catalysts. Adv. Energy Mater. 2017, 7, 1601275. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Estermann, T.; Newborough, M.; Sterner, M. Power-to-gas systems for absorbing excess solar power in electricity distribution networks. Int. J. Hydrog. Energy 2016, 41, 13950–13959. [Google Scholar] [CrossRef]
- Olivier, P.; Bourasseau, C.; Bouamama, P.B. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Qadrdan, M.; Abeysekera, M.; Chaudry, M.; Wu, J.; Jenkins, N. Role of power-to-gas in an integrated gas and electricity system in Great Britain. Int. J. Hydrog. Energy 2015, 40, 5763–5775. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Duch, J.; Sebastián, D.; Lázaro, M.J.; Kotarba, A. Carbon-Based Composites as Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. Materials 2021, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Setzler, B.P.; Lin, S.; Wang, J.; Zhao, Y.; Xu, H.; Pivovar, B.; Tian, B.; Chen, X.; Wu, G.; et al. A Roadmap to Low-Cost Hydrogen with Hydroxide Exchange Membrane Electrolyzers. Adv. Mater. 2019, 31, e1805876. [Google Scholar] [CrossRef]
- Ju, W.; Heinz, M.; Pusterla, L.; Hofer, M.; Fumey, B.; Castiglioni, R.; Pagani, M.; Battaglia, C.; Vogt, U.F. Lab-Scale Alkaline Water Electrolyzer for Bridging Material Fundamentals with Realistic Operation. ACS Sustain. Chem. Eng. 2018, 6, 4829–4837. [Google Scholar] [CrossRef]
- Guilbert, D.; Vitale, G. Hydrogen as a Clean and Sustainable Energy Vector for Global Transition from Fossil-Based to Zero-Carbon. Clean Technol. 2021, 3, 881–909. [Google Scholar] [CrossRef]
- Yodwong, B.; Guilbert, D.; Phattanasak, M.; Kaewmanee, W.; Hinaje, M.; Vitale, G. AC-DC Converters for Electrolyzer Applications: State of the Art and Future Challenges. Electronics 2020, 9, 912. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for Hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326, Corrigendum in Prog. Energy Combust. Sci. 2011, 37, 631. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Saur, G. Wind-To-Hydrogen Project: Electrolyzer Capital Cost Study; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2008. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Mojica, F.; Li, G.; Chuang, P.-Y.A. Experimental study and analytical modeling of an alkaline water electrolysis cell. Int. J. Energy Res. 2017, 41, 2365–2373. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of Hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen Crossover in PEM and Alkaline Water Electrolysis: Mechanisms, Direct Comparison and Mitigation Strategies. J. Electrochem. Soc. 2018, 165, F502–F513. [Google Scholar] [CrossRef]
- Schalenbach, M.; Tjarks, G.; Carmo, M.; Lueke, W.; Mueller, M.; Stolten, D. Acidic or Alkaline? Towards a New Perspective on the Efficiency of Water Electrolysis. J. Electrochem. Soc. 2016, 163, F3197–F3208. [Google Scholar] [CrossRef] [Green Version]
- Suermann, M.; Kiupel, T.; Schmidt, T.J.; Büchi, F.N. Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. J. Electrochem. Soc. 2017, 164, F1187–F1195. [Google Scholar] [CrossRef]
- Corgnale, C.; Greenway, S.; Motyka, T.; Sulic, M.; Hardy, B.; Molten, T.; Ludlow, D. Technical Performance of a Hybrid Thermo-Electrochemical System for High Pressure Hydrogen Compression. ECS Trans. 2017, 80, 41–54. [Google Scholar] [CrossRef]
- Vermeiren, P.; Leysen, R.; Beckers, H.; Moreels, J.P.; Claes, A. The influence of manufacturing parameters on the properties of macroporous Zirfon® separators. J. Porous Mater. 2008, 15, 259–264. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Moreels, J.P.; Leysen, R. Evaluation of the Zirfon® separator for use in alkaline water electrolysis and Ni-H2 batteries. Int. J. Hydrog. Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Schalenbach, M.; Zeradjanin, A.R.; Kasian, O.; Cherevko, S.; Mayrhofer, K.J. A perspective on low-temperature water electrolysis–challenges in alkaline and acidic technology. Int. J. Electrochem. Sci. 2018, 13, 1173–1226. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.H.; Yuk, J.; Kim, S.; Song, Y.; So, S.; Lee, K.T.; Kim, T.-H. Highly selective porous separator with thin skin layer for alkaline water electrolysis. J. Power Sources 2022, 524, 231059. [Google Scholar] [CrossRef]
- Brauns, J.; Schönebeck, J.; Kraglund, M.R.; Aili, D.; Hnát, J.; Žitka, J.; Mues, W.; Jensen, J.O.; Bouzek, K.; Turek, T. Evaluation of Diaphragms and Membranes as Separators for Alkaline Water Electrolysis. J. Electrochem. Soc. 2021, 168, 014510. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Lee, H.I.; Dung, D.T.; Kim, J.; Pak, J.H.; Kim, S.K.; Cho, H.S.; Cho, W.C.; Kim, C.H. The synthesis of a Zirfon-type porous separator with reduced gas crossover for alkaline electrolyzer. Int. J. Energy Res. 2020, 44, 1875–1885. [Google Scholar] [CrossRef]
- Lee, H.I.; Mehdi, M.; Kim, S.K.; Cho, H.S.; Kim, M.J.; Cho, W.C.; Rhee, Y.W.; Kim, C.H. Advanced Zirfon-type porous separator for a high-rate alkaline electrolyser operating in a dynamic mode. J. Membr. Sci. 2020, 616, 118541. [Google Scholar] [CrossRef]
- Alam, A.; Park, C.; Lee, J.; Ju, H. Comparative analysis of performance of alkaline water electrolyzer by using porous separator and ion-solvating polybenzimidazole membrane. Renew. Energy 2020, 166, 222–233. [Google Scholar] [CrossRef]
- Padaki, M.; Isloor, A.M.; Wanichapichart, P.; Ismail, A.F. Preparation and characterization of sulfonated polysulfone and N-phthloyl chitosan blend composite cation-exchange membrane for desalination. Desalination 2012, 298, 42–48. [Google Scholar] [CrossRef]
- Yunos, M.Z.; Harun, Z.; Basri, H.; Ismail, A.F. Studies on fouling by natural organic matter (NOM) on polysulfone membranes: Effect of polyethylene glycol (PEG). Desalination 2014, 333, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Renaud, R.; Leroy, R. Separator materials for use in alkaline water electrolysers. Int. J. Hydrog. Energy 1982, 7, 155–166. [Google Scholar] [CrossRef]
- Kukovičič, I.; Šoster, R.; Brumen, M.; Ribitsch, V.; Wiegel, D.; Arnold, K.; Stropnik, Č. Chemical modification and characterization of the surface of polysulfone membranes. Acta Chim. Slov. 2000, 47, 339–347. [Google Scholar]
- Zhu, L.; Song, H.; Zhang, D.; Wang, G.; Zeng, Z.; Xue, Q. Negatively charged polysulfone membranes with hydrophilicity and antifouling properties based on in situ cross-linked polymerization. J. Colloid Interface Sci. 2017, 498, 136–143. [Google Scholar] [CrossRef]
- Teotia, R.S.; Kalita, D.; Singh, A.K.; Verma, S.K.; Kadam, S.S.; Bellare, J.R. Bifunctional Polysulfone-Chitosan Composite Hollow Fiber Membrane for Bioartificial Liver. ACS Biomater. Sci. Eng. 2015, 1, 372–381. [Google Scholar] [CrossRef]
- Aerts, P.; Kuypers, S.; Genne, I.; Leysen, R.; Mewis, J.; Vankelecom, I.F.J.; Jacobs, P.A. Polysulfone−ZrO2 Surface Interactions. The Influence on Formation, Morphology and Properties of Zirfon-Membranes. J. Phys. Chem. B 2006, 110, 7425–7430. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; You, Y.; Zhang, S.; Zhao, Y. Polysulfone and zirconia composite separators for alkaline water electrolysis. Front. Chem. Sci. Eng. 2013, 7, 154–161. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Lee, J.; Kim, S.-K.; Cho, H.-S.; Kim, M.; Cho, W.; Joo, J.; Kim, C.-H. Cerium Oxide–Polysulfone Composite Separator for an Advanced Alkaline Electrolyzer. Polymers 2020, 12, 2821. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Ravichandran, S.; Davidson, D.J.; Vasudevan, S.; Sozhan, G. Polyvinyl Alcohol Based Membrane as Separator for Alkaline Water Electrolyzer. Sep. Sci. Technol. 2011, 46, 1563–1570. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic–inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Otero, J.; Sese, J.; Michaus, I.; Maria, M.S.; Guelbenzu, E.; Irusta, S.; Carrilero, I.; Arruebo, M. Sulphonated polyether ether ketone diaphragms used in commercial scale alkaline water electrolysis. J. Power Sources 2014, 247, 967–974. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Vinodh, R.; Purushothaman, M.; Sangeetha, D. Novel quaternized polysulfone/ZrO2 composite membranes for solid alkaline fuel cell applications. Int. J. Hydrog. Energy 2011, 36, 7291–7302. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.; Li, W.; You, Y.; Xu, W.; Zhang, S. The influence of manufacturing parameters and adding support layer on the properties of Zirfon® separators. Front. Chem. Sci. Eng. 2014, 8, 295–305. [Google Scholar] [CrossRef]
- Yeo, R.S.; McBreen, J.; Kissel, G.; Kulesa, F.; Srinivasan, S. Perfluorosulphonic acid (Nafion) membrane as a separator for an advanced alkaline water electrolyser. J. Appl. Electrochem. 1980, 10, 741–747. [Google Scholar] [CrossRef]
- Aili, D.; Hansen, M.K.; Andreasen, J.W.; Zhang, J.; Jensen, J.O.; Bjerrum, N.J.; Li, Q. Porous poly(perfluorosulfonic acid) membranes for alkaline water electrolysis. J. Membr. Sci. 2015, 493, 589–598. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochimica Acta 2014, 137, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Structural and Electrochemical Properties of Nanocomposite Polymer Electrolyte for Electrochemical Devices. Ind. Eng. Chem. Res. 2014, 53, 14993–15001. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ramakrishna, S.; Krishna, S.V.; Srilatha, K.; Devi, B.R.; Himabindu, V. Synthesis of titanium (IV) oxide composite membrane for Hydrogen production through alkaline water electrolysis. South Afr. J. Chem. Eng. 2018, 25, 54–61. [Google Scholar] [CrossRef]
- Diaz, L.; Coppola, R.; Abuin, G.; Escudero-Cid, R.; Herranz, D.; Ocón, P. Alkali-doped polyvinyl alcohol—Polybenzimidazole membranes for alkaline water electrolysis. J. Membr. Sci. 2017, 535, 45–55. [Google Scholar] [CrossRef]
- Bernäcker, C.I.; Rauscher, T.; Büttner, T.; Kieback, B.; Röntzsch, L. A Powder Metallurgy Route to Produce Raney-Nickel Electrodes for Alkaline Water Electrolysis. J. Electrochem. Soc. 2019, 166, F357–F363. [Google Scholar] [CrossRef]
- Nickell, R.A.; Zhu, W.H.; Payne, R.U.; Cahela, D.R.; Tatarchuk, B.J. Hg/HgO electrode and Hydrogen evolution potentials in aqueous sodium hydroxide. J. Power Sources 2006, 161, 1217–1224. [Google Scholar] [CrossRef]
- Kwon, G.-H.; Kim, T.W.; Lee, H.I.; Cho, W.C.; Kim, H. Synthesis of ZrO2 Nanorods and Their Application as Membrane Materials. J. Korean Ceram. Soc. 2019, 56, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Gao, F.; Wang, H.; Cheng, X.; Xie, Z.; Xing, F.; An, L. Hollow Alumina Microsphere Chain Networks. J. Am. Ceram. Soc. 2009, 92, 280–282. [Google Scholar] [CrossRef]
- Vermeiren, P.; Moreels, J.P.; Leysen, R. Porosity in composite zirfon® membranes. J. Porous Mater. 1996, 3, 33–40. [Google Scholar] [CrossRef]
- Schalenbach, M.; Lueke, W.; Stolten, D. Hydrogen Diffusivity and Electrolyte Permeability of the Zirfon PERL Separator for Alkaline Water Electrolysis. J. Electrochem. Soc. 2016, 163, F1480–F1488. [Google Scholar] [CrossRef] [Green Version]
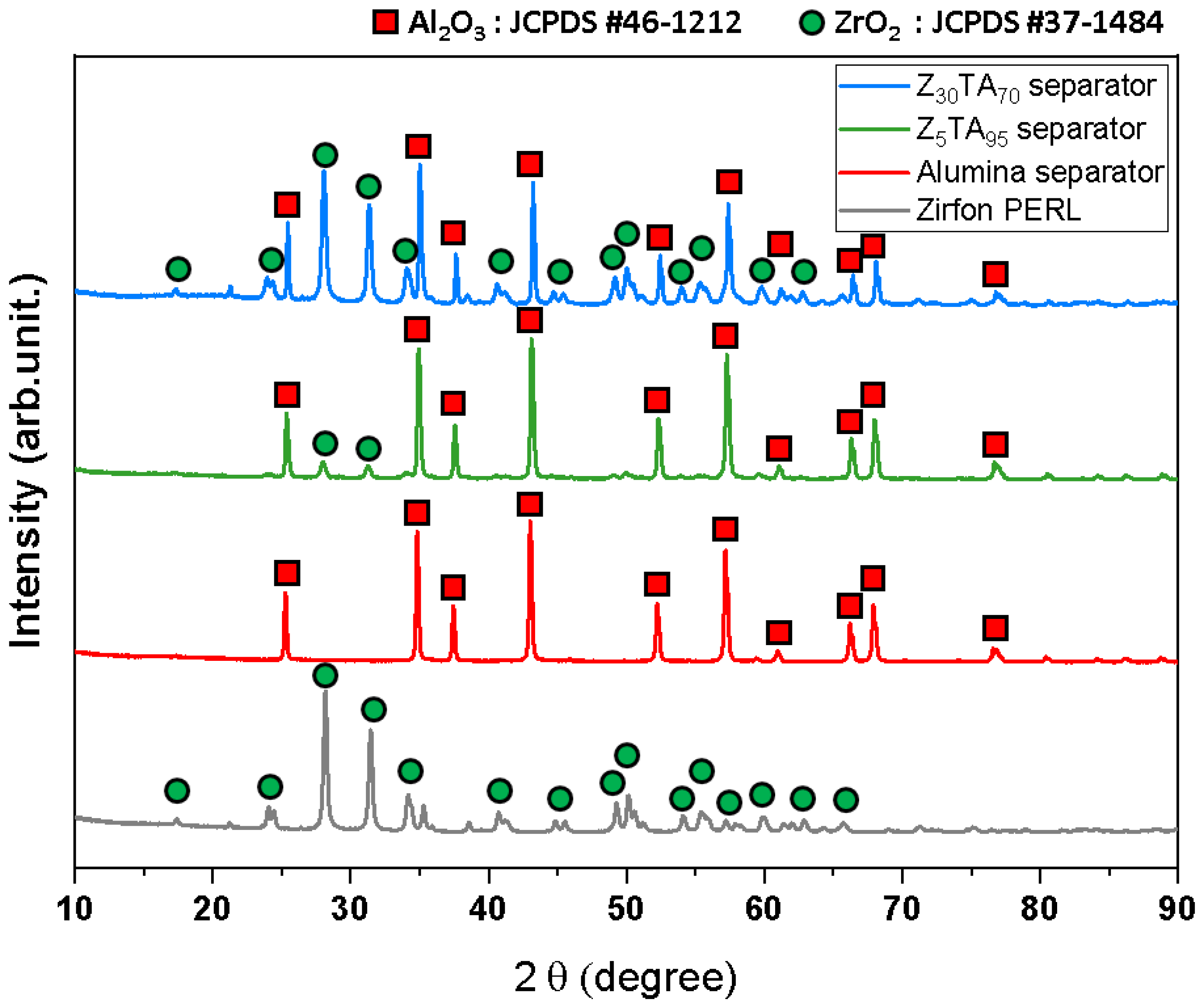


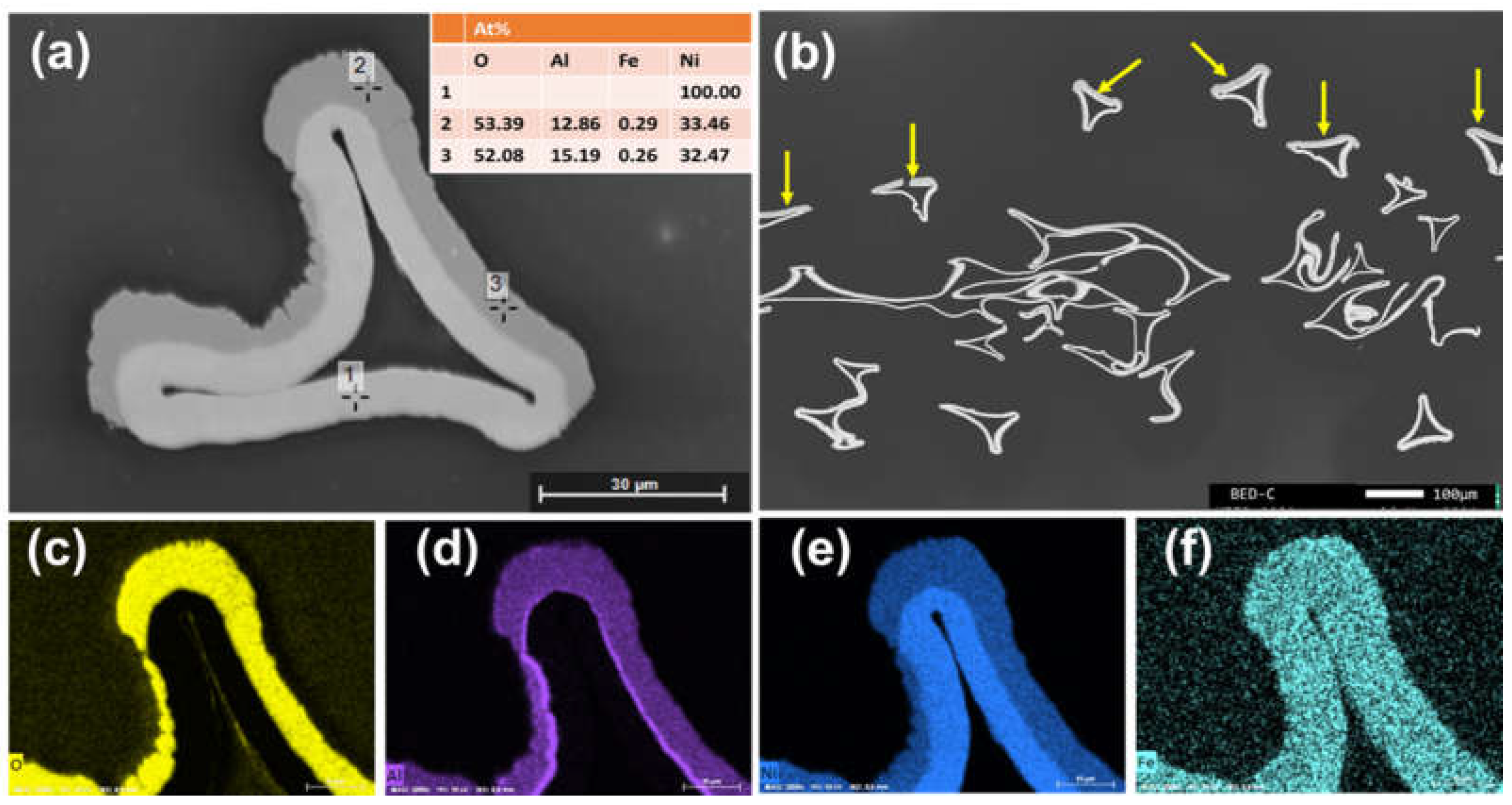

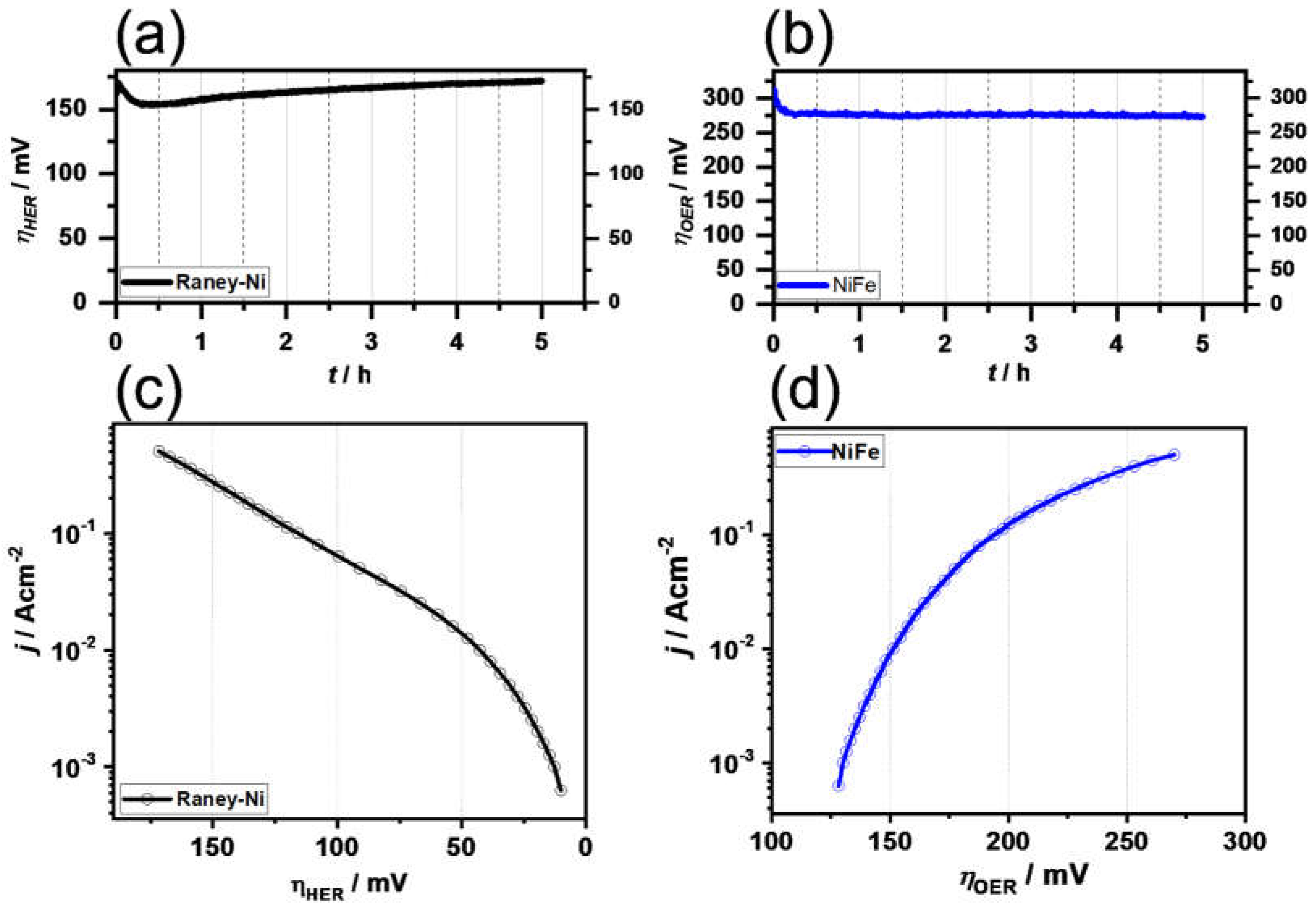
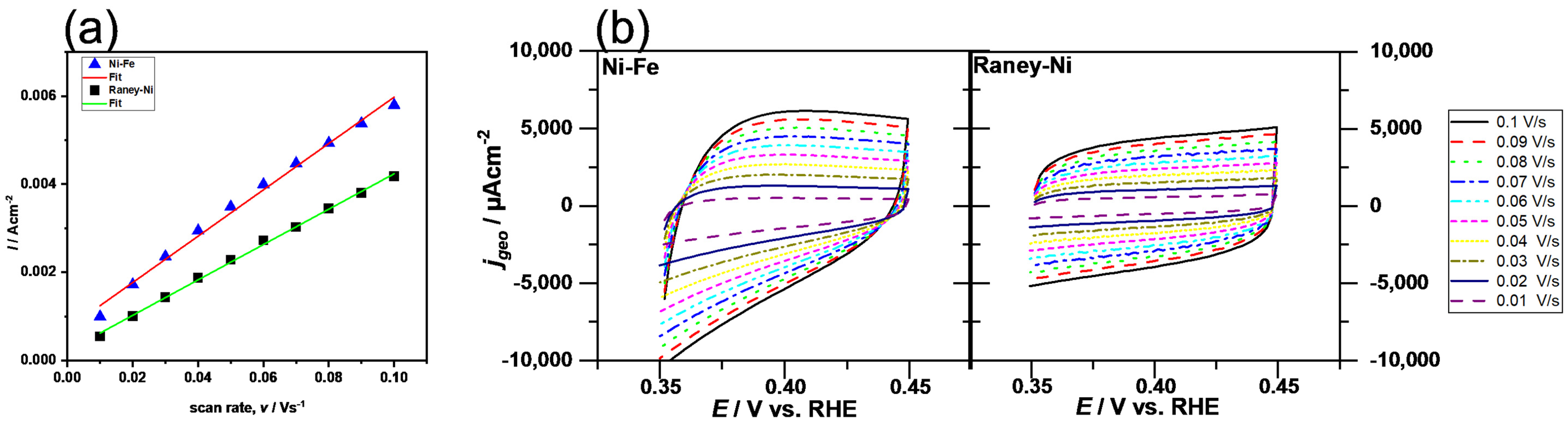


| Samples | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) |
|---|---|---|
| Al2O3:ZrO2 (70:30) Z30TA70 | 14 | 0.060 |
| Al2O3:ZrO2 (95:5) Z5TA95 | 9.82 | 0.027 |
| Al2O3:ZrO2 (100:0) | 8.87 | 0.025 |
| Unit | Z30TA70 | Z5TA95 | Al2O3:ZrO2 (100:0) | Zirfon® | |
|---|---|---|---|---|---|
| Total pore area | [m2 g−1] | 13.65 | 15.85 | 16.39 | 19.26 |
| Average pore diameter | [nm] | 125.10 | 81.68 | 79.63 | 99.06 |
| Bulk density | [g/mL] | 1.26 | 1.42 | 1.40 | 1.10 |
| Apparent density | [g/mL] | 2.75 | 2.64 | 2.58 | 2.34 |
| Porosity | [%] | 54.06 | 46.09 | 45.78 | 52.82 |
| Thickness | m | 430 ± 5 | 430 ± 15 | 460 ± 5 | 470 ± 10 |
| Sample | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Z30TA70 | 14 | 0.060 | 17 |
| Z30TA70 after 2 weeks | 14 | 0.065 | 19 |
| Z30TA70 after 4 weeks | 14 | 0.070 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.F.; Lee, H.I.; Bernäcker, C.I.; Weißgärber, T.; Lee, S.; Kim, S.-K.; Cho, W.-C. Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer. Polymers 2022, 14, 1173. https://doi.org/10.3390/polym14061173
Ali MF, Lee HI, Bernäcker CI, Weißgärber T, Lee S, Kim S-K, Cho W-C. Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer. Polymers. 2022; 14(6):1173. https://doi.org/10.3390/polym14061173
Chicago/Turabian StyleAli, Muhammad Farjad, Hae In Lee, Christian Immanuel Bernäcker, Thomas Weißgärber, Sechan Lee, Sang-Kyung Kim, and Won-Chul Cho. 2022. "Zirconia Toughened Alumina-Based Separator Membrane for Advanced Alkaline Water Electrolyzer" Polymers 14, no. 6: 1173. https://doi.org/10.3390/polym14061173








