Antifouling PVC Catheters by Gamma Radiation-Induced Zwitterionic Polymer Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
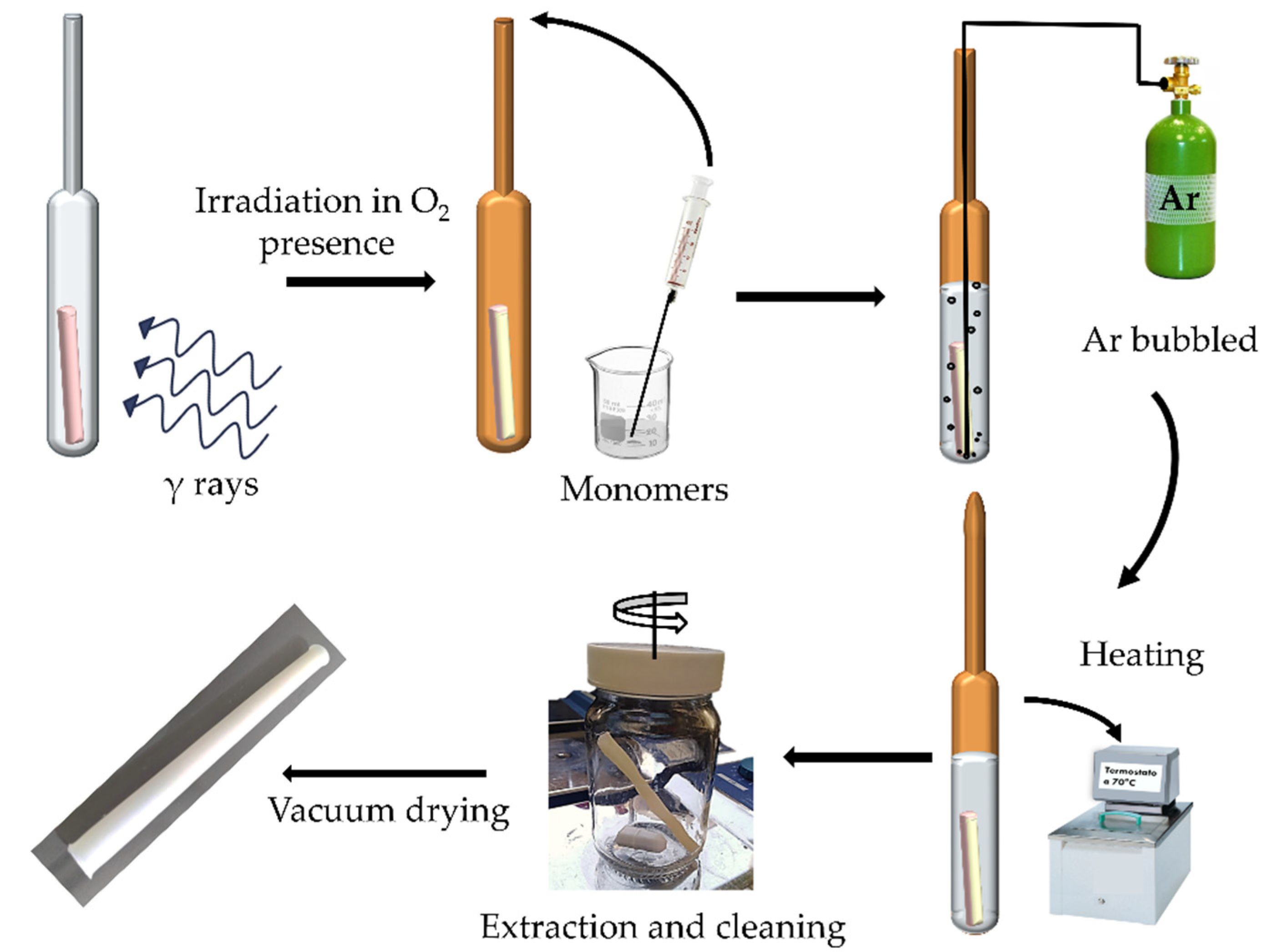
2.2. VP Graft on PVC Catheters (PVC-g-4VP)
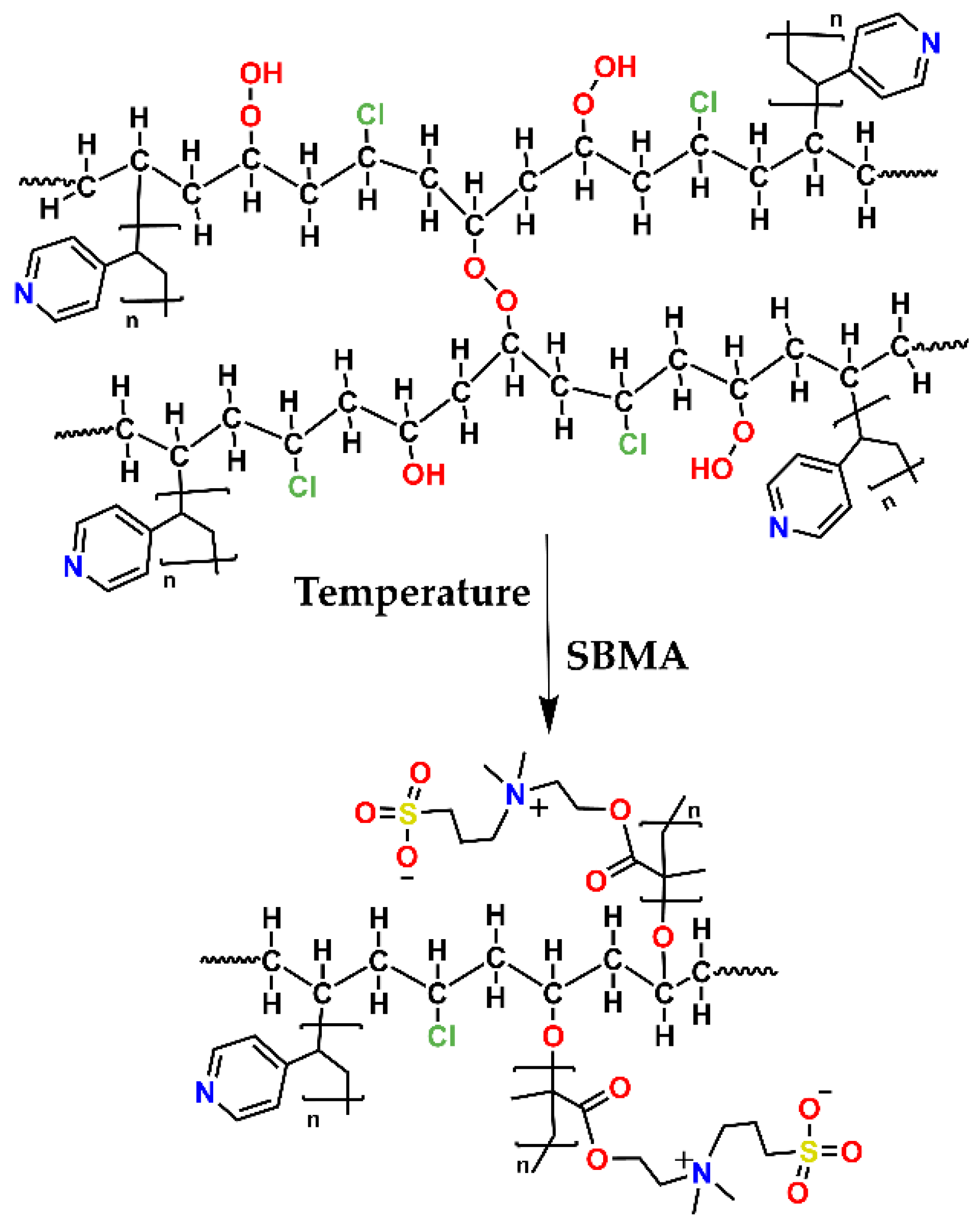
2.3. SBMA Graft on PVC-g-4VP Catheters [(PVC-g-4VP)-g-SBMA)]
2.4. FT-IR and Thermal Characterization
2.5. Wettability
2.5.1. Dynamic Contact Angle
2.5.2. Swelling
2.6. pH and Thermo Responsiveness
2.7. Protein Adsoption
2.7.1. Protein Extraction
2.7.2. Protein Quantification
3. Results
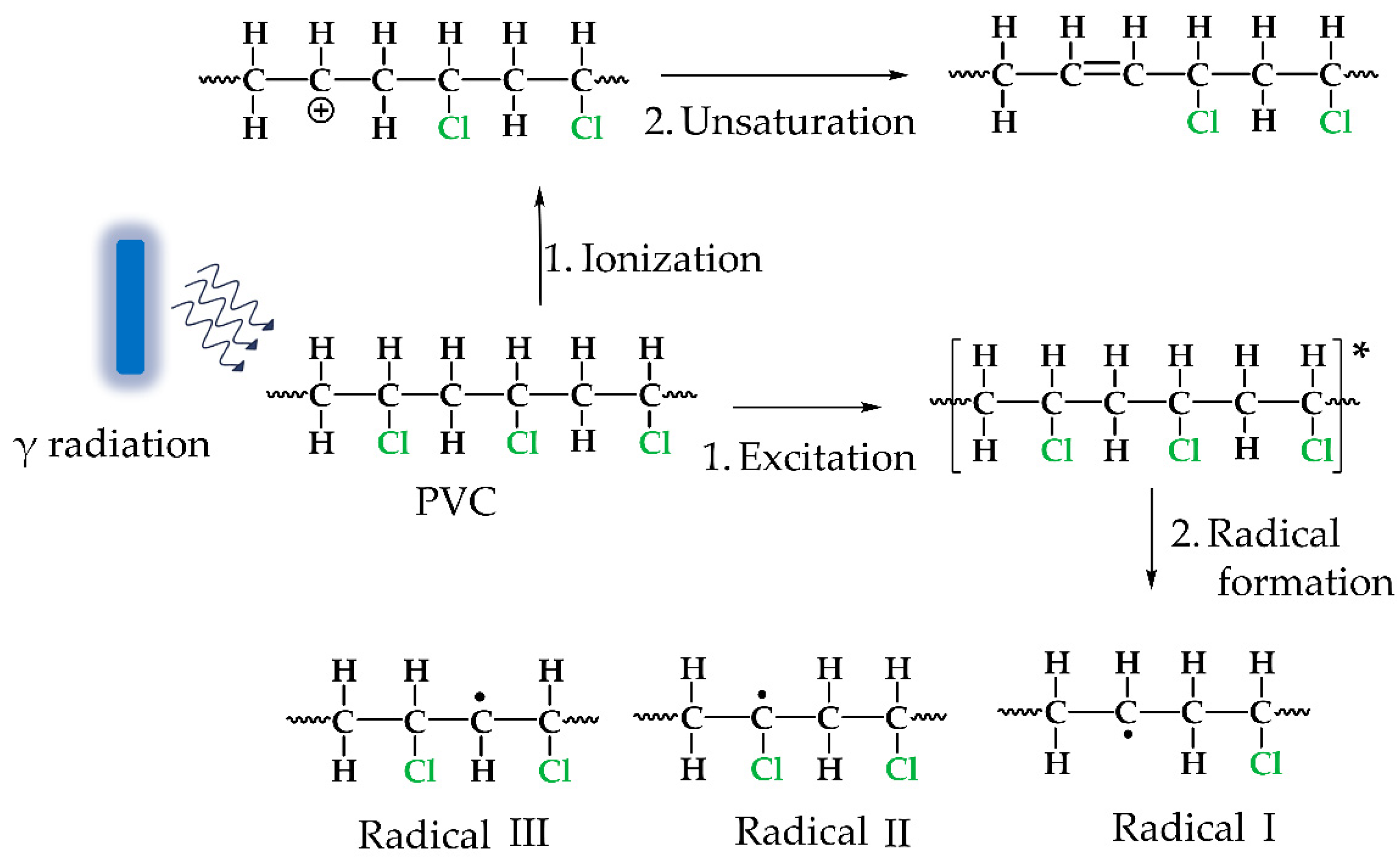
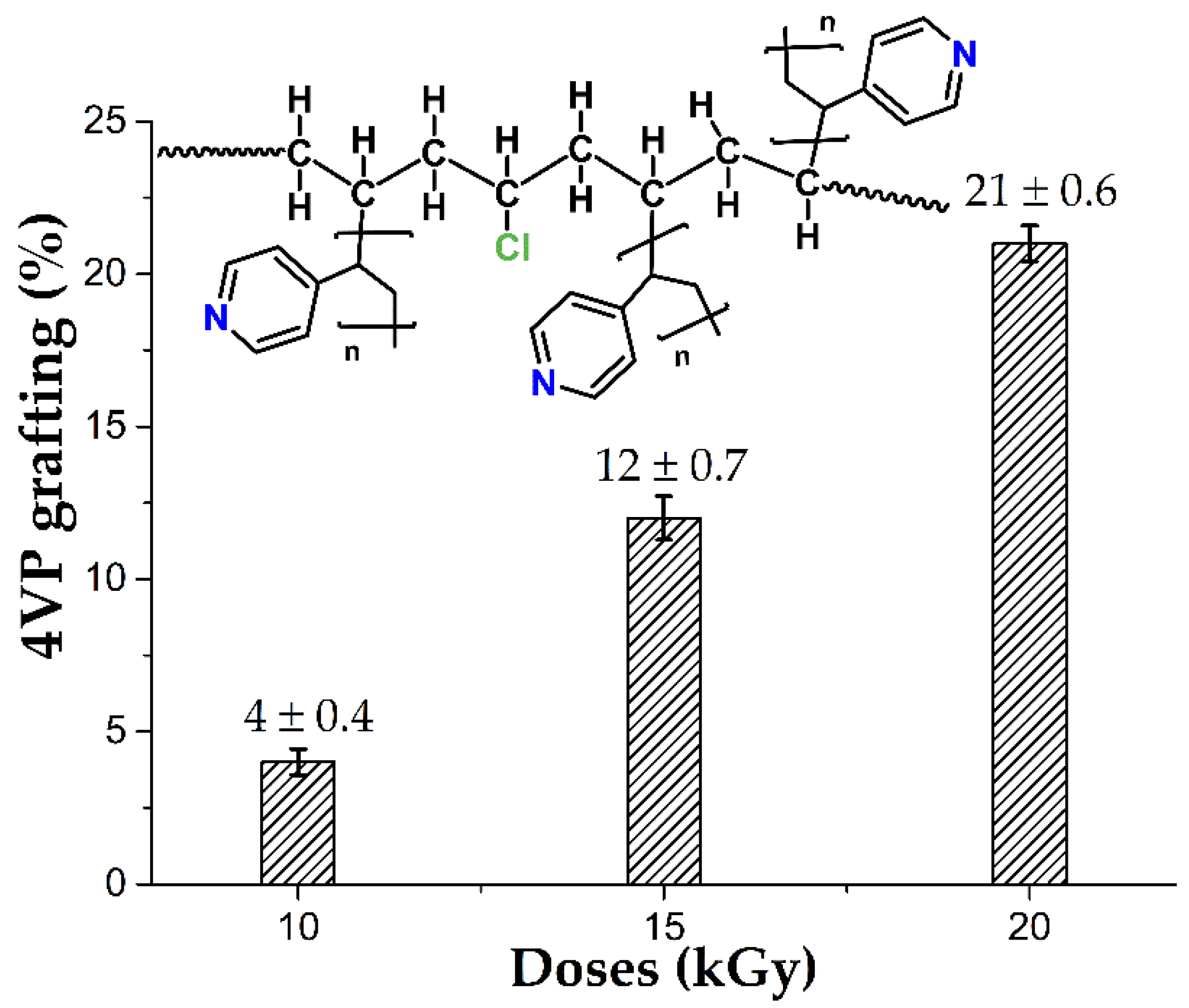
3.1. PVC-g-4VP Synthesis
3.2. SBMA Graft on PVC-g-4VP Catheters [(PVC-g-4VP)-g-SBMA)]
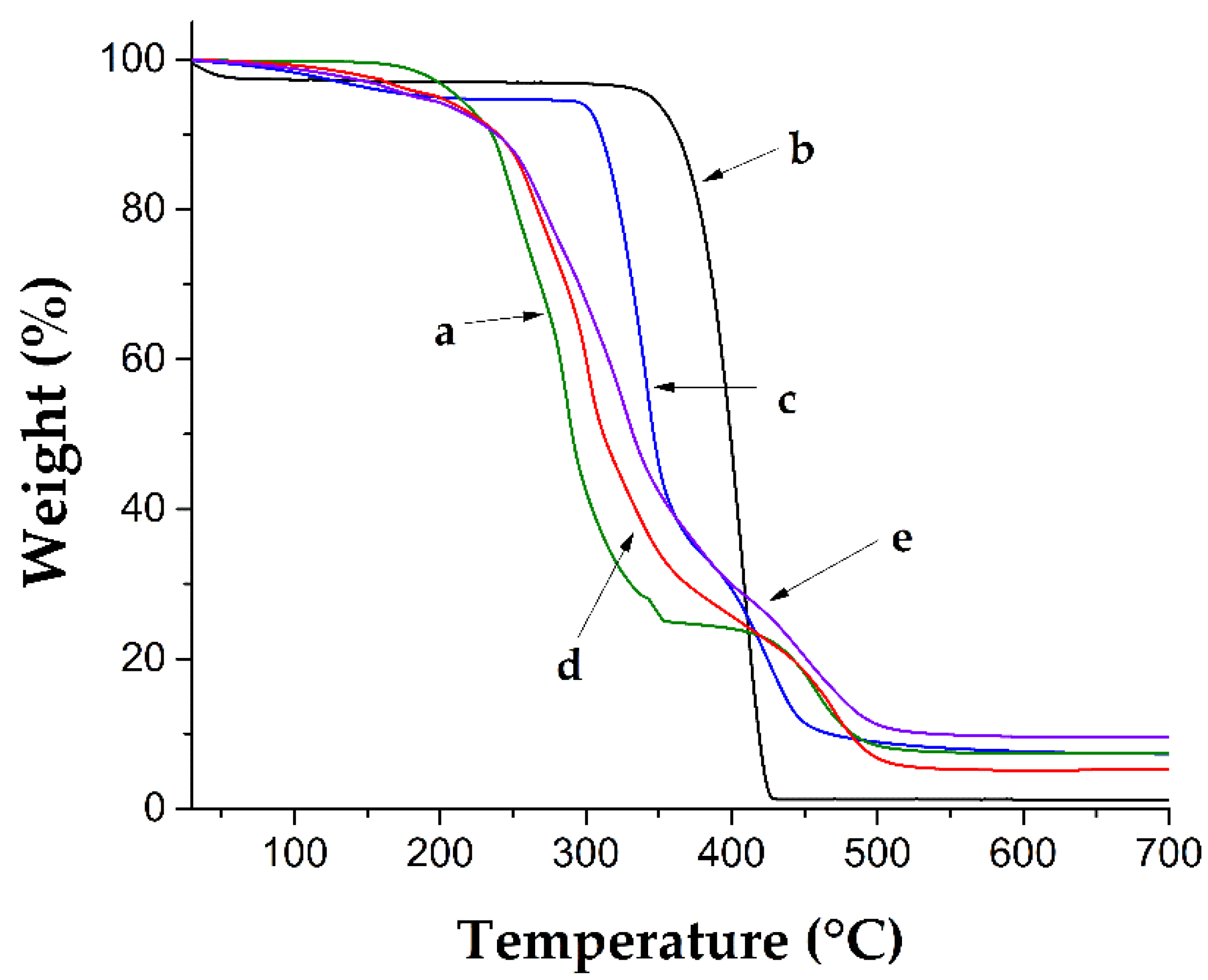
3.3. FT-IR and Thermal Characterization
3.4. Wettability
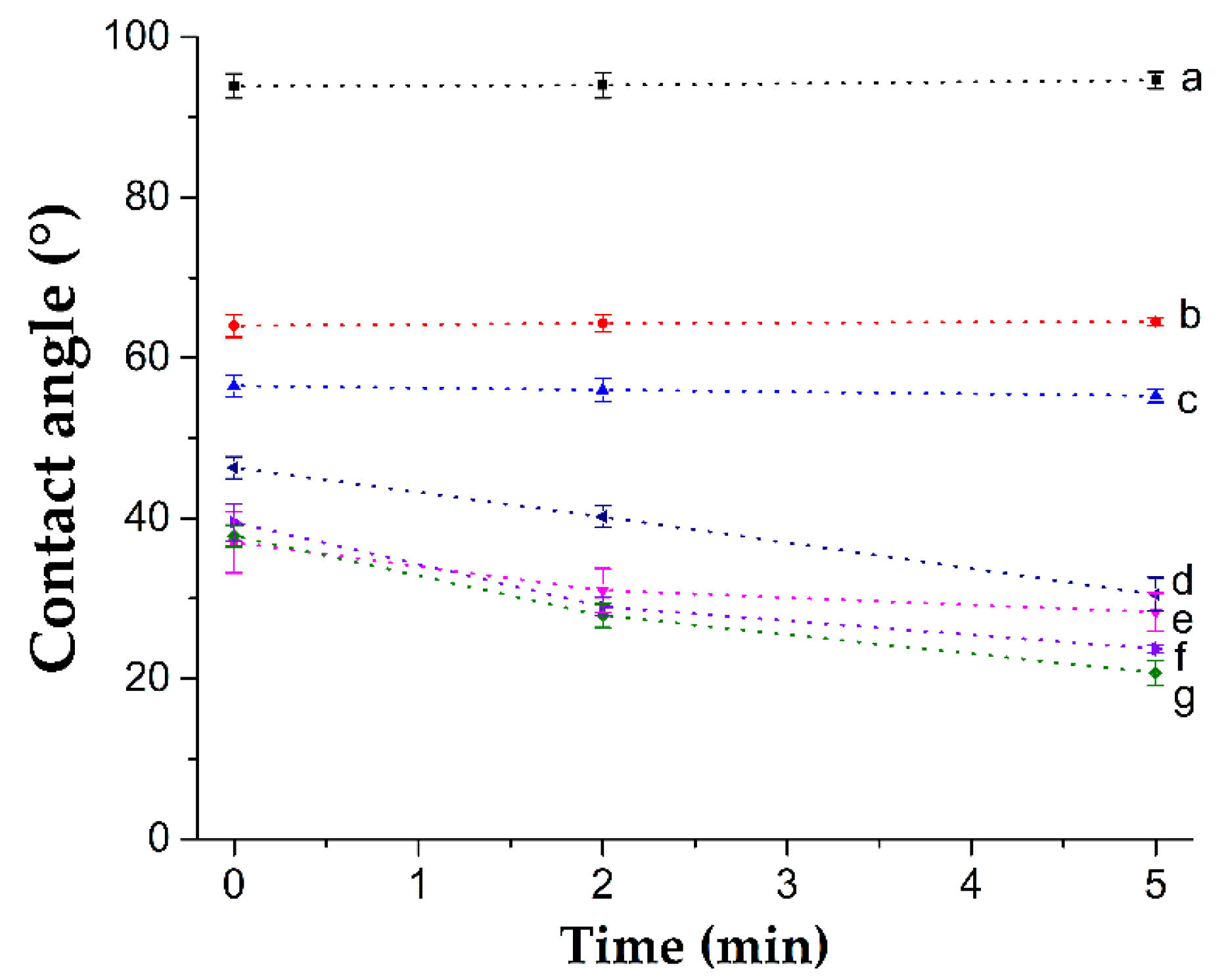
3.4.1. Dynamic Contact Angle
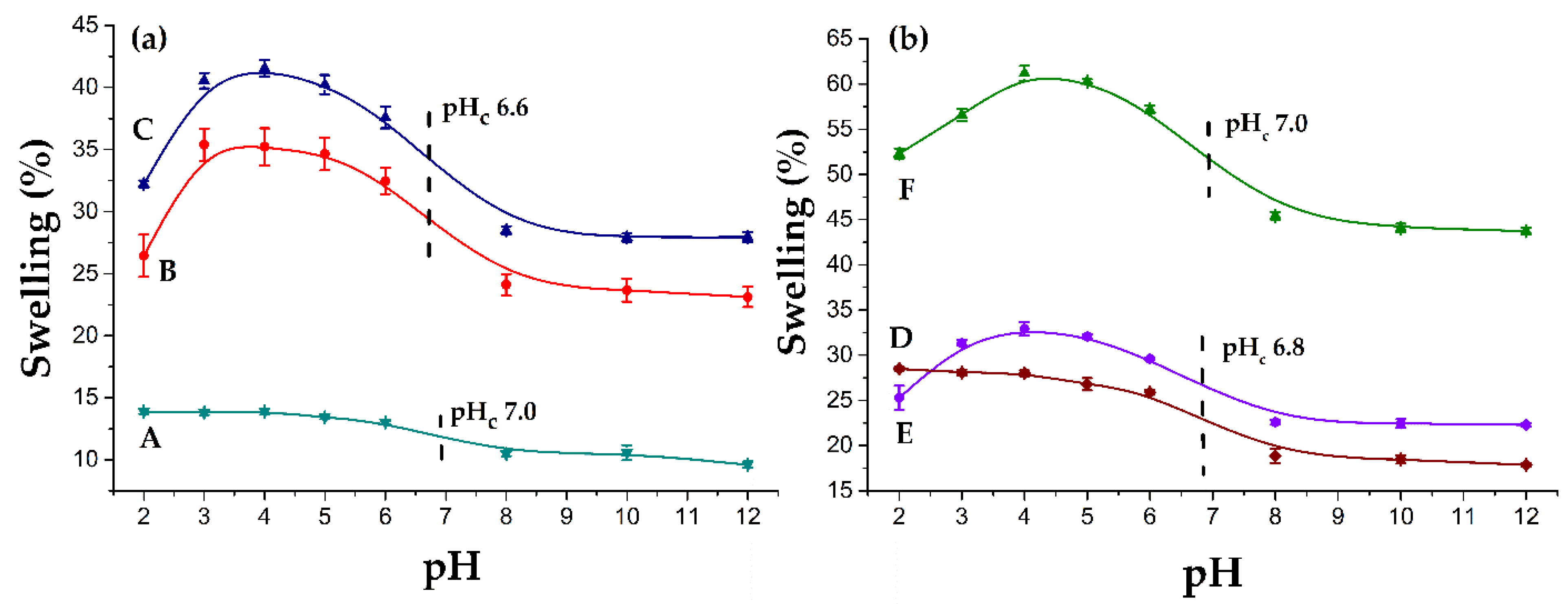
3.4.2. Swelling
3.5. pH Responsiveness
3.6. Protein Adsorption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Floyd, K.A.; Eberly, A.R.; Hadjifrangiskou, M. 3-Adhesion of bacteria to surfaces and biofilm formation on medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Amsterdan, The Netherlands, 2017; pp. 47–95. ISBN 978-0-08-100382-4. [Google Scholar]
- Lovetri, K.; Gawande, P.; Yakandawala, N.; Madhyastha, S. Biofouling and anti-fouling of medical devices. In Biofouling Types, Impact and Anti-Fouling; Chan, J., Wong, S., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 105–128. ISBN 978-1-60876-501-0. [Google Scholar]
- Zhang, Y.; Liu, Y.; Ren, B.; Zhang, D.; Xie, S.; Chang, Y.; Yang, J.; Wu, J.; Xu, L.; Zheng, J. Fundamentals and Applications of Zwitterionic Antifouling Polymers. J. Phys. D. Appl. Phys. 2019, 52, 403001. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial Infections: Epidemiology, Prevention, Control and Surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Sahin, F.; Celik, N.; Ceylan, A.; Pekdemir, S.; Ruzi, M.; Onses, M.S. Antifouling superhydrophobic surfaces with bactericidal and SERS activity. Chem. Eng. J. 2022, 431, 133445. [Google Scholar] [CrossRef]
- Peng, J.; Su, Y.; Chen, W.; Zhao, X.; Jiang, Z.; Dong, Y.; Zhang, Y.; Liu, J.; Fan, X. Antifouling Membranes Prepared by a Solvent-Free Approach via Bulk Polymerization of 2-Hydroxyethyl Methacrylate. Ind. Eng. Chem. Res. 2013, 52, 13137–13145. [Google Scholar] [CrossRef]
- Mrabet, B.; Nguyen, M.N.; Majbri, A.; Chergui, S.; Turmine, M.; Bakhrouf, A.; Chehimi, M. Anti-fouling poly(2-hydoxyethyl methacrylate) surface coatings with specific bacteria recognition capabilities. Surf. Sci.-Surf. SCI 2009, 603, 2422–2429. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, S.; Zhang, D.; Zhang, W.; Zhang, H.; Zou, J.; Mao, Z.; Yuan, Y.; Gao, C.; Liu, R. Impact of Antifouling PEG Layer on the Performance of Functional Peptides in Regulating Cell Behaviors. J. Am. Chem. Soc. 2019, 141, 16772–16780. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications Toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An Introduction To Zwitterionic Polymer Behavior and Applications in Solution and at Surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Klein, J. Control of Surface Forces through Hydrated Boundary Layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Chapter 14-Electrostatic forces between surfaces in liquids. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 291–340. ISBN 978-0-12-375182-9. [Google Scholar]
- Chang, Y.; Chang, W.-J.; Shih, Y.-J.; Wei, T.-C.; Hsiue, G.-H. Zwitterionic Sulfobetaine-Grafted Poly(Vinylidene Fluoride) Membrane with Highly Effective Blood Compatibility via Atmospheric Plasma-Induced Surface Copolymerization. ACS Appl. Mater. Interfaces 2011, 3, 1228–1237. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Sivakumar, M.; Ang, M.B.M.Y.; Patra, T.; Almodovar, J.; Wickramasinghe, S.R.; Hung, W.-S.; Lai, J.-Y. Zwitterionic Polymer Brush Grafted on Polyvinylidene Difluoride Membrane Promoting Enhanced Ultrafiltration Performance with Augmented Antifouling Property. Polymer 2020, 12, 1303. [Google Scholar] [CrossRef]
- Fan, Y.-J.; Pham, M.T.; Huang, C.-J. Development of Antimicrobial and Antifouling Universal Coating via Rapid Deposition of Polydopamine and Zwitterionization. Langmuir 2019, 35, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sundaram, H.S.; Ella, J.-R.; He, N.; Jiang, S. Low-Fouling Electrospun PLLA Films Modified with Zwitterionic Poly(Sulfobetaine Methacrylate)-Catechol Conjugates. Acta Biomater. 2016, 40, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Meng, J.; Xu, M.; Yuan, T.; Yang, N.; Sun, T.; Zhang, Y.; Feng, X.; Cheng, B. A Simple but Efficient Zwitterionization Method towards Cellulose Membrane with Superior Antifouling Property and Biocompatibility. J. Memb. Sci. 2015, 492, 547–558. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-Responsive Polymers and Their Applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef] [Green Version]
- Ortega, A.; Bucio, E.; Burillo, G. New Interpenetrating Polymer Networks of N-Isopropylacrylamide/N-Acryloxysuccinimide: Synthesis and characterization. Polym. Bull. 2008, 60, 515–524. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, H.; et al. Temperature-Responsive Properties of Poly(4-Vinylpyridine) Coatings: Influence of Temperature on the Wettability, Morphology, and Protein Adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sin, M.C.; Chen, S.H.; Chang, Y. Hemocompatibility of Zwitterionic Interfaces and Membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of Protein Adsorption: Surface-Induced Conformational Changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Duarte-Peña, L.; López-Saucedo, F.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Modification of Indwelling PVC Catheters by Ionizing Radiation with Temperature-and pH-Responsive Polymers for Antibiotic Delivery. Radiat. Phys. Chem. 2022, 193, 110005. [Google Scholar] [CrossRef]
- Shen, C.-H. Quantification and Analysis of Proteins. In Diagnostic Molecular Biology; Shen, C.-H., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 187–214. ISBN 978-0-12-802823-0. [Google Scholar]
- He, F. BCA (Bicinchoninic Acid) Protein Assay. Bio-Protocol 2011, 1, e44. [Google Scholar] [CrossRef]
- Miller, A.A. Radiation Chemistry of Polyvinyl Chloride. J. Phys. Chem. 1959, 63, 1755–1759. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.H.C.; Bucio, E. Radiation Grafting for the Functionalization and Development of Smart Polymeric Materials. Top. Curr. Chem. 2016, 374, 1–28. [Google Scholar] [CrossRef]
- Dispenza, C.; Alessi, S.; Spadaro, J. Radiation processing of polymers in aqueous media. In Applications of Ionizing Radiation in Materials Processing; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; pp. 291–326. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson/Prentice Hall: London, UK, 2005; ISBN 9780131291928. [Google Scholar]
- Shafi, H.Z.; Wang, M.; Gleason, K.K.; Khan, Z. Synthesis of Surface-Anchored Stable Zwitterionic Films for Inhibition of Biofouling. Mater. Chem. Phys. 2020, 239, 121971. [Google Scholar] [CrossRef]
- Lucio, D.S.V.; Rivera-Armenta, J.L.; Rivas-Orta, V.; Díaz-Zavala, N.P.; Páramo-García, U.; Rivas, N.V.G.; Cinco, M.Y.C. Manufacturing of Composites from Chicken Feathers and Polyvinyl Chloride (PVC). Handb. Compos. Renew. Mater. 2017, 1–8, 159–174. [Google Scholar] [CrossRef]
- Ibrahim, G.P.S.; Isloor, A.M.; Inamuddin; Asiri, A.M.; Ismail, N.; Ismail, A.F.; Ashraf, G.M. Novel, One-Step Synthesis of Zwitterionic Polymer Nanoparticles via Distillation-Precipitation Polymerization and Its Application for Dye Removal Membrane. Sci. Rep. 2017, 7, 15889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelachvili, J.N. 10-Unifying concepts in intermolecular and interparticle forces. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 191–204. ISBN 978-0-12-375182-9. [Google Scholar]
- Kan, K.H.M.; Li, J.; Wijesekera, K.; Cranston, E.D. Polymer-Grafted Cellulose Nanocrystals as pH-Responsive Reversible Flocculants. Biomacromolecules 2013, 14, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymer 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic Grafting of Sulfobetaine Methacrylate (SBMA) on Hydrophobic PVDF Membranes for Enhanced Anti-Fouling and Anti-Wetting in the Membrane Distillation of Oil Emulsions. J. Memb. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Q.; Hu, H.; Wang, X.; Zhou, F. Grafting Zwitterionic Polymer Brushes via Electrochemical Surface-Initiated Atomic-Transfer Radical Polymerization for Anti-Fouling Applications. J. Mater. Chem. B 2014, 2, 5352–5357. [Google Scholar] [CrossRef]
- Guo, Y.-S.; Mi, Y.-F.; Ji, Y.-L.; An, Q.-F.; Gao, C.-J. One-Step Surface Grafting Method for Preparing Zwitterionic Nanofiltation Membrane via in Situ Introduction of Initiator in Interfacial Polymerization. ACS Appl. Polym. Mater. 2019, 1, 1022–1033. [Google Scholar] [CrossRef]
- Harijan, M.; Singh, M. Zwitterionic Polymers in Drug Delivery: A Review. J. Mol. Recognit. 2022, 35, e2944. [Google Scholar] [CrossRef]
- Liu, P.; Xu, G.; Pranantyo, D.; Xu, L.Q.; Neoh, K.-G.; Kang, E.-T. pH-Sensitive Zwitterionic Polymer as an Antimicrobial Agent with Effective Bacterial Targeting. ACS Biomater. Sci. Eng. 2018, 4, 40–46. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte-Peña, L.; Bucio, E. Antifouling PVC Catheters by Gamma Radiation-Induced Zwitterionic Polymer Grafting. Polymers 2022, 14, 1185. https://doi.org/10.3390/polym14061185
Duarte-Peña L, Bucio E. Antifouling PVC Catheters by Gamma Radiation-Induced Zwitterionic Polymer Grafting. Polymers. 2022; 14(6):1185. https://doi.org/10.3390/polym14061185
Chicago/Turabian StyleDuarte-Peña, Lorena, and Emilio Bucio. 2022. "Antifouling PVC Catheters by Gamma Radiation-Induced Zwitterionic Polymer Grafting" Polymers 14, no. 6: 1185. https://doi.org/10.3390/polym14061185






