High Amylose-Based Bio Composites: Structures, Functions and Applications
Abstract
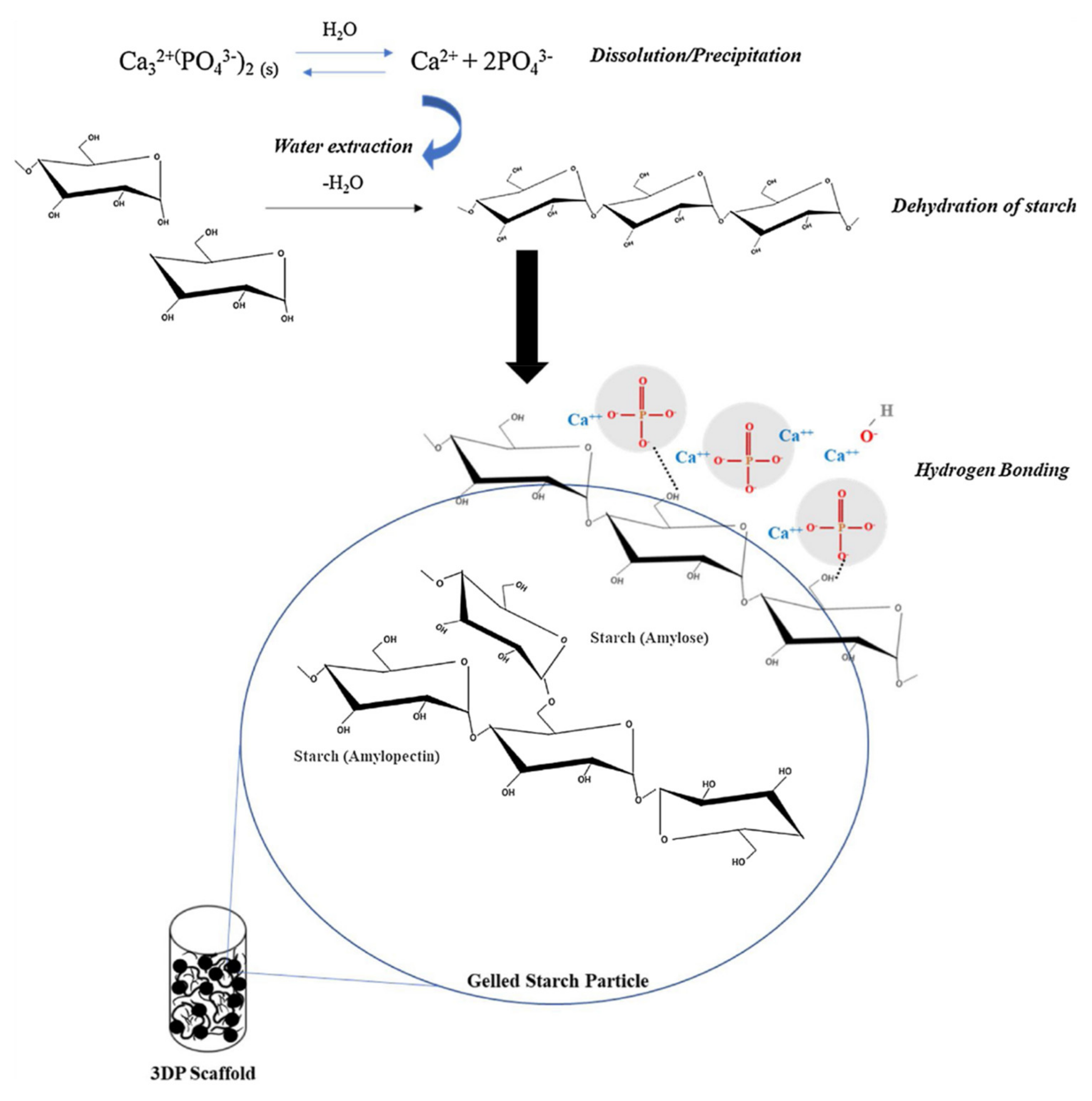
:1. Introduction
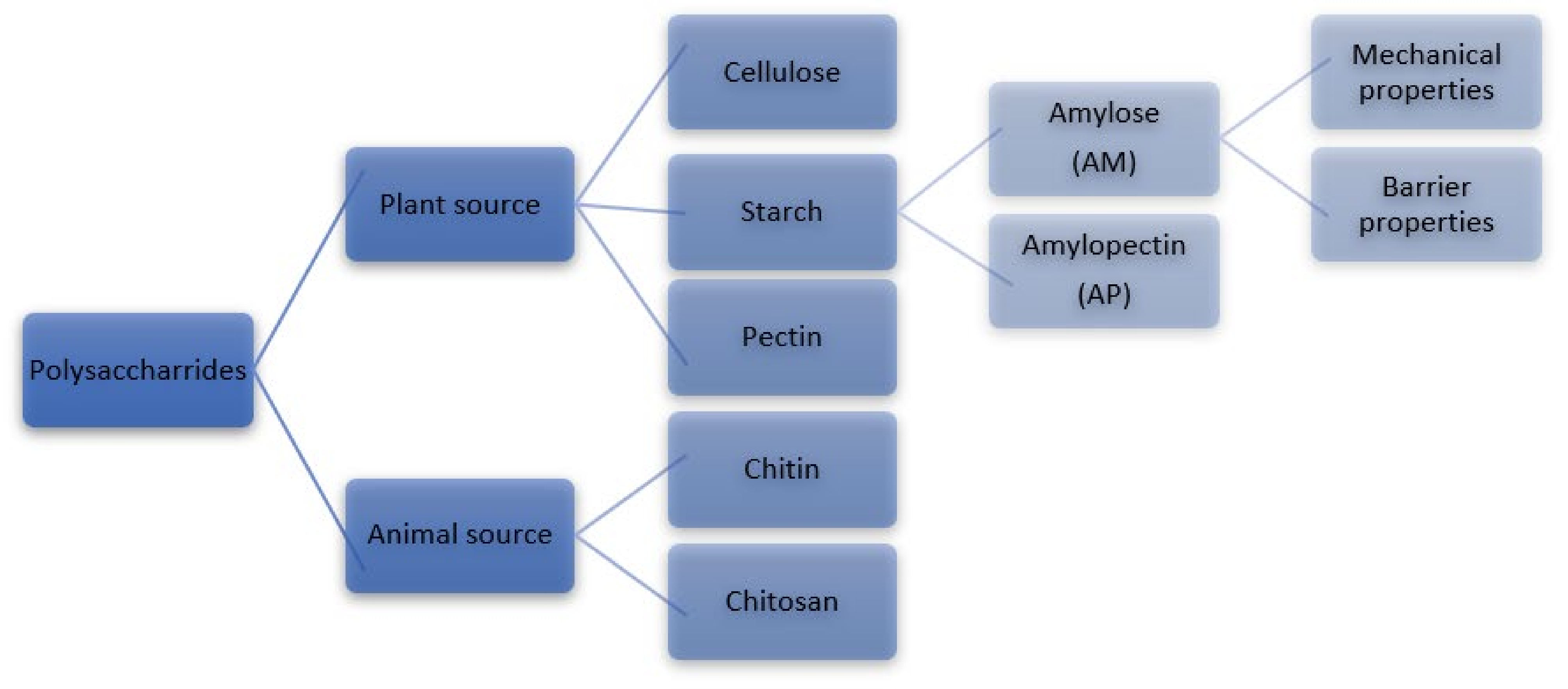
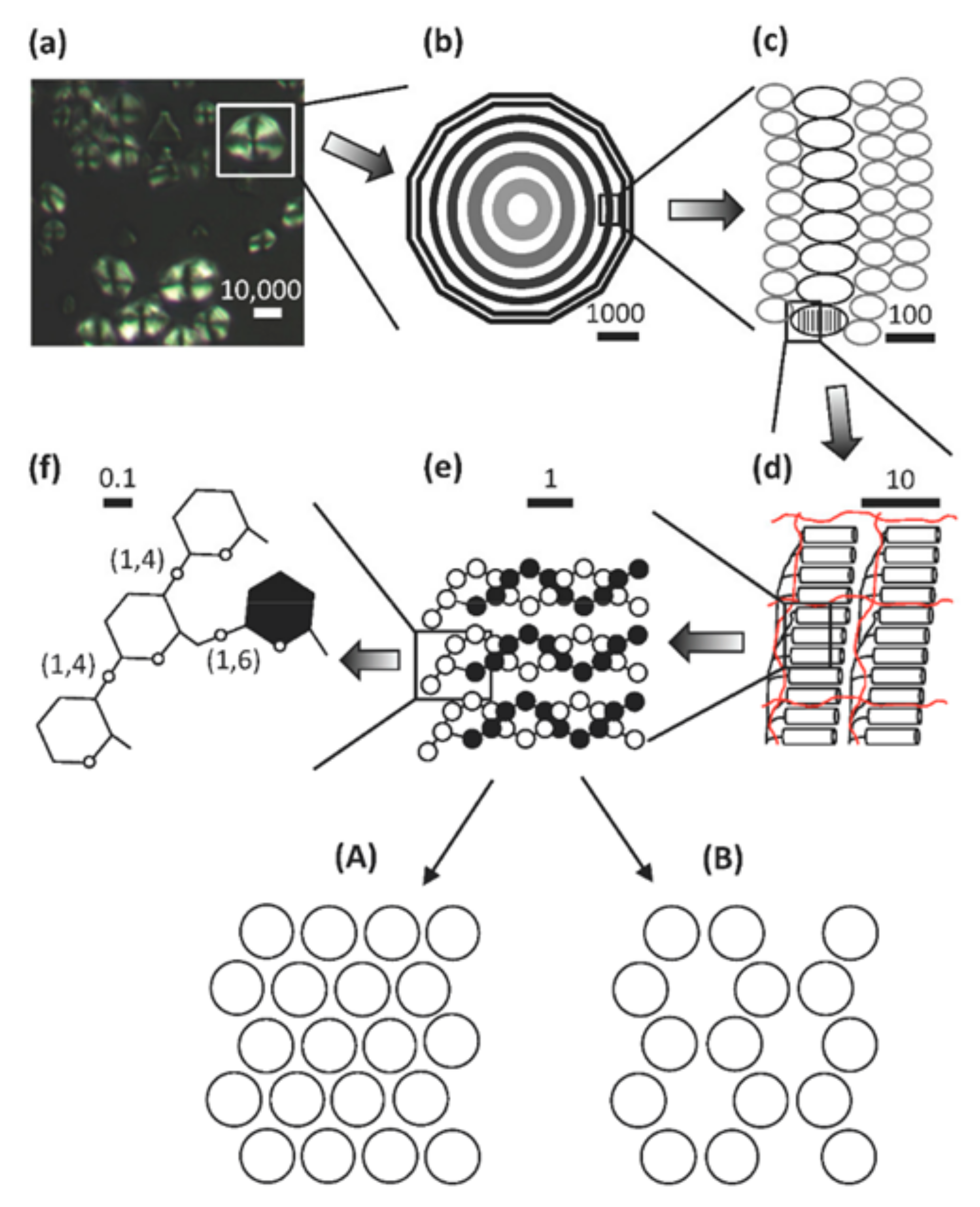
2. High Amylose Starch (HAS)
3. HAS-Based Films Production Technology and Protocols
4. Properties of HAS Films
4.1. Mechanical Properties
4.2. Plasticizing Agent Effect
4.3. Barrier Properties
| Starch | AM% | Plasticizer % | WVP × 1010 (g/ms Pa) | Gas Permeability O2 × 1010 (cm3/ms Pa) | CO2 × 109 (cm3/ms Pa) | References & Remarks |
|---|---|---|---|---|---|---|
| Amylomaize | 65 | 0 | 2.62 | 28.05 | 26.45 | [32] Gly and Sorbitol concentrations 20% |
| Gly | 2.14 | 3.21 | 3.85 | |||
| Gly + SO | 1.76 | 4.39 | 2.36 | |||
| Sorbitol | 1.21 | 2.96 | 2.28 | |||
| Sorbitol + SO | 0.97 | 3.43 | 2.18 | |||
| HAS | 80 | 0 | 0.52 | ND | ND | [26] Xylitol and glycerol concentrations 20%. |
| G | 0.43 | ND | ND | |||
| Xylitol | 0.11 (g mm/m2 h kPa) | ND | ND | |||
| Gly + Xylitol | 0.14 | ND | ND | |||
| AM-only | 99 | 0 | 0.351 (cm3 mm Pa/m2 24 h) | ND | ND | [10] |
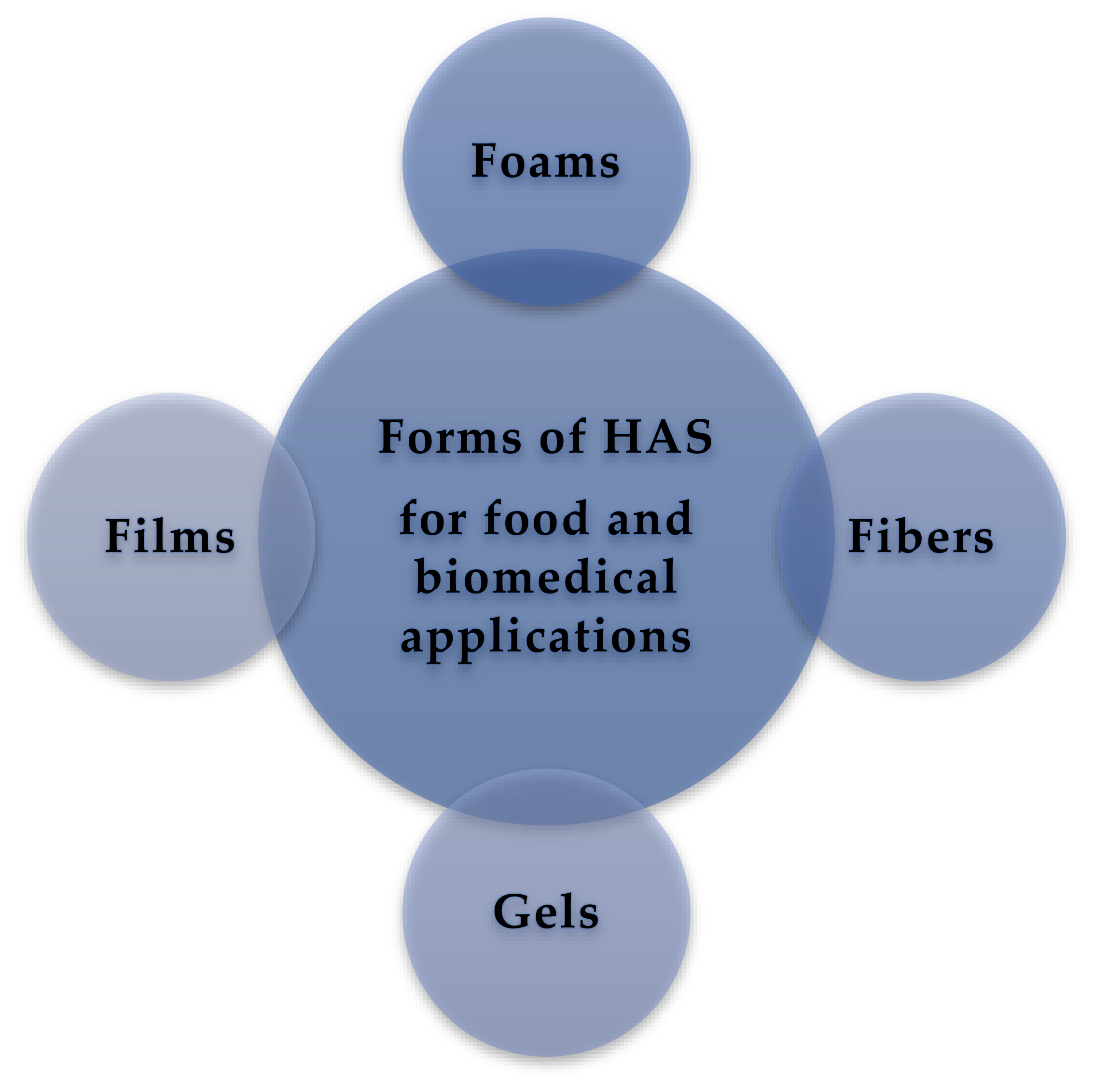
5. Forms of HAS
5.1. Foams
5.2. Starch Fibers
5.3. Gels
6. Uses of HAS
6.1. HAS Complexes
6.2. HAS Based Food Products
6.3. Applications of Starch-Based Films in Food Applications
6.4. Antimicrobial Food Packaging
6.5. Application of HAS as a Biomedical Material
| Starch | AM% | Method of Preparation | In Combination with | Forms and Application | Remarks | References |
|---|---|---|---|---|---|---|
| Amaizo5 Amylomaize | 50 70 | Baking mould | Foam | AAM content increased: density, foam flexibility decreased, trapped air bubbles, more pores produced. | [35] | |
| HAS | 50 | Baking mould |
| Foam | Strength increased at high and low humidities. Heavy, with irregular shapes. | [36] |
| HA-acetate (Acetate high amylose starch) | 50 | Baking mould |
| Foam | Lightweight with regular shape. | [36] |
| HP-HylonVII (Hydroxyl-propyl Hylon VII) | 70 | Baking mould | Foam | Viscosity and elasticity of the paste were too low to expand foam using the baking. | [36] | |
| HAS | 70 | Extrusion |
| Foam | Reduce shrinkage at 95% RH. Enhanced tensile properties. | [37] |
| HA-acetate | 70% | Twin-screw extruder |
| Foam | Corncob and cellulose enhanced hydrophobic properties. Bulk densities and strength increased. | [38] |
| HylonVII | 70% | Electro spinning |
| Fibers Diameter (200–700 nm) | Stable in water, non-toxic, 10 times more strength than uncross linked fibers. | [41] |
| Hylon VII | 70% | Electro spinning |
| Fibers Diameter (50 ± 5µm) | Uniform fibers obtained with small diameter at formic acid concentration (90%). | [40] |
| Gelose 80 | ̴80 | Electrospinning | Fibers diameter between (2.15–4.02 µm) | Better alignment occurred at higher rotational speed and lower ethanol concentration. Speed. | [42] | |
| Gelose 80 | 76 | Electrospinning |
| Fibers, Diameter (146 ± 50 nm) | Pullulan hindered starch association. Tensile strength of the nanofiber composite was found to be weaker than that of micro-sized pure starch fiber mats. | [43] |
| HylonVII | 70 | Electrospinning |
| Fibers, diameter (304 nm) at 100% formic acid and 84 nm at 80% FA. | Diameter decreased as water content increased | [39] |
| Hylon VII | 70 | Special pressure vessel at 140–165 °C | Hydrogel | Lost its rigidity, due to the degradation of AP | [45] | |
| Hylon VII | 61 | Mixing gelatinized starch then autoclave treatment | Alginate matrix | Macro gels | A high AM amount in the starch the produce gels with less degradation after digestibility compared with common starches and high AP starches. | [46] |
| Hylon VII | −70 | MTGase | Gel | MTGase treated gels can withstand high temperature. Hylon VII added to the gels supplied tighter, stronger, and denser protein network. | [47] | |
| Hylon VII | −71 | Heated in high pressure reactor apparatus | Guar gum/Xanthan gum | Gel | Guar and xanthan gums affected the pasting properties of normal maize starch more than those of waxy maize starch. no new covalent bonds were formed between the guar and xanthan gums and the starches (normal, waxy and high-AM). | [48] |
| Amylomaize VII | 70 | Starch cold gelatinized | Glycerol | Coating | HAS, coating reduced strawberries weight loss and decay. Maintain freshness compared to medium AM starch. | [59] |
| HP-HAS (hydroxyl propyl high AM starch) | 80 | Blending and casting |
| Composite film | Improved transparency and mechanical properties. | [61] |
| HAS | 55 | Blending and casting |
| Composite film | Permeability of gas and water increased. Mechanical properties decreased. | [62] |
| HAS | 85.5 | Blending and casting |
| Composite film/Packaging film | Phase separation, high WVP permeability | [63] |
| HAS | 85.5 | Blending and casting |
| Active composite packaging film | Enhanced WVP, Mechanical properties. Reduced moisture content. | [64] |
| HAS | 80 | Blending and casting |
| Composite film | Anti-plasticization effect at 2.5% glycerol accompanied with visual cracks. | [65] |
| AM-only | 99 | Blending and casting |
| Composite film/ Food packaging | Better mechanical properties. Anti-plasticization at 15% of glycerol. | [10] |
| Hylon VII | −70 |
| Starch –flavor complex preparation. | HAS for flavor encapsulation by inclusion technique, effectively entrapped low water solubility flavors. | [51] | |
| Amylomaize | −56 | Formation of V-AM molecular inclusion complexes |
| Starch –flavor Complex | Oleic acid in the form of Hylon VII starch complex is efficiently protected against oxidation as well as thermal degradation for at least up to 100 °C | [52] |
| HAS | 72 | Blending and casting |
| Composite film | Thickness and transparency increased. Enhanced mechanical properties and water solubility of gelatin films | [66] |
| HP-HAS | 80 | Blending and casting |
| Anti-bacterial, edible composite film/Food industries | It was found that the developed films demonstrated good antibacterial properties against both S. aureus and Salmonella, and enhanced the mechanical behavior. | [69] |
| HAS | 72 | Blending and casting |
| Composite film/Collagen applications | Improvement of mechanical, thermal properties and water solubility. | [70] |
| HAS | 25 | 3D printing (SFFF) |
| Composite Scaffold Bone tissue | Enhanced mechanical properties | [79] |
| Amylomaize | 80 | Extrusion |
| Tissue engineering | Low tissue response of the host, due to degradation of amylomaize | [25] |
| Acetylated/hydroxypropylate HASs | - | Casting and blending |
| Drug delivery | Potential sites specific for coating colon | [78] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Amylose |
| AP | Amylopectin |
| HAS | High amylose starch |
| AM-only | Amylose extracted from barely with amylose content 98% |
| Amaizo | high amylose maize containing approximately 50% amylose |
| Amylomaize VII | high amylose maize containing approximately 70% amylose |
| Hylon V | high amylose corn containing approximately 55% amylose |
| Hylon VII | high amylose corn containing approximately 70% amylose |
| Gelose 50 | high amylose corn containing approximately 50% amylose |
| Gelose 70 | high amylose corn containing approximately 70% amylose |
| Gelose 80 | high amylose corn containing approximately 80% amylose |
| HAS acetate | Acetylated high amylose starch |
| HP-HAS | Hydroxyl propyl high amylose starch |
| Gly | glycerol |
| SO | sunflower oil |
| [Emim] [OAc] | 1-ethyl-3-methylimidazolium acetate |
| DMSO | Dimethyl sulfoxide |
| β-CD | β-cyclodextrin |
| KGM | Konjac glucomannan |
References
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Pages, G.; Gilbert, R.G.; Kuchel, P.W. Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Light, J.M. Modified food starches: Why, what, where and how. Cereal Foods World 1990, 1081–1092. Available online: https://agris.fao.org/agris-search/search.do?recordID=US9121352 (accessed on 6 February 2022).
- Sjöö, M.; Nilsson, L. Chapter 4—Starch Bioengineering. In Starch in Food, Structure, Function and Applications, 2nd ed.; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Sawston, UK, 2017; p. 916. ISBN 9780081008966. [Google Scholar]
- Sorndech, W.; Nakorn, K.N.; Tongta, S.; Blennow, A. Isomalto-oligosaccharides: Recent insights in production technology and their use for food and medical applications. Lwt 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Wang, J.; Hu, P.; Chen, Z.; Liu, Q.; Wei, C. Progress in high-amylose cereal crops through inactivation of starch branching enzymes. Front. Plant Sci. 2017, 8, 469. [Google Scholar] [CrossRef] [Green Version]
- Carciofi, M.; Blennow, A.; Jensen, S.L.; Shaik, S.S.; Henriksen, A.; Buléon, A.; Holm, P.B.; Hebelstrup, K.H. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol. 2012, 12, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hebelstrup, K.H.; Sagnelli, D.; Blennow, A. The future of starch bioengineering: GM microorganisms or GM plants? Front. Plant Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Sagnelli, D.; Faisal, M.; Perzon, A.; Taresco, V.; Mais, M.; Giosafatto, C.V.L.; Hebelstrup, K.H.; Ulvskov, P.; Jørgensen, B.; et al. Amylose/cellulose nanofiber composites for all-natural, fully biodegradable and flexible bioplastics. Carbohydr. Polym. 2021, 253, 117277. [Google Scholar] [CrossRef]
- Pagella, C.; Spigno, G.; De Faveri, D.M. Characterization of starch based edible coatings. Food Bioprod. Process. Trans. Inst. Chem. Eng. Part C 2002, 80, 193–198. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Encapsulation of fruit flavor compounds through interaction with polysaccharides. Molecules 2021, 26, 4207. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion processing and characterization of edible starch films with different amylose contents. J. Food Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Influence of Nanocellulose Additive on the Film Properties of Native Rice Starch-based Edible Films for Food Packaging. Recent Pat. Nanotechnol. 2019, 13, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, L.; Liu, H.; Chen, L.; Li, L. In situ thermal decomposition of starch with constant moisture in a sealed system. Polym. Degrad. Stab. 2008, 93, 260–262. [Google Scholar] [CrossRef]
- López, O.V.; Zaritzky, N.E.; Grossmann, M.V.E.; García, M.A. Acetylated and native corn starch blend films produced by blown extrusion. J. Food Eng. 2013, 116, 286–297. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Varavinit, S.; Shobsngob, S.; Varanyanond, W.; Chinachoti, P.; Naivikul, O. Effect of amylose content on gelatinization, retrogradation and pasting properties of flours from different cultivars of thai rice. Starch/Staerke 2003, 55, 410–415. [Google Scholar] [CrossRef]
- Lourdin, D.; Della Valle, G.; Colonna, P. Influence of amylose content on starch films and foams. Carbohydr. Polym. 1995, 27, 261–270. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, R.; McKnight, S.; Guo, Q.; Adhikari, B. The physicochemical characteristics and hydrophobicity of high amylose starch-glycerol films in the presence of three natural waxes. J. Food Eng. 2013, 119, 205–219. [Google Scholar] [CrossRef]
- Palav, T.; Seetharaman, K. Mechanism of starch gelatinization and polymer leaching during microwave heating. Carbohydr. Polym. 2006, 65, 364–370. [Google Scholar] [CrossRef]
- Karim, A.A.; Nadiha, M.Z.; Chen, F.K.; Phuah, Y.P.; Chui, Y.M.; Fazilah, A. Pasting and retrogradation properties of alkali-treated sago (Metroxylon sagu) starch. Food Hydrocoll. 2008, 22, 1044–1053. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S.; Herald, T.J. Barrier and mechanical properties of starch-clay nanocomposite films. Cereal Chem. 2008, 85, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, D.; Pavon-Djavid, G.; Chaunier, L.; Meddahi-Pellé, A.; Lourdin, D. Effect of crystallinity and plasticizer on mechanical properties and tissue integration of starch-based materials from two botanical origins. Carbohydr. Polym. 2015, 124, 180–187. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D.S. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Zullo, R.; Iannace, S. The effects of different starch sources and plasticizers on film blowing of thermoplastic starch: Correlation among process, elongational properties and macromolecular structure. Carbohydr. Polym. 2009, 77, 376–383. [Google Scholar] [CrossRef]
- Xie, F.; Flanagan, B.M.; Li, M.; Sangwan, P.; Truss, R.W.; Halley, P.J.; Strounina, E.V.; Whittaker, A.K.; Gidley, M.J.; Dean, K.M.; et al. Characteristics of starch-based films plasticised by glycerol and by the ionic liquid 1-ethyl-3-methylimidazolium acetate: A comparative study. Carbohydr. Polym. 2014, 111, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- McHugh, T.H.; Avena-Bustillos, R.; Krochta, J.M. Hydrophilic Edible Films: Modified Procedure for Water Vapor Permeability and Explanation of Thickness Effects. J. Food Sci. 1993, 58, 899–903. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Rhim, J.W.; Roh, H.J.; Kim, S.S. Preparation and physical properties of zein-coated high-amylose corn starch film. LWT-Food Sci. Technol. 2002, 35, 680–686. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Composite starch-based coatings applied to strawberries (Fragaria ananassa). Nahrung-Food 2001, 45, 267–272. [Google Scholar] [CrossRef]
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582. [Google Scholar] [CrossRef]
- Chinnaswamy, R.; Hanna, M. Relationship between amylose content and extrusion-expansion properties of com starches. Cereal Chem. 1988, 65, e147. [Google Scholar]
- Shogren, R.L.; Lawton, J.W.; Doane, W.M.; Tiefenbacher, K.F. Structure and morphology of baked starch foams. Polymer 1998, 39, 6649–6655. [Google Scholar] [CrossRef]
- Shogren, R.L.; Lawton, J.W.; Tiefenbacher, K.F. Baked starch foams: Starch modifications and additives improve process parameters, structure and properties. Ind. Crops Prod. 2002, 16, 69–79. [Google Scholar] [CrossRef]
- Finkenstadt, V.L.; Felker, F.C.; Fanta, G.F.; Kenar, J.A.; Selling, G.W.; Hornback, K.J.; Fisk, D.L. Extruded foams prepared from high amylose starch with sodium stearate to form amylose inclusion complexes. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Guan, J.; Hanna, M.A. Functional properties of extruded foam composites of starch acetate and corn cob fiber. Ind. Crops Prod. 2004, 19, 255–269. [Google Scholar] [CrossRef]
- Lancuški, A.; Vasilyev, G.; Putaux, J.L.; Zussman, E. Rheological Properties and Electrospinnability of High-Amylose Starch in Formic Acid. Biomacromolecules 2015, 16, 2529–2536. [Google Scholar] [CrossRef]
- Xu, W.; Yang, W.; Yang, Y. Electrospun starch acetate nanofibers: Development, properties, and potential application in drug delivery. Biotechnol. Prog. 2009, 25, 1788–1795. [Google Scholar] [CrossRef]
- Wang, W.; Jin, X.; Zhu, Y.; Zhu, C.; Yang, J.; Wang, H.; Lin, T. Effect of vapor-phase glutaraldehyde crosslinking on electrospun starch fibers. Carbohydr. Polym. 2016, 140, 356–361. [Google Scholar] [CrossRef]
- Wang, H.; Kong, L.; Ziegler, G.R. Aligned wet-electrospun starch fiber mats. Food Hydrocoll. 2019, 90, 113–117. [Google Scholar] [CrossRef]
- Wang, H.; Ziegler, G.R. Electrospun nanofiber mats from aqueous starch-pullulan dispersions: Optimizing dispersion properties for electrospinning. Int. J. Biol. Macromol. 2019, 133, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Picone, C.S.F.; Cunha, R.L. Influence of pH on formation and properties of gellan gels. Carbohydr. Polym. 2011, 84, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Vesterinen, E.; Suortti, T.; Autio, K. Effects of preparation temperature on gelation properties and molecular structure of high-amylose maize starch. Cereal Chem. 2001, 78, 442–446. [Google Scholar] [CrossRef]
- Feltre, G.; Almeida, F.S.; Sato, A.C.K.; Dacanal, G.C.; Hubinger, M.D. Alginate and corn starch mixed gels: Effect of gelatinization and amylose content on the properties and in vitro digestibility. Food Res. Int. 2020, 132, 109069. [Google Scholar] [CrossRef]
- Shahsavani Mojarrad, L.; Rafe, A.; Sadeghian, A.; Niazmand, R. Effects of high amylose corn starch and microbial transglutaminase on the textural and microstructural properties of wheat flour composite gels at high temperatures. J. Texture Stud. 2017, 48, 624–632. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; You, X.; Fang, F.; Li, B. Impacts of guar and xanthan gums on pasting and gel properties of high-amylose corn starches. Int. J. Biol. Macromol. 2020, 146, 1060–1068. [Google Scholar] [CrossRef]
- Jørgensen, A.D.; Jensen, S.L.; Ziegler, G.; Pandeya, A.; Buléon, A.; Svensson, B.; Blennow, A. Structural and physical effects of aroma compound binding to native starch granules. Starch/Staerke 2012, 64, 461–469. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Tapanapunnitikul, O.; Chaiseri, S.; Peterson, D.G.; Thompson, D.B. Water solubility of flavor compounds influences formation of flavor inclusion complexes from dispersed high-amylose maize starch. J. Agric. Food Chem. 2008, 56, 220–226. [Google Scholar] [CrossRef]
- Marinopoulou, A.; Papastergiadis, E.; Raphaelides, S.N. An investigation into the structure, morphology and thermal properties of amylomaize starch-fatty acid complexes prepared at different temperatures. Food Res. Int. 2016, 90, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hamaker, B.R. Sorghum (Sorghum bicolor L. Moench) flour pasting properties influenced by free fatty acids and protein. Cereal Chem. 2005, 82, 534–540. [Google Scholar] [CrossRef]
- Escobar-Puentes, A.A.; García-Gurrola, A.; Rincón, S.; Zepeda, A.; Martínez-Bustos, F. Effect of amylose/amylopectin content and succinylation on properties of corn starch nanoparticles as encapsulants of anthocyanins. Carbohydr. Polym. 2020, 250, 116972. [Google Scholar] [CrossRef]
- Fortunati, P.; Gallo, A. High Amylose Starches and Legume Flours as Interesting Ingredients in Gluten-Free Food Formulation High Amylose Starches and Legume Flours as Interesting Ingredients in Gluten-Free Food Formulation. Int. J. Nutr. Sci. 2016, 1, 1–2. [Google Scholar]
- Pham, V.H.; Yamamori, M.; Morita, N. Formation of enzyme-resistant starch in bread as affected by high-amylose wheat flour substitutions. Cereal Chem. 2005, 82, 690–694. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, H.; Liang, W.; Li, X.; Liu, L.; Zhang, X.; Yue, H.; Xue, J.; Liu, X.; Guo, D. High-amylose starch as a new ingredient to balance nutrition and texture of food. J. Cereal Sci. 2018, 81, 8–14. [Google Scholar] [CrossRef]
- Sagnelli, D.; Chessa, S.; Mandalari, G.; Di Martino, M.; Sorndech, W.; Mamone, G.; Vincze, E.; Buillon, G.; Nielsen, D.S.; Wiese, M.; et al. Low glycaemic index foods from wild barley and amylose-only barley lines. J. Funct. Foods 2018, 40, 408–416. [Google Scholar] [CrossRef]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Starch-based coatings: Effect on refrigerated strawberry (Fragaria ananassa) quality. J. Sci. Food Agric. 1998, 76, 411–420. [Google Scholar] [CrossRef]
- Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Effect of nanocellulose as a filler on biodegradable thermoplastic starch films from tuber, cereal and legume. Carbohydr. Polym. 2017, 157, 1094–1104. [Google Scholar] [CrossRef]
- Ali, A.; Xie, F.; Yu, L.; Liu, H.; Meng, L.; Khalid, S.; Chen, L. Preparation and characterization of starch-based composite films reinfoced by polysaccharide-based crystals. Compos. Part B Eng. 2018, 133, 122–128. [Google Scholar] [CrossRef]
- Feng, Q.; Hu, F.; Qiu, L. Microstructure and characteristics of high-amylose corn starch-chitosan film as affected by composition. Food Sci. Technol. Int. 2013, 19, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, C.; Cui, B.; Liu, P.; Wu, Z.; Zhao, H. Formation of high amylose corn starch/konjac glucomannan composite film with improved mechanical and barrier properties. Carbohydr. Polym. 2021, 251, 117039. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Yuan, C.; Cui, B.; Sha, H.; Liu, P.; Lu, L.; Wu, Z. High-Amylose Corn Starch/Konjac Glucomannan Composite Film: Reinforced by Incorporating β-Cyclodextrin. J. Agric. Food Chem. 2021, 69, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Adhikari, R.; Guo, Q.; Adhikari, B. Preparation and characterization of glycerol plasticized (high-amylose) starch-chitosan films. J. Food Eng. 2013, 116, 588–597. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Xiao, J.; Liu, Y.; Zhao, Y.; Liu, A. Performance of high amylose starch-composited gelatin films influenced by gelatinization and concentration. Int. J. Biol. Macromol. 2017, 94, 258–265. [Google Scholar] [CrossRef]
- Corrales, M.; Han, J.H.; Tauscher, B. Antimicrobial properties of grape seed extracts and their effectiveness after incorporation into pea starch films. Int. J. Food Sci. Technol. 2009, 44, 425–433. [Google Scholar] [CrossRef]
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoǧlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Liu, A.; Xiao, J.; Liu, Y.; Zhao, Y. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef]
- Bartneck, M.; Heffels, K.H.; Pan, Y.; Bovi, M.; Zwadlo-Klarwasser, G.; Groll, J. Inducing healing-like human primary macrophage phenotypes by 3D hydrogel coated nanofibres. Biomaterials 2012, 33, 4136–4146. [Google Scholar] [CrossRef]
- Lancuški, A.; Abu Ammar, A.; Avrahami, R.; Vilensky, R.; Vasilyev, G.; Zussman, E. Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydr. Polym. 2017, 158, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ashford, M.; Fell, J.; Attwood, D.; Sharma, H.; Woodhead, P. Studies on pectin formulations for colonic drug delivery. J. Control. Release 1994, 30, 225–232. [Google Scholar] [CrossRef]
- Milojevic, S.; Newton, J.M.; Cummings, J.H.; Gibson, G.R.; Louise Botham, R.; Ring, S.G.; Stockham, M.; Allwood, M.C. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using 5-aminosalicylic acid pellets. J. Control. Release 1996, 38, 75–84. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Satyanarayana, S.; Rama Prasad, Y.V.; Narasimha Rao, S. Evaluation of guar gum as a compression coat for drug targeting to colon. Int. J. Pharm. 1998, 171, 137–146. [Google Scholar] [CrossRef]
- Kim, S.; Willett, J.L. Isolation of amylose from starch solutions by phase separation. Starch/Staerke 2004, 56, 29–36. [Google Scholar] [CrossRef]
- Cristina Freire, A.; Fertig, C.C.; Podczeck, F.; Veiga, F.; Sousa, J. Starch-based coatings for colon-specific drug delivery. Part I: The influence of heat treatment on the physico-chemical properties of high amylose maize starches. Eur. J. Pharm. Biopharm. 2009, 72, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Karrout, Y.; Neut, C.; Wils, D.; Siepmann, F.; Deremaux, L.; Desreumaux, P.; Siepmann, J. Characterization of ethylcellulose: Starch-based film coatings for colon targeting. Drug Dev. Ind. Pharm. 2009, 35, 1190–1200. [Google Scholar] [CrossRef]
- Koski, C.; Bose, S. Effects of amylose content on the mechanical properties of starch-hydroxyapatite 3D printed bone scaffolds. Addit. Manuf. 2019, 30, 100817. [Google Scholar] [CrossRef]
| Starch | AM% | Plasticizer Content % | Mechanical Properties TS (MPa) E (MPa) | EAB % | References | |
|---|---|---|---|---|---|---|
| AM-only | 99 | 15% glycerol | 27 * | 2200 * | 2.8 * | [10] |
| Amylomaize | 70 | 20% glycerol | ND | 83 | ND | [25] |
| HAS | >51 | 30% glycerol | 2.04 | 11.83 | 0.24 | [27] |
| HAS | >51 | 30% urea formamide | 2.02 | 9.94 | 0.97 | [27] |
| Gelose 80 | 82.9 | 9% [Emim] [OAc] | 37 * | 1180 | 12 * | [28] |
| Gelose 80 | 82.9 | 9% glycerol | 36 * | 1000 | 14 * | [28] |
| Corn Starch | 80 | 20% glycerol | 30.65 | 1079.67 | 4.60 | [26] |
| Corn starch | 80 | 20% Xylitol | 37.10 | 1177.57 | 4.03 | [26] |
| Corn starch | 80 | 20% glycerol + Xylitol | 37.29 | 1127.79 | 4.10 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Kou, T.; Zhong, Y.; Blennow, A. High Amylose-Based Bio Composites: Structures, Functions and Applications. Polymers 2022, 14, 1235. https://doi.org/10.3390/polym14061235
Faisal M, Kou T, Zhong Y, Blennow A. High Amylose-Based Bio Composites: Structures, Functions and Applications. Polymers. 2022; 14(6):1235. https://doi.org/10.3390/polym14061235
Chicago/Turabian StyleFaisal, Marwa, Tingting Kou, Yuyue Zhong, and Andreas Blennow. 2022. "High Amylose-Based Bio Composites: Structures, Functions and Applications" Polymers 14, no. 6: 1235. https://doi.org/10.3390/polym14061235
APA StyleFaisal, M., Kou, T., Zhong, Y., & Blennow, A. (2022). High Amylose-Based Bio Composites: Structures, Functions and Applications. Polymers, 14(6), 1235. https://doi.org/10.3390/polym14061235








