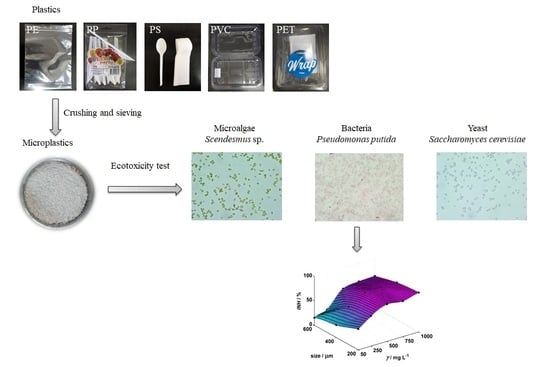
Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Experiment
2.2. Preparation of Microplastics
2.3. Preparation of Test Microorganisms
2.4. Toxicity Tests
2.5. Response Surface Modeling
3. Results and Discussion
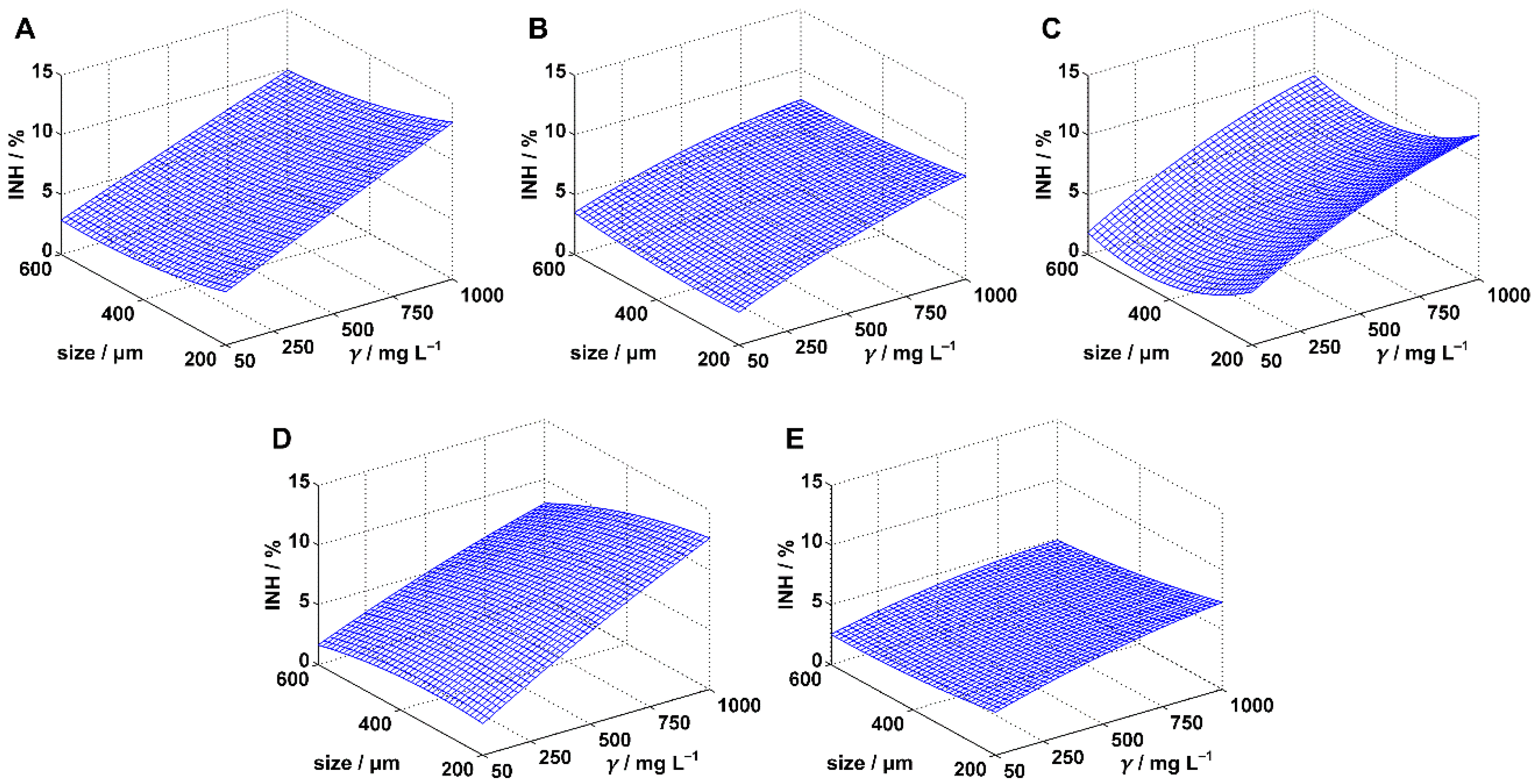
3.1. Inhibition of Scenedesmus sp.
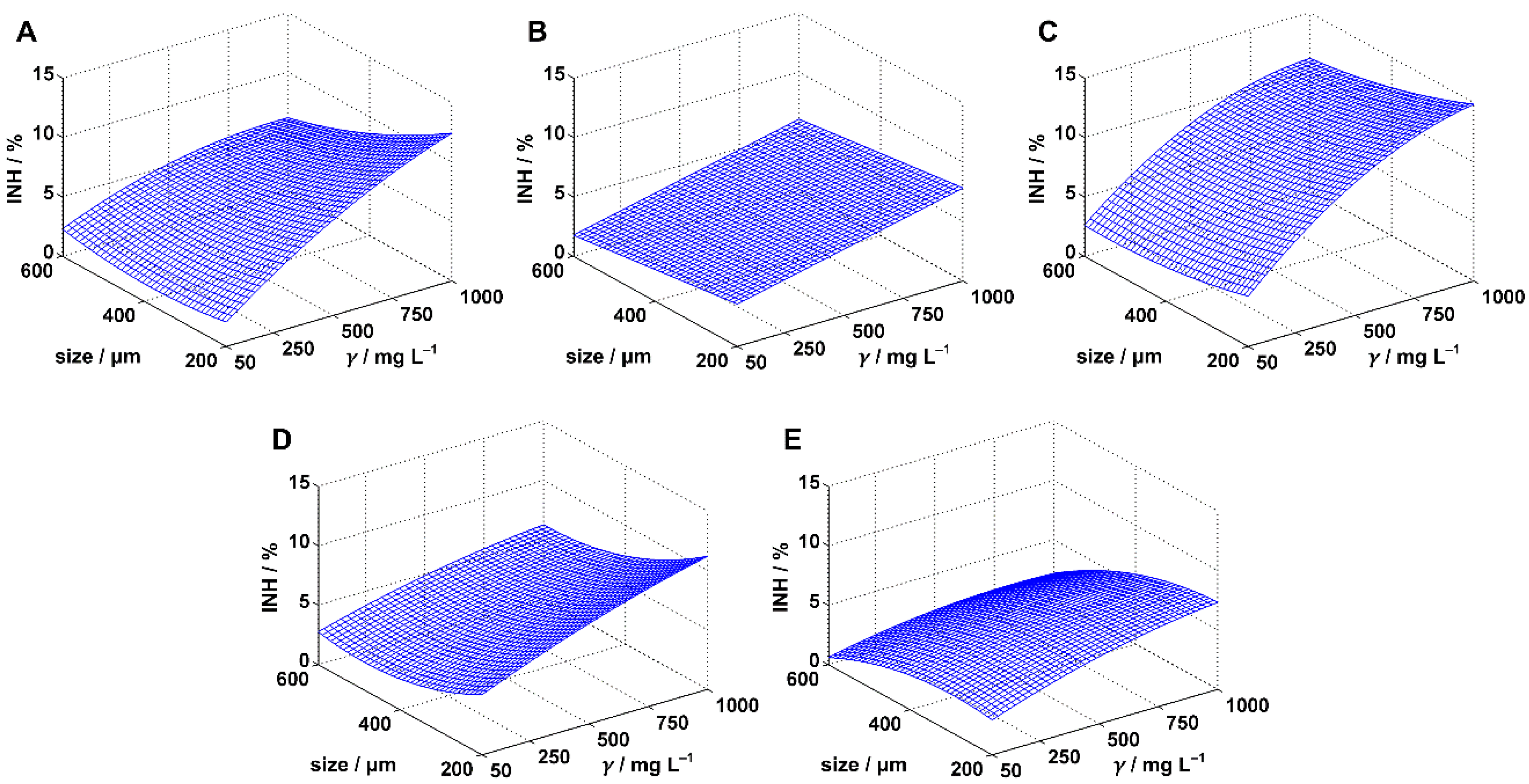
3.2. Inhibition of Pseudomonas putida
3.3. Inhibition of Saccharomyces cerevisiae
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calabrò, P.S.; Grosso, M. Bioplastics and waste management. Waste Manag. 2018, 78, 800–801. [Google Scholar] [CrossRef]
- Kumar, R.; Pandit, P.; Kumar, D.; Patel, Z.; Pandya, L.; Kumar, M.; Joshi, C.; Joshi, M. Landfill microbiome harbour plastic degrading genes: A metagenomic study of solid waste dumping site of Gujarat, India. Sci. Total Environ. 2021, 779, 146184. [Google Scholar] [CrossRef] [PubMed]
- Erceg, M.; Tutman, P.; Bojanić Varezić, D.; Bobanović, A. Characterization of Microplastics in Prapratno Beach Sediment. Kem. Ind. 2020, 69, 253–260. [Google Scholar] [CrossRef]
- Bule, K.; Zadro, K.; Tolić, A.; Radin, E.; Miloloža, M.; Ocelić Bulatović, V.; Kučić Grgić, D. Microplastics in the Marine Environment of the Adriatic Sea. Kem. Ind. 2020, 69, 303–310. [Google Scholar] [CrossRef]
- Plastics—the Facts 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 26 January 2022).
- Reichel, A.; Trier, X.; Fernandez, R.; Bakas, I.; Zeiger, B. Plastics, the Circular Economy and Europe’s Environment—A Priority for Action; European Environmental Agency: Copenhagen, Denmark, 2021. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2020, 407, 124357. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and environmental pollutants: Key interaction and toxicology in aquatic and soil environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef]
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef]
- Esiukova, E.E.; Lobchuk, O.I.; Volodina, A.A.; Chubarenko, I.P. Marine macrophytes retain microplastics. Mar. Pollut. Bull. 2021, 171, 112738. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Huang, D.; Zeng, G.; Deng, R.; Liu, R.; Zhou, Z.; Tao, J.; Xiao, R.; Pan, H. Microplastics retention by reeds in freshwater environment. Sci. Total Environ. 2021, 790, 148200. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Chen, Y.; Zhang, W.; Zhao, J.; He, S.; Yanga, C.; Zhang, T.; Tang, C.; Yang, Z. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2020, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, J.; Zang, L.; Nishimura, N.; Shimada, Y. Zebrafish: An emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. [Google Scholar] [CrossRef] [PubMed]
- Miloloža, M.; Kučić Grgić, D.; Bolanča, T.; Ukić, Š.; Cvetnić, M.; Ocelić Bulatović, V.; Dionysiou, D.D.; Kušić, H. Ecotoxicological assessment of microplastics in freshwater sources—A review. Water 2021, 13, 56. [Google Scholar] [CrossRef]
- Canniff, P.M.; Hoang, T.C. Microplastic ingestion by Daphnia magna and its enhancement on algal growth. Sci. Total Environ. 2018, 633, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, A.; Martins, A.; Guilhermino, L. Toxicological interactions induced by chronic exposure to gold nanoparticles and microplastics mixtures in Daphnia magna. Sci. Total Environ. 2018, 628–629, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, J. Adverse outcome pathways potentially related to hazard identification of microplastics based on toxicity mechanisms. Chemosphere 2019, 231, 249–255. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of microplastics on microalgae populations: A critical review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Tunali, M.; Uzoefuna, E.N.; Tunali, M.M.; Yenigün, O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci. Total Environ. 2020, 743, 140479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ. Pollut. 2016, 220, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, F.; Olivier, O.; Zanella, M.; Daniel, P.; Hiard, S.; Caruso, A. Microplastic interactions with freshwater microalgae: Hetero-aggregation and changes in plastic density appear strongly dependent on polymer type. Environ. Pollut. 2016, 215, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Malapascua, J.R.F.; Jerez, C.G.; Sergejevová, M.; Figueroa, F.L.; Masojídek, J. Photosynthesis monitoring to optimize growth of microalgal mass cultures: Application of chlorophyll fluorescence techniques. Aquat. Biol. 2014, 22, 123–140. [Google Scholar] [CrossRef] [Green Version]
- den Besten, P.J.; Munawar, M.; Suter, G. Ecotoxicological testing of marine and freshwater ecosystems: Emerging techniques, trends and strategies. Integr. Environ. Assess. Manag. 2007, 3, 305–306. [Google Scholar] [CrossRef]
- Ribeiro, I.C.; Veríssimo, I.; Moniz, L.; Cardoso, H.; Sousa, M.J.; Soares, A.M.V.M.; Leão, C. Yeasts as a model for assessing the toxicity of the fungicides Penconazol, Cymoxanil and Dichlofluanid. Chemosphere 2000, 41, 1637–1642. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Miyazaki, J.; Miyamoto, A.; Kuriyama, Y.; Tokumoto, H.; Konishi, Y. Exposure of the yeast Saccharomyces cerevisiae to functionalized polystyrene latex nanoparticles: Influence of surface charge on toxicity. Environ. Sci. Technol. 2013, 47, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- ISO 10712; Water Quality—Pseudomonas Putida Growth Inhibition Test Pseudomonas Cell Multiplication Inhibition Test. International Organization for Standardization: Geneva, Switzerland, 1995.
- Hafner, C. Pseudomonas putida growth inhibition test. In Ecotoxicological Characterization of Waste; Moser, H., Römbke, J., Eds.; Springer: New York, NY, USA, 2009; pp. 153–159. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Alga, Growth Inhibition Test, OECD Guidelines for Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1984. [Google Scholar]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef] [Green Version]
- Stilinović, B. Saccharomyces cerevisiae test (YTT) as the water toxicity determination method. Acta Bot. Croat. 1981, 40, 127–131. [Google Scholar]
- Hrenovic, J.; Stilinović, B.; Dvoracek, L. Use of prokaryotic and eukaryotic biotests to assess. Acta Chim. Slov. 2005, 52, 119–125. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Washington, S.; Karlaftis, M.G.; Mannering, F.; Anastasopoulos, P. Statistical and Econometric Methods for Transportation Data Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2020. [Google Scholar]
- Garrido, S.; Linares, M.; Campillo, J.M.; Albentosa, M. Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol. Environ. Saf. 2019, 173, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical Adsorption of Charged Plastic Nanoparticles Affects Algal Photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, W.; Li, J.; Liu, W.; Qi, P.; Ye, Y.; Guo, B.; Zhang, J.; Qu, C. Effects of microplastics on growth, phenanthrene stress, and lipid accumulation in a diatom. Phaeodactylum Tricornutum. Environ. Pollut. 2019, 257, 113628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Wang, J.; Wang, Y.; Mu, J.; Wang, P.; Lin, X.; Ma, D. Microplastic pollution in the surface waters of the Bohai Sea. China Environ. Pollut. 2017, 231, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Moog, D.; Schmitt, J.; Senger, J.; Zarzycki, J.; Rexer, K.H.; Linne, U.; Erb, T.; Maier, U.G. Using a marine microalga as a chassis for polyethylene terephthalate (PET) degradation. Microb. Cell. Fact. 2019, 18, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Park, S.B.; Tran, Q.G.; Cho, D.H.; Choi, D.Y.; Lee, Y.J.; Kim, H.S. Functional expression of polyethylene terephthalate-degrading enzyme (PETase) in green microalgae. Microb. Cell. Fact. 2020, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Falah, W.; Chen, F.; Zeb, B.S.; Hayat, M.T.; Mahmood, Q.; Ebadi, A.; Toughani, M.; Li, E. Polyethylene Terephthalate Degradation by Microalga Chlorella vulgaris along with Pretreatment. Mater. Plast. 2020, 57, 260–270. [Google Scholar] [CrossRef]
- Sikkema, J.; Poolman, B.; Konings, W.N.; de Bont, J.A.M. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J. Bacteriol. 1992, 174, 2986–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019, 52, 35–41. [Google Scholar] [CrossRef]
- Li, W.J.; Jayakody, L.N.; Franden, M.A.; Wehrmann, M.; Daun, T.; Hauer, B.; Blank, L.M.; Beckham, G.T.; Klebensberger, J.; Wierckx, N. Laboratory evolution reveals the metabolic and regulatory basis of ethylene glycol metabolism by Pseudomonas putida KT2440. Environ. Microbiol. 2019, 21, 3669–3682. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current status and application aspects. Acs Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Roberts, C.; Edwards, S.; Vague, M.; León-Zayas, R.; Scheffer, H.; Chan, G.; Swartz, N.A.; Melliesa, J.L. Environmental consortium containing Pseudomonas and Bacillus species synergistically degrades polyethylene terephthalate plastic. Appl. Environ. Sci. 2020, 5, e01151-20. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S.; Höppner, A.; Ferrer, M.; Gohlke, H.; Smits, S.H.J.; Jaeger, K.E. A Novel Polyester Hydrolase from the Marine Bacterium Pseudomonas aestusnigri—Structural and Functional Insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Size/μm | Conc./mg/L | Scenedesmus sp. | Pseudomonas putida | Saccharomyces cerevisiae | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | PP | PS | PVC | PET | PE | PP | PS | PVC | PET | PE | PP | PS | PVC | PET | ||
| INH/% | INH/% | INH/% | ||||||||||||||
| 200 | 50 | 3.61 | 3.03 | 4.56 | 2.13 | 4.11 | 1.87 | 2.57 | 3.62 | 4.49 | 2.75 | 49.63 | 42.86 | 50.00 | 63.24 | 35.24 |
| 250 | 7.24 | 4.03 | 6.27 | 5.16 | 4.51 | 5.04 | 4.97 | 8.70 | 7.35 | 4.88 | 65.63 | 61.86 | 64.29 | 66.67 | 52.13 | |
| 500 | 8.08 | 6.55 | 9.26 | 8.43 | 5.89 | 6.53 | 5.65 | 11.60 | 8.56 | 5.31 | 80.31 | 76.29 | 69.05 | 73.33 | 68.09 | |
| 750 | 11.41 | 6.74 | 11.34 | 10.50 | 5.89 | 12.19 | 6.31 | 12.90 | 9.00 | 6.48 | 98.44 | 73.20 | 95.24 | 83.09 | 88.10 | |
| 1000 | 12.99 | 9.13 | 11.34 | 12.10 | 7.63 | 12.35 | 8.48 | 14.74 | 11.89 | 7.42 | 98.76 | 80.95 | 100.00 | 100.00 | 91.30 | |
| 400 | 50 | 3.61 | 2.98 | 2.49 | 3.10 | 2.43 | 1.35 | 2.33 | 2.90 | 2.83 | 1.73 | 39.06 | 25.77 | 41.62 | 20.59 | 20.83 |
| 250 | 4.93 | 4.62 | 3.27 | 4.13 | 3.47 | 5.37 | 4.57 | 4.87 | 4.35 | 4.99 | 58.44 | 54.76 | 52.38 | 32.35 | 43.48 | |
| 500 | 6.97 | 5.18 | 4.56 | 6.54 | 4.51 | 5.85 | 4.66 | 10.39 | 5.37 | 5.52 | 73.44 | 74.23 | 66.67 | 35.90 | 58.33 | |
| 750 | 7.81 | 6.55 | 6.94 | 10.23 | 5.89 | 6.67 | 5.23 | 11.60 | 5.83 | 5.30 | 79.06 | 75.26 | 88.10 | 42.86 | 68.64 | |
| 1000 | 11.15 | 8.22 | 9.26 | 10.5 | 5.89 | 8.00 | 7.17 | 12.49 | 7.37 | 5.72 | 90.63 | 76.19 | 88.59 | 76.92 | 76.19 | |
| 600 | 50 | 2.73 | 2.70 | 0.72 | 2.24 | 2.43 | 1.54 | 1.93 | 3.10 | 2.33 | 1.02 | 7.810 | 15.46 | 9.520 | 16.67 | 7.450 |
| 250 | 4.36 | 5.18 | 4.14 | 3.10 | 3.47 | 4.52 | 3.10 | 5.85 | 3.77 | 1.45 | 29.06 | 24.74 | 26.19 | 30.95 | 23.85 | |
| 500 | 5.75 | 6.74 | 6.27 | 4.13 | 4.51 | 5.03 | 3.65 | 8.10 | 5.52 | 1.52 | 40.48 | 57.73 | 54.76 | 33.33 | 37.23 | |
| 750 | 8.76 | 6.95 | 9.26 | 6.54 | 4.51 | 5.26 | 4.76 | 9.75 | 5.33 | 1.52 | 61.54 | 62.89 | 64.29 | 46.67 | 47.14 | |
| 1000 | 9.86 | 6.95 | 9.26 | 8.43 | 4.51 | 7.03 | 5.56 | 11.60 | 6.44 | 2.84 | 73.44 | 64.29 | 88.10 | 72.06 | 54.76 | |
| MPs | Model | Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R2 | R2adj | F | p | Coefficient | Value | Lower 95% | Upper 95% | p | Significant Term? | Influential Factor | |
| PE | MODEL I | 0.9535 | 0.9458 | 123.17 | 0.00 | a0 | 5.45 | 4.19 | 6.70 | 0.00 | concentration & size | |
| a1 (10−3) | 8.26 | 7.04 | 9.47 | 0.00 | YES | |||||||
| a2 (10−3) | −5.93 | −8.46 | −3.41 | 0.00 | YES | |||||||
| MODEL II | 0.9618 | 0.9465 | 62.95 | 0.00 | a0 | 7.37 | 4.07 | 10.67 | 0.00 | concentration | ||
| a1 (10−2) | 0.84 | 0.35 | 1.33 | 0.00 | YES | |||||||
| a2 (10−2) | −1.76 | −3.56 | 0.03 | 0.94 | ||||||||
| a3 (10−6) | −0.14 | −4.64 | 4.35 | 0.17 | ||||||||
| a4 (10−5) | 1.46 | −0.76 | 3.68 | 0.05 | ||||||||
| MODEL III | 0.9680 | 0.9502 | 54.40 | 0.00 | a0 | 6.50 | 2.93 | 10.07 | 0.00 | concentration | ||
| a1 (10−2) | 1.01 | 0.45 | 1.57 | 0.00 | YES | |||||||
| a2 (10−2) | −1.54 | −3.34 | 0.25 | 0.08 | ||||||||
| a3 (10−6) | −0.14 | −4.55 | 4.26 | 0.22 | ||||||||
| a4 (10−5) | 1.46 | −0.71 | 3.64 | 0.94 | ||||||||
| a5 (10−5) | −0.43 | −1.17 | 0.31 | 0.16 | ||||||||
| PP | MODEL I | 0.8824 | 0.8628 | 45.01 | 0.00 | a0 | 3.25 | 2.02 | 4.48 | 0.00 | concentration | |
| a1 (10−3) | 5.19 | 3.99 | 6.38 | 0.00 | YES | |||||||
| a2 (10−3) | −0.48 | −2.96 | 2.00 | 0.68 | ||||||||
| MODEL II | 0.9055 | 0.8677 | 23.96 | 0.00 | a0 | 3.79 | 0.58 | 6.99 | 0.02 | concentration | ||
| a1 (10−2) | 0.80 | 0.33 | 1.28 | 0.00 | YES | |||||||
| a2 (10−2) | −0.63 | −2.37 | 1.11 | 0.44 | ||||||||
| a3 (10−6) | −2.70 | −7.07 | 1.67 | 0.20 | ||||||||
| a4 (10−5) | 0.72 | −1.43 | 2.88 | 0.47 | ||||||||
| MODEL III | 0.9279 | 0.8878 | 23.15 | 0.00 | a0 | 2.76 | −0.55 | 6.06 | 0.09 | concentration | ||
| a1 (10−2) | 1.00 | 0.48 | 1.52 | 0.00 | YES | |||||||
| a2 (10−2) | −0.37 | −2.04 | 1.30 | 0.63 | ||||||||
| a3 (10−6) | −2.70 | −6.79 | 1.39 | 0.17 | ||||||||
| a4 (10−5) | 0.72 | −1.29 | 2.74 | 0.44 | ||||||||
| a5 (10−5) | −0.51 | −1.19 | 0.18 | 0.13 | ||||||||
| PS | MODEL I | 0.8570 | 0.8332 | 35.95 | 0.00 | a0 | 5.12 | 2.83 | 7.41 | 0.00 | concentration & size | |
| a1 (10−2) | 0.80 | 0.58 | 1.03 | 0.00 | YES | |||||||
| a2 (10−2) | −0.66 | −1.12 | −0.19 | 0.01 | YES | |||||||
| MODEL II | 0.9525 | 0.9335 | 50.11 | 0.00 | a0 | 1.10 | 0.71 | 1.48 | 0.00 | concentration & size | ||
| a1 (10−2) | 1.20 | 0.63 | 1.77 | 0.00 | YES | |||||||
| a2 (10−2) | −4.53 | −6.62 | −2.45 | 0.00 | YES | |||||||
| a3 (10−6) | −3.78 | −9.01 | 1.45 | 0.14 | ||||||||
| a4 (10−5) | 4.84 | 2.26 | 7.43 | 0.00 | YES | |||||||
| MODEL III | 0.9565 | 0.9323 | 39.54 | 0.00 | a0 (10) | 1.17 | 0.74 | 1.60 | 0.00 | concentration & size | ||
| a1 (10−2) | 1.06 | 0.37 | 1.74 | 0.01 | YES | |||||||
| a2 (10−2) | −4.72 | −6.90 | −2.53 | 0.00 | YES | |||||||
| a3 (10−6) | −3.78 | −9.14 | 1.58 | 0.14 | ||||||||
| a4 (10−5) | 4.85 | 2.20 | 7.49 | 0.00 | YES | |||||||
| a5 (10−5) | 0.36 | −0.54 | 1.26 | 0.39 | ||||||||
| PVC | MODEL I | 0.9331 | 0.9219 | 83.67 | 0.00 | a0 | 4.87 | 3.26 | 6.48 | 0.00 | concentration & size | |
| a1 (10−2) | 0.86 | 0.71 | 1.02 | 0.00 | YES | |||||||
| a2 (10−2) | −0.69 | −1.02 | −0.37 | 0.00 | YES | |||||||
| MODEL II | 0.9435 | 0.9208 | 41.71 | 0.00 | a0 | 2.53 | −1.76 | 6.82 | 0.22 | concentration | ||
| a1 (10−2) | 1.03 | 0.40 | 1.66 | 0.00 | YES | |||||||
| a2 (10−2) | 0.55 | −1.78 | 2.89 | 0.61 | ||||||||
| a3 (10−6) | −1.62 | −7.47 | 4.23 | 0.55 | ||||||||
| a4 (10−5) | −1.56 | −4.44 | 1.32 | 0.26 | ||||||||
| MODEL III | 0.9705 | 0.9541 | 59.18 | 0.00 | a0 | 0.57 | −3.09 | 4.23 | 0.73 | concentration & size | ||
| a1 (10−2) | 1.42 | 0.84 | 2.00 | 0.00 | YES | |||||||
| a2 (10−2) | 1.04 | −0.80 | 2.89 | 0.23 | ||||||||
| a3 (10−6) | −1.62 | −6.14 | 2.90 | 0.44 | ||||||||
| a4 (10−5) | −1.56 | −3.79 | 0.67 | 0.15 | ||||||||
| a5 (10−5) | −0.96 | −1.72 | −0.20 | 0.02 | YES | |||||||
| PET | MODEL I | 0.8845 | 0.8653 | 45.95 | 0.00 | a0 | 4.75 | 3.86 | 5.63 | 0.00 | concentration & size | |
| a1 (10−3) | 3.17 | 2.31 | 4.03 | 0.00 | YES | |||||||
| a2 (10−3) | −4.30 | −6.09 | −2.51 | 0.00 | YES | |||||||
| MODEL II | 0.9136 | 0.8790 | 26.42 | 0.00 | a0 | 5.46 | 3.23 | 7.69 | 0.00 | concentration | ||
| a1 (10−3) | 5.23 | 1.93 | 8.53 | 0.00 | YES | |||||||
| a2 (10−2) | −1.05 | −2.26 | 0.17 | 0.08 | ||||||||
| a3 (10−6) | −1.96 | −5.01 | 1.09 | 0.18 | ||||||||
| a4 (10−5) | 0.77 | −0.73 | 2.27 | 0.28 | ||||||||
| MODEL III | 0.9335 | 0.8965 | 25.25 | 0.00 | a0 | 4.75 | 2.43 | 7.06 | 0.00 | concentration | ||
| a1 (10−2) | 0.66 | 0.30 | 1.03 | 0.00 | YES | |||||||
| a2 (10−2) | −0.87 | −2.03 | 0.30 | 0.13 | ||||||||
| a3 (10−6) | −1.96 | −4.82 | 0.90 | 0.15 | ||||||||
| a4 (10−5) | 0.77 | −0.64 | 2.18 | 0.25 | ||||||||
| a5 (10−6) | −3.48 | −8.27 | 1.32 | 0.13 | ||||||||
| MPs | Model | Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R2 | R2adj | F | p | Coefficient | Value | Lower 95% | Upper 95% | p | Significant Term? | Influentia Factor | |
| PE | MODEL I | 0.8002 | 0.7669 | 24.03 | 0.00 | a0 | 5.01 | 2.32 | 7.69 | 0.00 | concentration & size | |
| a1 (10−2) | 0.75 | 0.49 | 1.01 | 0.00 | YES | |||||||
| a2 (10−2) | −0.73 | −1.27 | −0.19 | 0.01 | YES | |||||||
| MODEL II | 0.8306 | 0.7628 | 12.26 | 0.00 | a0 (10) | 0.65 | −0.06 | 1.37 | 0.07 | concentration | ||
| a1 (10−2) | 1.25 | 0.18 | 2.31 | 0.03 | YES | |||||||
| a2 (10−2) | –2.11 | –6.01 | 1.80 | 0.26 | ||||||||
| a3 (10−5) | −0.47 | −1.45 | 0.51 | 0.31 | ||||||||
| a4 (10−5) | 1.72 | −3.11 | 6.55 | 0.45 | ||||||||
| MODEL III | 0.9228 | 0.8799 | 21.52 | 0.00 | a0 | 3.04 | −2.68 | 8.76 | 0.26 | concentration & size | ||
| a1 (10−2) | 1.93 | 1.03 | 2.83 | 0.00 | YES | |||||||
| a2 (10−2) | −1.23 | −4.11 | 1.65 | 0.36 | ||||||||
| a3 (10−5) | −0.47 | −1.18 | 0.23 | 0.16 | ||||||||
| a4 (10−5) | 1.72 | −1.77 | 5.21 | 0.23 | ||||||||
| a5 (10−5) | −1.72 | −2.90 | −0.53 | 0.01 | YES | |||||||
| PP | MODEL I | 0.9176 | 0.9039 | 66.83 | 0.00 | a0 | 4.25 | 3.28 | 5.21 | 0.00 | concentration & size | |
| a1 (10−3) | 4.47 | 3.53 | 5.41 | 0.00 | YES | |||||||
| a2 (10−3) | −4.49 | −6.44 | −2.54 | 0.00 | YES | |||||||
| MODEL II | 0.9192 | 0.8869 | 28.44 | 0.00 | a0 | 3.84 | 1.06 | 6.62 | 0.01 | concentration | ||
| a1 (10−3) | 5.08 | 0.98 | 9.19 | 0.02 | YES | |||||||
| a2 (10−2) | −0.26 | −1.77 | 1.25 | 0.71 | ||||||||
| a3 (10−6) | −0.59 | −4.38 | 3.20 | 0.74 | ||||||||
| a4 (10−5) | −0.23 | −2.10 | 1.63 | 0.78 | ||||||||
| MODEL III | 0.9379 | 0.9035 | 27.20 | 0.00 | a0 | 2.95 | 0.08 | 5.83 | 0.04 | concentration | ||
| a1 (10−2) | 0.68 | 0.23 | 1.14 | 0.01 | YES | |||||||
| a2 (10−2) | −0.04 | −1.49 | 1.41 | 0.95 | ||||||||
| a3 (10−6) | −0.59 | −4.14 | 2.97 | 0.72 | ||||||||
| a4 (10−5) | −0.23 | −1.99 | 1.52 | 0.77 | ||||||||
| a5 (10−5) | −0.43 | −1.03 | 0.62 | 0.13 | ||||||||
| PS | MODEL I | 0.9159 | 0.9019 | 65.37 | 0.00 | a0 | 6.30 | 4.22 | 8.38 | 0.00 | concentration & size | |
| a1 (10−2) | 1.01 | 0.81 | 1.21 | 0.00 | YES | |||||||
| a2 (10−2) | −0.66 | −1.08 | −0.24 | 0.00 | YES | |||||||
| MODEL II | 0.9656 | 0.9519 | 70.26 | 0.00 | a0 (10) | 0.68 | 0.29 | 1.06 | 0.00 | concentration | ||
| a1 (10−2) | 1.91 | 1.34 | 2.48 | 0.00 | YES | |||||||
| a2 (10−2) | −1.75 | −3.85 | 0.35 | 0.09 | ||||||||
| a3 (10−5) | −0.86 | −1.38 | −0.33 | 0.00 | YES | |||||||
| a4 (10−5) | 1.36 | −1.24 | 3.97 | 0.27 | ||||||||
| MODEL III | 0.9723 | 0.9570 | 63.29 | 0.00 | a0 | 5.62 | 1.53 | 9.72 | 0.01 | concentration | ||
| a1 (10−2) | 2.13 | 1.48 | 2.77 | 0.00 | YES | |||||||
| a2 (10−2) | −1.47 | −3.53 | 0.60 | 0.14 | ||||||||
| a3 (10−5) | −0.86 | −1.36 | −0.35 | 0.00 | YES | |||||||
| a4 (10−5) | 1.36 | −1.13 | 3.86 | 0.25 | ||||||||
| a5 (10−5) | −0.55 | −1.40 | 0.29 | 0.17 | ||||||||
| PVC | MODEL I | 0.8626 | 0.8397 | 37.66 | 0.00 | a0 | 7.02 | 5.30 | 8.75 | 0.00 | concentration & size | |
| a1 (10−3) | 5.07 | 3.40 | 6.74 | 0.00 | YES | |||||||
| a2 (10−2) | −0.90 | −1.24 | −0.55 | 0.00 | YES | |||||||
| MODEL I | 0.9340 | 0.9076 | 35.37 | 0.00 | a0 (10) | 1.11 | 0.76 | 1.46 | 0.00 | concentration & size | ||
| a1 (10−2) | 0.72 | 0.21 | 1.24 | 0.01 | YES | |||||||
| a2 (10−2) | −3.53 | −5.42 | −1.64 | 0.00 | YES | |||||||
| a3 (10−6) | −2.05 | −6.78 | 2.68 | 0.36 | ||||||||
| a4 (10−5) | 3.29 | 0.96 | 5.63 | 0.01 | YES | |||||||
| MODEL III | 0.9589 | 0.9360 | 41.97 | 0.00 | a0 (10) | 0.97 | 0.64 | 1.29 | 0.00 | concentration & size | ||
| a1 (10−2) | 1.00 | 0.49 | 1.51 | 0.00 | YES | |||||||
| a2 (10−2) | −3.18 | −4.81 | −1.55 | 0.00 | YES | |||||||
| a3 (10−6) | −2.05 | −6.05 | 1.95 | 0.28 | ||||||||
| a4 (10−5) | 3.29 | 1.32 | 5.27 | 0.00 | YES | |||||||
| a5 (10−5) | −0.69 | −1.36 | −0.02 | 0.04 | YES | |||||||
| PET | MODEL I | 0.8016 | 0.7686 | 24.25 | 0.00 | a0 | 6.00 | 4.24 | 7.75 | 0.00 | concentration & size | |
| a1 (10−3) | 3.13 | 1.43 | 4.84 | 0.00 | YES | |||||||
| a2 (10−2) | −0.92 | −1.28 | −0.57 | 0.00 | YES | |||||||
| MODEL II | 0.8850 | 0.8389 | 19.23 | 0.00 | a0 | 1.77 | −2.12 | 5.66 | 0.33 | concentration & size | ||
| a1 (10−2) | 0.61 | 0.04 | 1.19 | 0.04 | YES | |||||||
| a2 (10−2) | 1.34 | –0.77 | 3.46 | 0.19 | ||||||||
| a3 (10−6) | −2.83 | −8.14 | 2.47 | 0.26 | ||||||||
| a4 (10−5) | −2.83 | −5.45 | −0.22 | 0.04 | YES | |||||||
| MODEL III | 0.9246 | 0.8827 | 22.07 | 0.00 | a0 | 0.26 | -3.46 | 3.97 | 0.88 | concentration & size | ||
| a1 (10−2) | 0.91 | 0.32 | 1.49 | 0.01 | YES | |||||||
| a2 (10−2) | 1.72 | −0.15 | 3.59 | 0.07 | ||||||||
| a3 (10−6) | −2.83 | −7.43 | 1.76 | 0.20 | ||||||||
| a4 (10−5) | −2.83 | −5.10 | −0.57 | 0.02 | YES | |||||||
| a5 (10−5) | −0.74 | −1.51 | 0.03 | 0.06 | ||||||||
| MPs | Model | Coefficients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | R2 | R2adj | F | p | Coefficient | Value | Lower 95% | Upper 95% | p | Significant Term? | Influential Factor | |
| PE | MODEL I | 0.9449 | 0.9358 | 102.97 | 0.00 | a0 (10) | 6.97 | 5.83 | 8.10 | 0.00 | concentration & size | |
| a1 (10−2) | 5.78 | 4.68 | 6.87 | 0.00 | YES | |||||||
| a2 (10−1) | −0.90 | −1.13 | −0.67 | 0.00 | YES | |||||||
| MODEL II | 0.9804 | 0.9725 | 124.86 | 0.00 | a0 (10) | 3.90 | 1.93 | 5.86 | 0.00 | concentration & size | ||
| a1 (10−1) | 0.93 | 0.64 | 1.22 | 0.00 | YES | |||||||
| a2 (10−1) | 0.62 | −0.45 | 1.69 | 0.22 | ||||||||
| a3 (10−5) | −3.35 | −6.03 | −0.67 | 0.02 | YES | |||||||
| a4 (10−4) | −1.90 | −3.23 | −0.58 | 0.01 | YES | |||||||
| MODEL III | 0.9860 | 0.9782 | 126.77 | 0.00 | a0 (10) | 4.59 | 2.63 | 6.55 | 0.00 | concentration & size | ||
| a1 (10−1) | 0.79 | 0.48 | 1.10 | 0.00 | YES | |||||||
| a2 (10−1) | 0.45 | −0.54 | 1.43 | 0.33 | ||||||||
| a3 (10−5) | −3.35 | −5,77 | −0.93 | 0.01 | YES | |||||||
| a4 (10−4) | −1.90 | −3.10 | −0.71 | 0.01 | YES | |||||||
| a5 (10−5) | 3.42 | −0.65 | 7.48 | 0.09 | ||||||||
| PP | MODEL I | 0.8100 | 0.7784 | 25.58 | 0.00 | a0 (10) | 5.57 | 3.87 | 7.27 | 0.00 | concentration & size | |
| a1 (10−2) | 4.72 | 3.07 | 6.37 | 0.00 | YES | |||||||
| a2 (10−2) | −5.50 | −8.93 | −2.07 | 0.00 | YES | |||||||
| MODEL II | 0.9387 | 0.9142 | 28.28 | 0.00 | a0 (10) | 2.65 | −0.16 | 5.46 | 0.06 | concentration | ||
| a1 (10−1) | 1.25 | 0.83 | 1.67 | 0.00 | YES | |||||||
| a2 (10−1) | 0.49 | −1.03 | 2.02 | 0.49 | ||||||||
| a3 (10−4) | −0.74 | −1.13 | −0.36 | 0.00 | YES | |||||||
| a4 (10−4) | −1.30 | −3.19 | 0.59 | 0.15 | ||||||||
| MODEL III | 0.9580 | 0.9347 | 41.05 | 0.00 | a0 (10) | 3.69 | 0.95 | 6.44 | 0.01 | concentration | ||
| a1 (10−1) | 1.05 | 0.61 | 1.48 | 0.00 | YES | |||||||
| a2 (10−1) | 0.23 | −1.15 | 1.62 | 0.71 | ||||||||
| a3 (10−4) | −0.74 | −1.08 | −0.40 | 0.00 | YES | |||||||
| a4 (10−4) | −1.30 | −2.98 | 0.37 | 0.11 | ||||||||
| a5 (10−4) | 0.51 | −0.06 | 1.08 | 0.07 | ||||||||
| PS | MODEL I | 0.9334 | 0.9223 | 84.09 | 0.00 | a0 (10) | 5.88 | 4.65 | 7.11 | 0.00 | concentration & size | |
| a1 (10−2) | 6.32 | 5.13 | 7.52 | 0.00 | YES | |||||||
| a2 (10−2) | −6.79 | −9.27 | −0.04 | 0.00 | YES | |||||||
| MODEL II | 0.9468 | 0.9255 | 44.50 | 0.00 | a0 (10) | 3.86 | 0.65 | 7.06 | 0.02 | concentration | ||
| a1 (10−1) | 0.79 | 0.32 | 1.27 | 0.00 | YES | |||||||
| a2 (10−1) | 0.39 | −1.36 | 2.13 | 0.63 | ||||||||
| a3 (10−5) | −1.55 | −5.92 | 2.82 | 0.45 | ||||||||
| a4 (10−4) | −1.33 | −3.49 | 0.82 | 0.20 | ||||||||
| MODEL III | 0.9687 | 0.9514 | 55.76 | 0.00 | a0 (10) | 5.22 | 2.32 | 8.12 | 0.00 | concentration & size | ||
| a1 (10−2) | 5.28 | 0.71 | 9.85 | 0.03 | YES | |||||||
| a2 (10−1) | 0.05 | −1.42 | 1.51 | 0.94 | ||||||||
| a3 (10−5) | −1.55 | −5.14 | 2.04 | 0.35 | ||||||||
| a4 (10−4) | −1.33 | −3.10 | 0.44 | 0.12 | ||||||||
| a5 (10−4) | 0.67 | 0.06 | 1.27 | 0.03 | YES | |||||||
| PVC | MODEL I | 0.8314 | 0.8033 | 29.59 | 0.00 | a0 (10) | 6.61 | 4.71 | 8.52 | 0.00 | concentration & size | |
| a1 (10−2) | 4.74 | 2.89 | 6.58 | 0.00 | YES | |||||||
| a2 (10−1) | −0.93 | −1.32 | −0.55 | 0.00 | YES | |||||||
| MODEL II | 0.9672 | 0.9541 | 73.75 | 0.00 | a0 (102) | 1.29 | 1.05 | 1.54 | 0.00 | concentration & size | ||
| a1 (10−2) | 0.10 | −3.51 | 3.71 | 0.95 | ||||||||
| a2 (10−1) | −4.31 | −5.64 | −2.98 | 0.00 | YES | |||||||
| a3 (10−5) | 4.42 | 1.09 | 7.75 | 0.01 | YES | |||||||
| a4 (10−4) | 4.22 | 2.57 | 5.86 | 0.00 | YES | |||||||
| MODEL III | 0.9746 | 0.9605 | 69.01 | 0.00 | a0 (102) | 1.37 | 1.12 | 1.63 | 0.00 | concentration & size | ||
| a1 (10−2) | −1.40 | −5.41 | 2.60 | 0.45 | ||||||||
| a2 (10−1) | −4.50 | −5.78 | −3.22 | 0.00 | YES | |||||||
| a3 (10−5) | 4.42 | 1.28 | 7.56 | 0.01 | YES | |||||||
| a4 (10−4) | 4.22 | 2.67 | 5.77 | 0.00 | YES | |||||||
| a5 (10−5) | 3.76 | −1.51 | 9.03 | 0.14 | ||||||||
| PET | MODEL I | 0.9596 | 0.9528 | 142.33 | 0.00 | a0 (10) | 5.61 | 4.71 | 6.52 | 0.00 | concentration & size | |
| a1 (∙10−2) | 5.54 | 4.66 | 6.42 | 0.00 | YES | |||||||
| a2 (∙10−1) | −0.82 | −1.00 | −0.64 | 0.00 | YES | |||||||
| MODEL II | 0.9889 | 0.9844 | 222.23 | 0.00 | a0 (10) | 3.98 | 2.59 | 5.36 | 0.00 | concentration | ||
| a1 (10−1) | 0.98 | 0.78 | 1.19 | 0.00 | YES | |||||||
| a2 (10−2) | −2.29 | −9.81 | 5.23 | 0.51 | ||||||||
| a3 (10−5) | −4.08 | −5.96 | −2.19 | 0.00 | YES | |||||||
| a4 (10−4) | −0.74 | −1.67 | 0.19 | 0.11 | ||||||||
| MODEL III | 0.9944 | 0.9913 | 318.85 | 0.00 | a0 (10) | 3.33 | 2.17 | 4.49 | 0.00 | concentration & size | ||
| a1 (10−1) | 1.11 | 0.93 | 1.29 | 0.00 | YES | |||||||
| a2 (10−2) | −0.68 | −6.53 | 5.17 | 0.80 | ||||||||
| a3 (10−5) | −4.08 | −5.51 | −2.64 | 0.00 | YES | |||||||
| a4 (10−4) | −0.74 | −1.45 | −0.03 | 0.04 | YES | |||||||
| a5 (10−5) | −3.16 | −5.56 | −0.75 | 0.02 | YES | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miloloža, M.; Bule, K.; Prevarić, V.; Cvetnić, M.; Ukić, Š.; Bolanča, T.; Kučić Grgić, D. Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae. Polymers 2022, 14, 1246. https://doi.org/10.3390/polym14061246
Miloloža M, Bule K, Prevarić V, Cvetnić M, Ukić Š, Bolanča T, Kučić Grgić D. Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae. Polymers. 2022; 14(6):1246. https://doi.org/10.3390/polym14061246
Chicago/Turabian StyleMiloloža, Martina, Kristina Bule, Viktorija Prevarić, Matija Cvetnić, Šime Ukić, Tomislav Bolanča, and Dajana Kučić Grgić. 2022. "Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae" Polymers 14, no. 6: 1246. https://doi.org/10.3390/polym14061246
APA StyleMiloloža, M., Bule, K., Prevarić, V., Cvetnić, M., Ukić, Š., Bolanča, T., & Kučić Grgić, D. (2022). Assessment of the Influence of Size and Concentration on the Ecotoxicity of Microplastics to Microalgae Scenedesmus sp., Bacterium Pseudomonas putida and Yeast Saccharomyces cerevisiae. Polymers, 14(6), 1246. https://doi.org/10.3390/polym14061246