Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Reagents
2.2. Instruments
2.3. Synthesis of Liquid Crystal Epoxy Resin
2.3.1. 4,4′-Biphenol Diglycidyl Ether (BPE)
2.3.2. 4,4′-Bis(4-hydroxybenzylidene)diaminophenylene (SBPDA)
2.3.3. 4,4′-Bis(4-hydroxybenzylidene)diaminophenylene Diglycidyl Ether (SBPDAE)
2.3.4. 1,4-Bis(4-formylbenzoyloxy)butane (BDA)
2.3.5. 1,4-Bis{[4-(4-hydroxy)phenyliminomethylidene]-benzoyloxyl}butane (SBBDA)
2.3.6. 1,4-Bis{[4-(2,3-epoxypropoxy)phenyliminomethylidene]-benzoyloxyl}butane (SBBDAE)
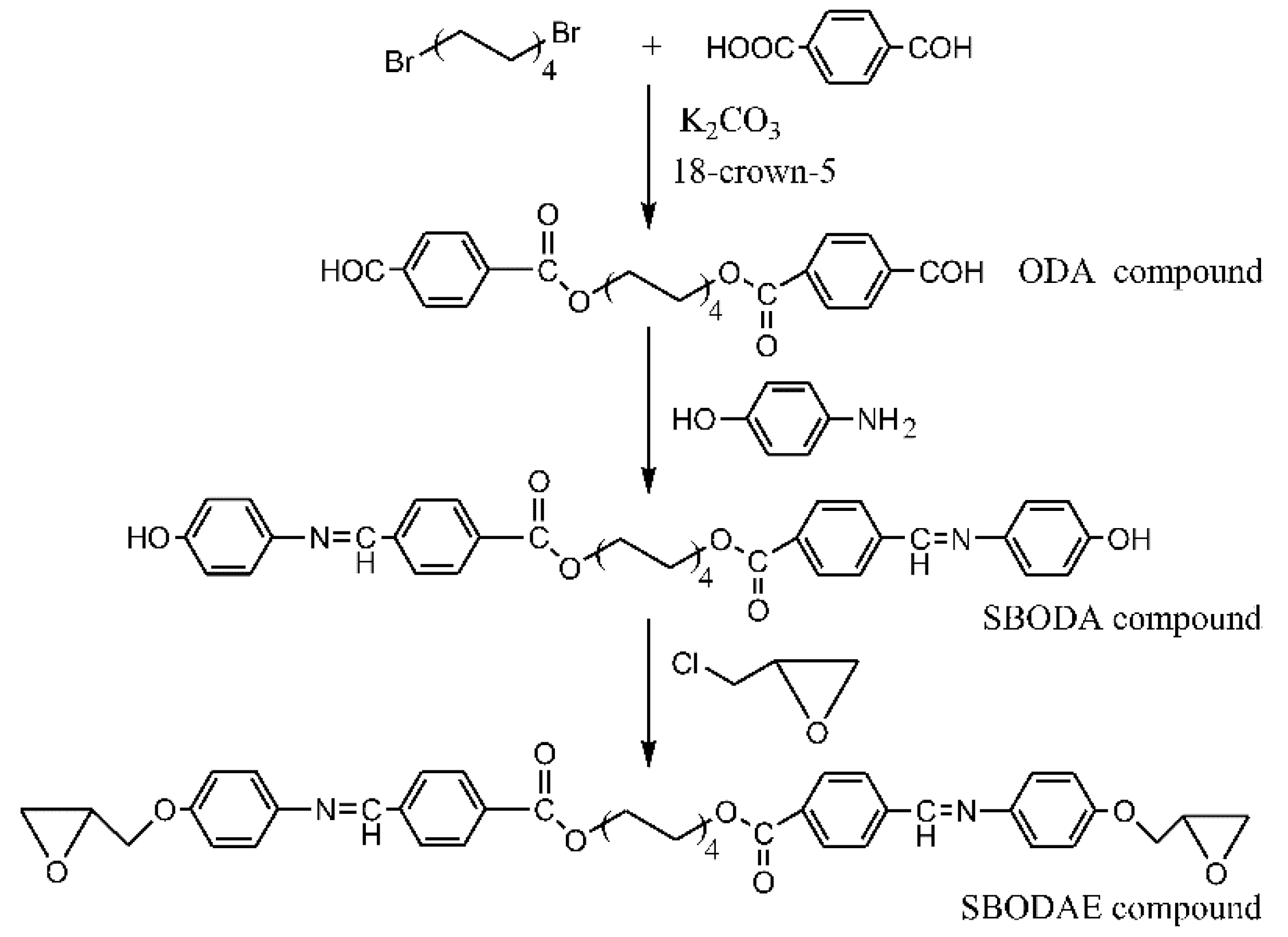
2.3.7. 1,8-Bis(4-formylbenzoyloxy)octane (ODA)
2.3.8. 1,8-Bis{[4-(4-hydroxy)phenyliminomethylidene]-benzoyloxyl}octane (SBODA)
2.3.9. 1,8-Bis{[4-(2,3-epoxypropoxy)phenyliminomethylidene]-benzoyloxyl}octane (SBODAE)
2.4. Epoxy Sample Preparation
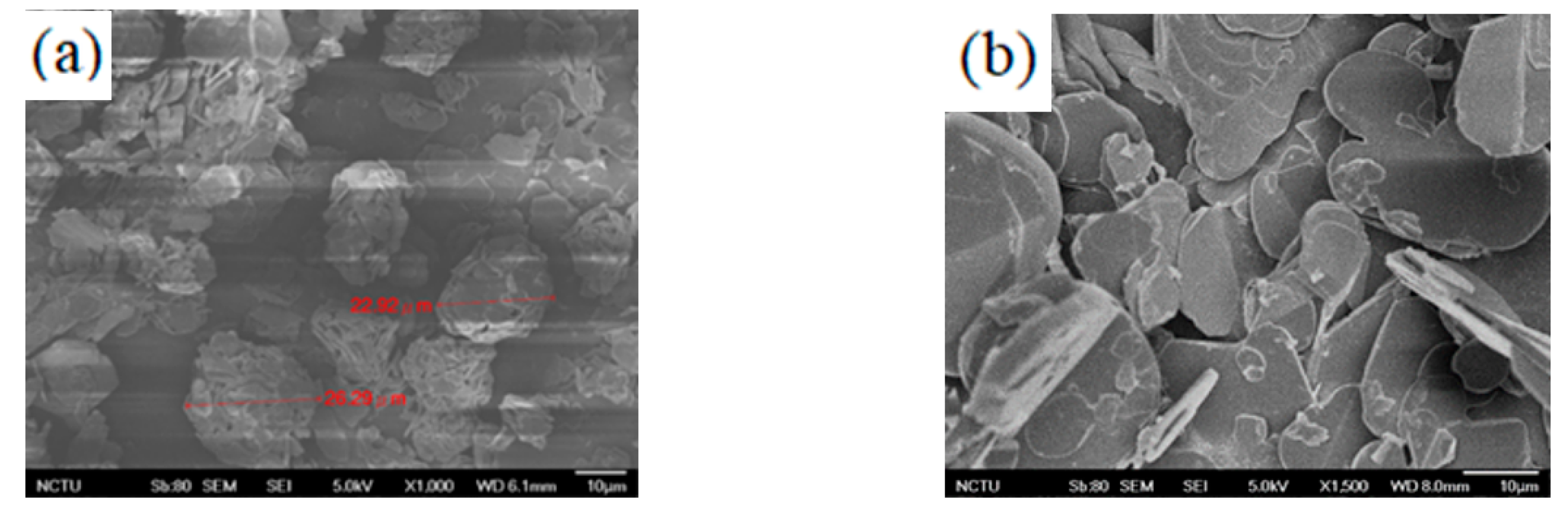
2.5. Epoxy Resin Boron Nitride Sample Preparation
2.6. Measurement of Thermal Conductivity
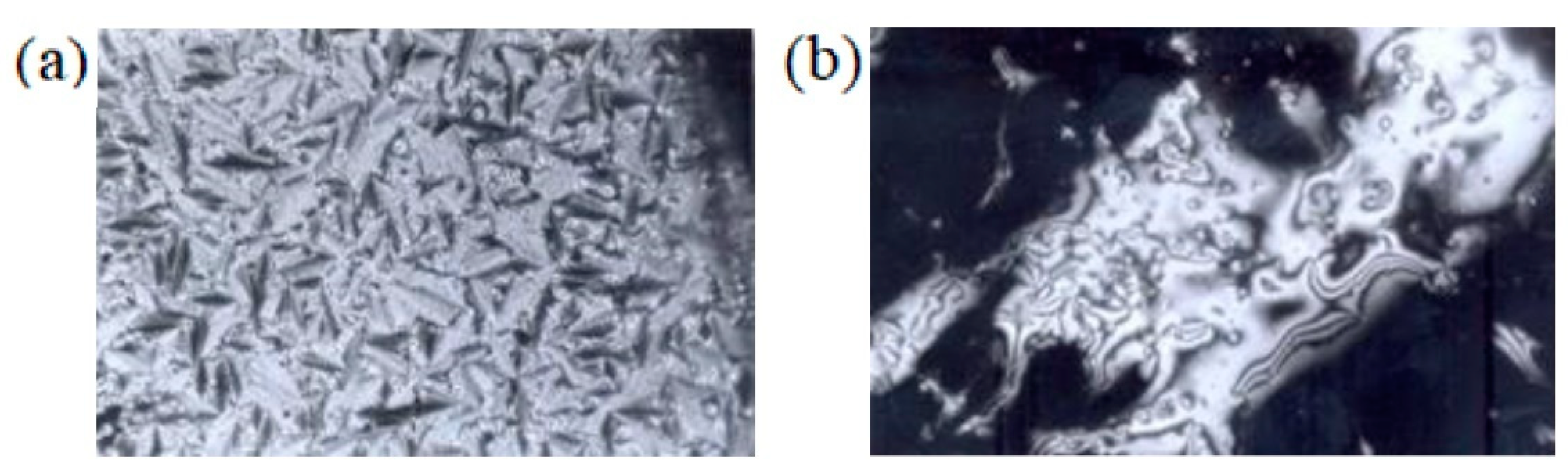
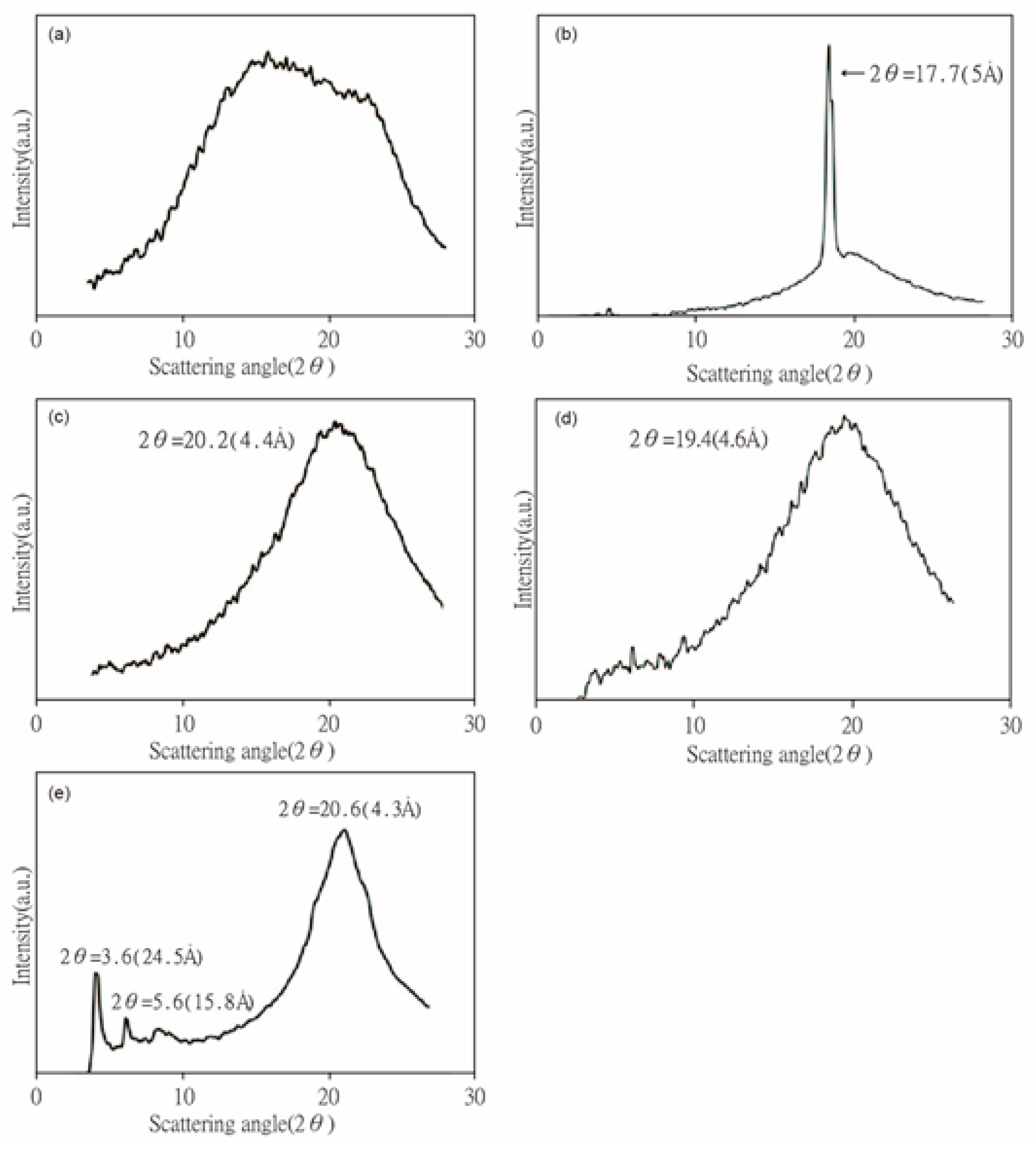
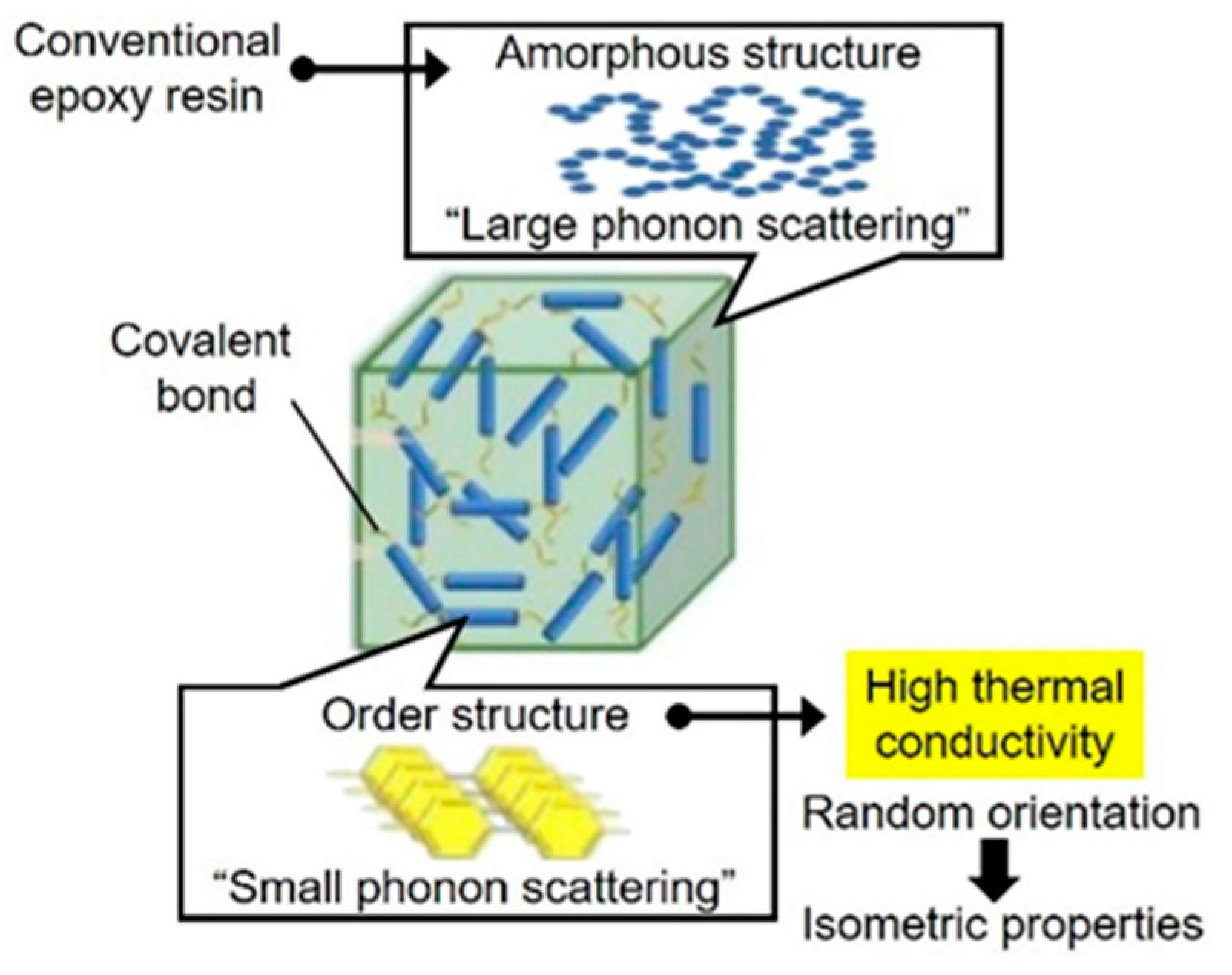
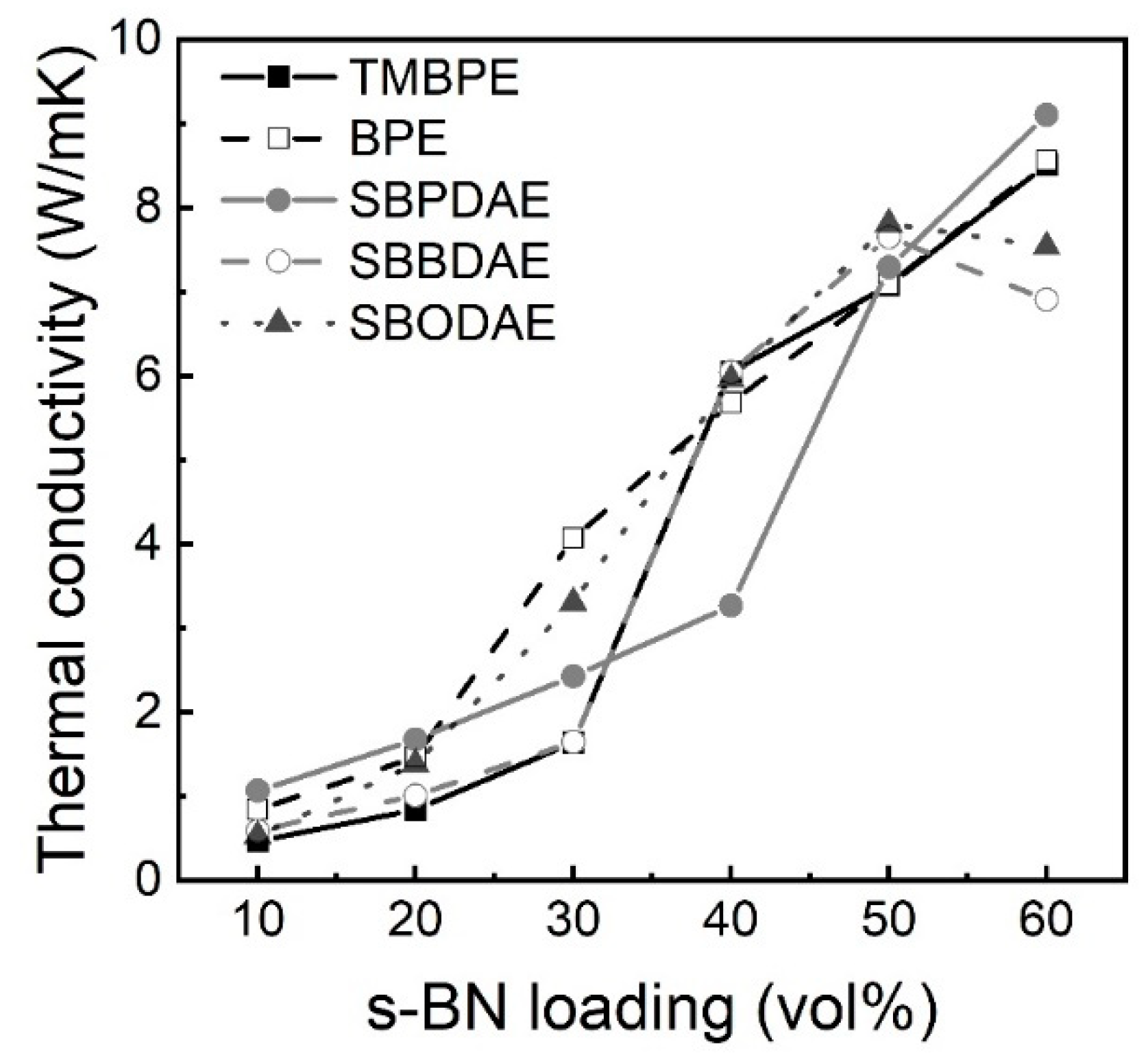
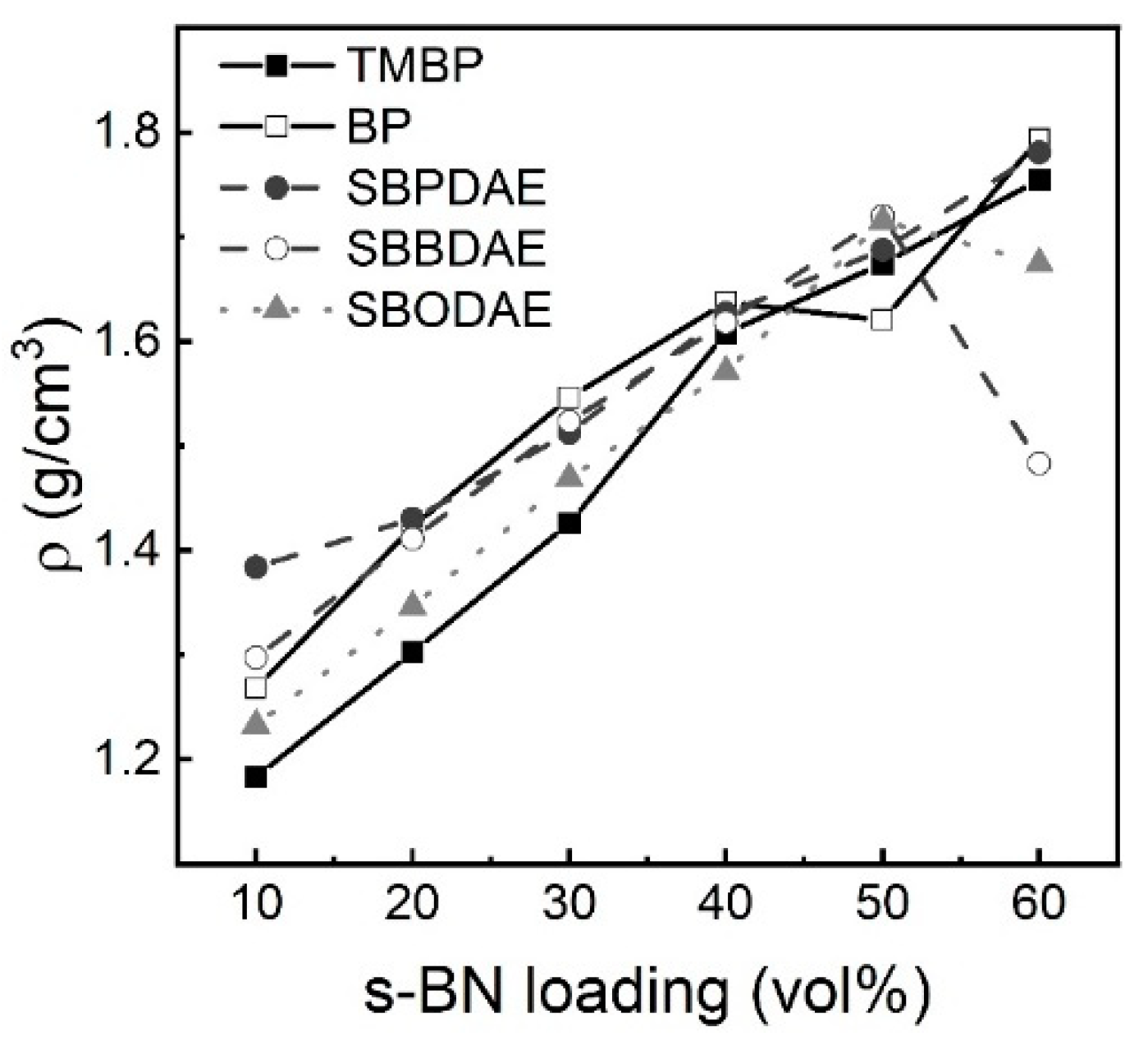
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nie, L.; Burgess, A.; Ryan, A. Moisture Permeation in Liquid Crystalline Epoxy Thermosets. Macromol. Chem. Phys. 2013, 214, 225–235. [Google Scholar] [CrossRef]
- Qi, B.; Lu, S.R.; Xiao, X.E.; Pan, L.L.; Tan, F.Z.; Yu, J.H. Enhanced thermal and mechanical properties of epoxy composites by mixing thermotropic liquid crystalline epoxy grafted graphene oxide. Express Polym. Lett. 2014, 8, 467–479. [Google Scholar] [CrossRef]
- Hsu, C.S.; Chen, H.L. Preparation of Liquid-Crystal Thermosets: In Situ Photopolymerization of Oriented Liquid-Crystal Duacrylates. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3929–3935. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Watanabe, T.; Whitehead, J.B. Anisotropic Network Formation by Photopolymerization of Liquid Crystal Monomers in a Low Strength Magnetic Field. Macromolecules 1995, 27, 6581–6588. [Google Scholar] [CrossRef]
- Vadivelu, M.A.; Kumar, C.R.; Joshi, G.M. Polymer Composites for Thermal Management: A Review. Compos. Interfaces 2016, 23, 847–872. [Google Scholar] [CrossRef]
- Huang, X.Y.; Jiang, P.K.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Henry, A. Thermal transport in polymers. Annu. Rev. Heat Transf. 2013, 17, 485–520. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal Conductivity of Polymer-Based Composites: Fundamentals and Applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Agari, Y.; Ueda, A.; Nagai, S. Thermal Conductivity of a Polymer Composite. J. Appl. Polym. Sci. 1993, 49, 1625–1634. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Hojo, F.; Takezawa, Y. Highly Thermoconductive Polymer Nanocomposite with a Nanoporous α-Alumina Sheet. ACS Appl. Mater. Interfaces 2009, 1, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Yamada, I.; Watari, K.; Harada, I.; Mitsuishi, K. Highthermal-conductivity AlN Filler for Polymer/Ceramics Composites. J. Am. Ceram. Soc. 2009, 92, S153–S156. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, S.M.; Shanefield, D.J.; Cannon, W.R. Enhanced Thermal Conductivity of Polymer Matrix Composite via High Solids Loading of Aluminum Nitride on Epoxy Resin. J. Am. Ceram. Soc. 2008, 91, 1169–1174. [Google Scholar] [CrossRef]
- Tanaka, S.; Hojo, F.; Kagawa, H.; Takezawa, Y. Fabrication of Highly Water-Resistant AlN Particles with Hybrid α-Al2O3/Organic Layers. J. Ceram. Soc. Jpn. 2014, 122, 211–215. [Google Scholar] [CrossRef][Green Version]
- Sato, K.; Horie, H.; Shirai, T.; Hotta, Y.; Nakano, H.; Nagai, H.; Mitsui, K.; Watari, K. Thermally Conductive Composite Films of Hexagonal Boron Nitride and Polyimide with Affinity-Enhanced Interfaces. J. Mater. Chem. 2010, 20, 2749–2751. [Google Scholar] [CrossRef]
- Li, S.; Qi, S.; Liu, N.; Cao, P. Study on Thermal Conductive BN/ Novolac Resin Composites. Thermochim. Acta 2011, 523, 111–115. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Thermal Conductivity and Mechanical Properties of BN-Filled Epoxy Composite: Effects of Filler Content, Mixing Conditions, and BNAg glomerate Size. J. Compos. Mater. 2011, 45, 1967–1980. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Yao, Y.; Pan, G.; Sun, J.; Zeng, J.; Sun, R.; Xu, J.B.; Song, B.; Wong, C.P. Polymer Composite with Improved Thermal Conductivity by Constructing a Hierarchically Ordered Three-Dimensional Interconnected Network of BN. ACS Appl. Mater. Interfaces 2017, 9, 13544–13553. [Google Scholar] [CrossRef]
- Harada, M.; Hamaura, N.; Ochi, M.; Agari, Y. Thermal conductivity of liquid crystalline epoxy/BN filler composites having ordered network structure. Compos. Part B 2013, 55, 306–313. [Google Scholar] [CrossRef]
- Hong, J.P.; Yoon, S.W.; Hwang, T.; Oh, J.S.; Hong, S.C.; Lee, Y.; Nam, J.D. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Jo, I.; Pettes, M.T.; Kim, J.; Watanabe, K.; Taniguchi, T.; Yao, Z.; Shi, L. Thermal Conductivity and Phonon Transport in Suspended Few-Layer Hexagonal Boron Nitride. Nano Lett. 2013, 13, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Guerra, V.; Wan, C.; McNally, T. Thermal conductivity of 2D nano-structured boron nitride (BN) and its composites with polymers. Prog. Mater. Sci. 2019, 100, 170–186. [Google Scholar] [CrossRef]
- Hoyt, A.E.; Benicewicz, B.C. Rigid Rod Molecules as Liquid Crystal Thermosets. I. Rigid Rod Amides. J. Polym. Sci. Part A Polym. Chem. 1990, 28, 3403–3415. [Google Scholar] [CrossRef]
- Douglas, E.P.; Langlois, D.A.; Benicewicz, B.C. Synthesis, Phase Behavior, and Curing Studies of Bisacetylene Rigid-Rod Thermosets. Chem. Mater. 1994, 6, 1925–1933. [Google Scholar] [CrossRef]
- Ober, C.K.; Shiota, A. Synthesis and Curing of Novel LC Twin Epoxy Monomers for Liquid Crystal Thermosets. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1291–1303. [Google Scholar]
- Carfagna, C.; Amendola, E.; Giamberini, M. Liquid Crystalline Epoxy Based Thermosetting Polymers. Prog. Polym. Sci. 1997, 22, 1607–1647. [Google Scholar] [CrossRef]
- Wang, B.; Yin, X.; Peng, D.; Zhang, Y.; Wu, W.; Gu, X.; Na, B.; Ruihua Lv, R.; Liu, H. Highly thermally conductive PVDF-based ternary dielectric composites via engineering hybrid filler networks. Compo. Part B 2020, 191, 107978. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.J.; Kuang, W.Y.; Tang, Z.H.; Guo, B.C. Coating polyrhodanine onto boron nitride nanosheets for thermally conductive elastomer composites. Compos. A Appl. Sci. 2017, 94, 77–85. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, W.; Gong, X.; Sun, Q.; Cao, X.; Su, Y.; Yu, B.; Li, R.K.Y.; Vellaisamy, R.A.L. Surface decoration of Halloysite nanotubes with POSS for fire-safe thermoplastic polyurethane nanocomposites. J. Mat. Sci. Technol. 2022, 101, 107–117. [Google Scholar] [CrossRef]
- Lakshmi, B.; Shivananda, K.N.; Mahendra, K.N. Synthesis, Characterization and Curing Studies of Thermosetting Epoxy Resin with Amines. Bull. Korean Chem. Soc. 2010, 31, 2272–2278. [Google Scholar] [CrossRef][Green Version]
- Hong, Y.; Goh, M. Advances in Liquid Crystalline Epoxy Resins for High Thermal Conductivity. Polymers 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Ribera, D.; Mantecon, A.; Serra, A. Synthesis and Crosslinking of a Series of Dimeric Liquid Crystalline Epoxy Resins Containing Imine Mesogens. Macromol Chem and Phys. 2001, 202, 1658–1671. [Google Scholar] [CrossRef]
- Heise, M.S.; Martin, G.C. Curing Mechanism and Thermal Properties of Epoxy-Imidazole Systems. Macromolecules 1989, 22, 99–104. [Google Scholar] [CrossRef]

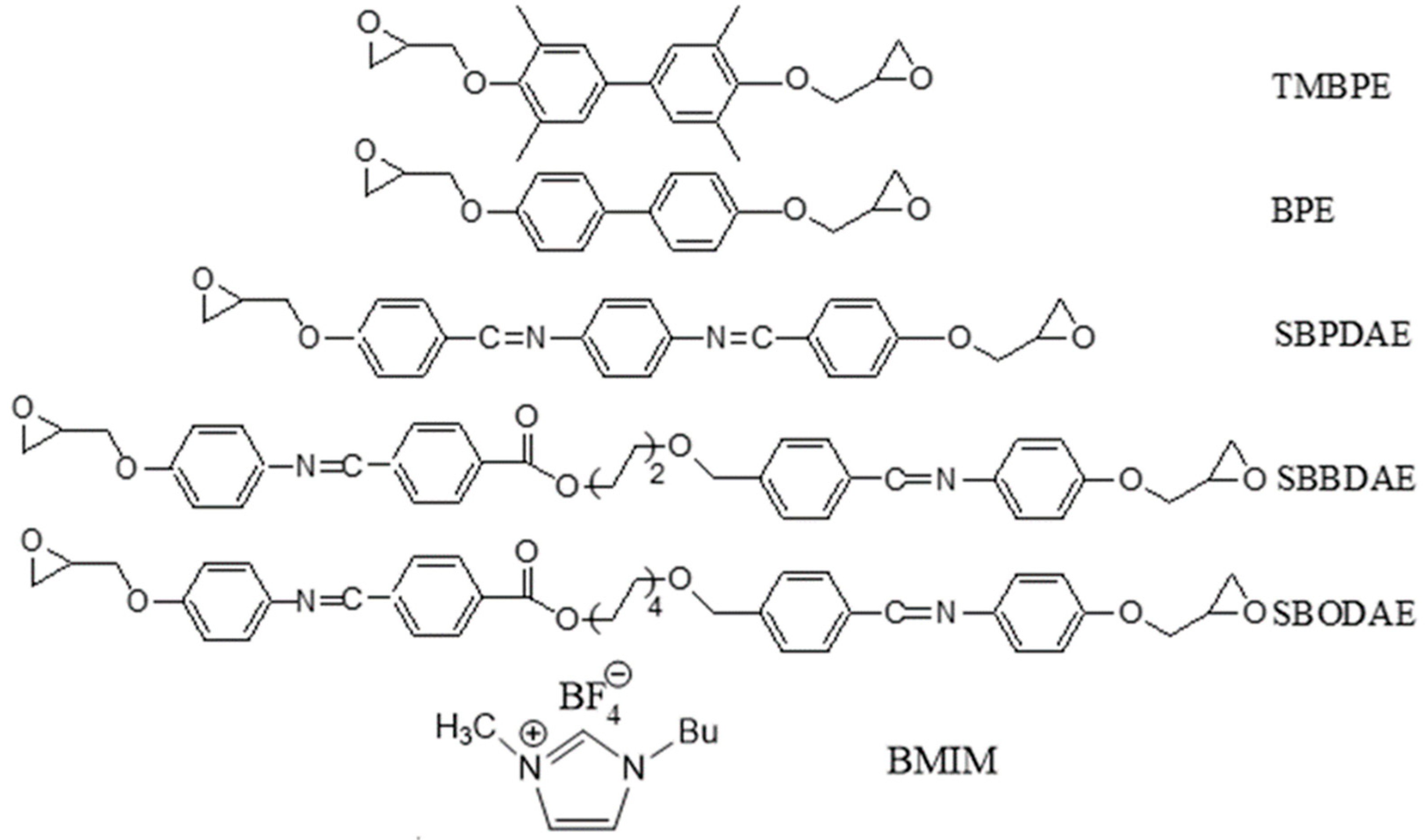

| SBPDAE, SBBDAE and SBODAE | |
|---|---|
| liquid crystal epoxy | Phase transitions |
| TMBPE | K 105.2 I |
| BPE | K 125.2 SA 158.3 I |
| SBPDAE | K 185.5 SA 262.9 I |
| SBBDAE | K 129.3 SA 173.8 N 258.6 I |
| SBODAE | K 142.3 SA 161.7 I |
K = crystal, SA = smectic A, N = nematic, I = isotropic
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.-C.; Huang, Y.-W.; Yeh, Y.-M.; Lin, C.-H. Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite. Polymers 2022, 14, 1252. https://doi.org/10.3390/polym14061252
Wu L-C, Huang Y-W, Yeh Y-M, Lin C-H. Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite. Polymers. 2022; 14(6):1252. https://doi.org/10.3390/polym14061252
Chicago/Turabian StyleWu, Li-Chuan, Yi-Wen Huang, Yao-Ming Yeh, and Chih-Hung Lin. 2022. "Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite" Polymers 14, no. 6: 1252. https://doi.org/10.3390/polym14061252
APA StyleWu, L.-C., Huang, Y.-W., Yeh, Y.-M., & Lin, C.-H. (2022). Characteristic and Synthesis of High-Temperature Resistant Liquid Crystal Epoxy Resin Containing Boron Nitride Composite. Polymers, 14(6), 1252. https://doi.org/10.3390/polym14061252






