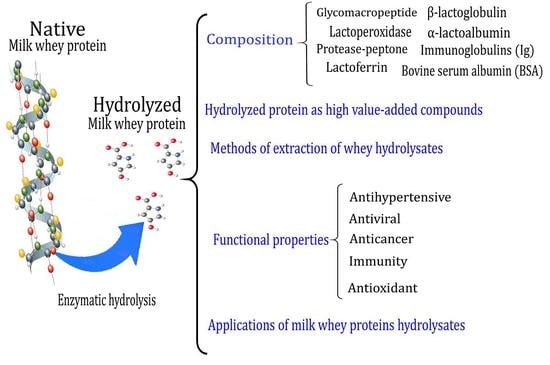
Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications
Abstract
:1. Introduction
2. Intrinsic Properties and Composition of Milk Whey Native Proteins
2.1. β-Lactoglobulin
2.2. α-Lactoalbumin
2.3. Immunoglobulins (Ig)
2.4. Bovine Serum Albumin (BSA)
2.5. Lactoferrin
2.6. Lactoperoxidase
2.7. Protease–Peptone
2.8. Glycomacropeptide
3. Hydrolyzed Protein from Milk Whey as High Value-Added Compounds
4. Methods of Extraction of Whey Hydrolysates
| Methods of Extraction | General Characteristics | Advantages | References |
|---|---|---|---|
| Chemical | Difficult to control and generates hydrolysates with modified amino acids. | Easy access to reagents. | [83,84,85] |
| Fermentation | It involves some acid lactic bacteria (BAL), no need to use acid or alkaline media | Bioactive peptides obtained can be purified without further hydrolysis. | [17,88] |
| Ultrasound | >20 kHz induced the unfolding of whey protein by high cavitation | Improves the enzymatic hydrolysis producing bioactive peptides from proteins presents in whey. | [89,90,91,92] |
| Enzymatic | Takes place under relatively mild operating conditions | Not addition of chemical reagents, nutritional value is maintained, control of the process (time, temperature and pH), most common method. | [93,94,95,110] |
| Green technology | Can be thermal treatments and high hydrostatic | Reduce time of hydrolysis, no generation of chemical waste. | [96,106,107,109] |
5. Functional Properties of Hydrolyzed Milk Whey Proteins
5.1. Antihypertensive
5.2. Antiviral
5.3. Anticancer
5.4. Immunity
5.5. Antioxidant
6. Applications of Milk Whey Proteins Hydrolysates
| Product | Functionality | Reference |
|---|---|---|
| Flavored milk beverage | Antioxidant activity | [154] |
| Whey MWH food supplementation in post-menopausal women | Increase muscle mass and strength | [165] |
| Apple juice | Low sedimentation | [156] |
| Beverage enriched white flaxseed oil | Increased of flavor, odor | [157] |
| MWH food supplementation in college-aged males | Increase mixed muscle and protein synthesis | [163] |
| MWH food supplementation | Improved recovery of muscle function and flexibility | [166] |
| Whey-raspberry flavored beverage | Antioxidant capacity and ACE inhibition | [148] |
| MWH food supplementation in athletes | Excellent source of nutritious | [162] |
| Whey protein-based beverage | Antioxidant and antimicrobial activity, no affecting physicochemical properties | [155] |
| Protein supplementation | Increasing mixed muscle and protein synthesis and lean body mass | [164] |
7. Future Considerations of Milk Whey Hydrolysates
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kaminarides, S.; Zagari, H.; Zoidou, E. Effect of whey fat content on the properties and yields of whey cheese and serum. J. Hell. Vet. Med. Soc. 2020, 71, 2149–2156. [Google Scholar] [CrossRef]
- Guo, M.; Wang, G. History of whey production and whey protein manufacturing. In Whey Protein Production, Chemistry, Functionality, and Applications; Guo, M., Ed.; John Wiley & Sons: Chichester, UK, 2019; pp. 1–12. [Google Scholar]
- Abd AL-Razaq, A.H. Whey applications in plants. Plant Arch. 2019, 19, 45–48. [Google Scholar]
- Shenana, M.; El-Alfy, M.; El-Nagar, G.; El-Barbary, A. Physico-chemical and functional properties of functional yoghurt made with different types of whey protein concentrates (Wpc). In Proceedings of the 5th International Conference on Biotechnology Applications in Agriculture (ICBAA), Hurghada, Egypt, 8–11 April 2020. [Google Scholar]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Tunick, M.H. Whey protein production and utilization: A brief history. In Whey Processing, Functionality and Health Benefits; Onwulata, C.I., Huth, P.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2008; pp. 1–13. [Google Scholar]
- Lee, H.; Song, M.; Hwang, S. Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process Biochem. 2003, 38, 1685–1693. [Google Scholar] [CrossRef]
- Khedkar, R.; Singh, K. Food industry waste: A panacea or pollution hazard? In Paradigms in Pollution Prevention; Springer: Berlin, Germany, 2018; pp. 35–47. [Google Scholar]
- Arsić, S.; Bulatović, M.; Zarić, D.; Kokeza, G.; Subić, J.; Rakin, M. Functional fermented whey carrot beverage-qualitative, nutritive and techno-economic analysis. Rom. Biotechnol. Lett. 2018, 23, 13496–13504. [Google Scholar]
- León-López, A.; Pérez-Marroquín, X.A.; Campos-Lozada, G.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Characterization of Whey-Based Fermented Beverages Supplemented with Hydrolyzed Collagen: Antioxidant Activity and Bioavailability. Foods 2020, 9, 1106. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef] [Green Version]
- Aoi, W.; Naito, Y.; Yoshikawa, T. Dietary exercise as a novel strategy for the prevention and treatment of metabolic syndrome: Effects on skeletal muscle function. J. Nutr. Metab. 2011, 2011, 676208. [Google Scholar] [CrossRef]
- Dullius, A.; Fassina, P.; Giroldi, M.; Goettert, M.I.; Volken de Souza, C.F. A biotechnological approach for the production of branched chain amino acid containing bioactive peptides to improve human health: A review. Food Res. Int. 2020, 131, 109002. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Wang, X.; Wang, H. Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases. LWT 2022, 159, 113257. [Google Scholar] [CrossRef]
- Kareb, O.; Aïder, M. Whey and its derivatives for probiotics, prebiotics, synbiotics, and functional foods: A critical review. Probiotics Antimicrob. Proteins 2019, 11, 348–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khemariya, P.; Rai, A. Process optimization for the manufacture of lemon based beverage from hydrolyzed whey. J. Food Sci. Technol. 2014, 51, 691–699. [Google Scholar] [CrossRef] [Green Version]
- Deeth, H.; Bansal, N. Chapter 1—Whey proteins: An overview. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: London, UK, 2019; pp. 1–50. [Google Scholar]
- Kumar, R.; Chauhan, S.K.; Shinde, G.; Subramanian, V.; Nadanasabapathi, S. Whey Proteins: A potential ingredient for food industry—A review. Asian J. Dairy Food Res. 2018, 37, 283–290. [Google Scholar]
- Fang, T.; Guo, M. Physicochemical, texture properties, and microstructure of yogurt using polymerized whey protein directly prepared from cheese whey as a thickening agent. J. Dairy Sci. 2019, 102, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Aguero, R.; Bringas, E.; San Roman, M.F.; Ortiz, I.; Ibanez, R. Membrane Processes for Whey Proteins Separation and Purification—A Review. Curr. Org. Chem. 2017, 21, 1740–1752. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Zaripov, O.G. Beta–lactoglobulin–nutrition allergen and nanotransporter of different nature ligands therapy with therapeutic action. Res. Vet. Sci. 2020, 133, 17–25. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Bianchini, R.; Afify, S.M.; Hofstetter, G.; Winkler, S.; Ahlers, S.; Altemeier, T.; Mayerhofer, H.; Hufnagl, K.; Korath, A.D. Secretory protein beta-lactoglobulin in cattle stable dust may contribute to the allergy-protective farm effect. Clin. Transl. Allergy 2022, 12, e12125. [Google Scholar] [CrossRef]
- Schlatterer, B.; Baeker, R.; Schlatterer, K. Improved purification of β-lactoglobulin from acid whey by means of ceramic hydroxyapatite chromatography with sodium fluoride as a displacer. J. Chromatogr. B 2004, 807, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Macwan, S.R.; Dabhi, B.K.; Parmar, S.; Aparnathi, K. Whey and its utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: Current options and future scenario—A critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Król, J.; Brodziak, A.; Zaborska, A.; Litwińczuk, Z. Comparison of whey proteins and lipophilic vitamins between four cow breeds maintained in intensive production system. Mljekarstvo Dairy 2017, 67, 17–24. [Google Scholar]
- Narayanan, R. Health augmenting properties of whey. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 152–154. [Google Scholar]
- Boscaini, S.; Cabrera-Rubio, R.; Nychyk, O.; Speakman, J.R.; Cryan, J.F.; Cotter, P.D.; Nilaweera, K.N. Age and duration-dependent effects of whey protein on high-fat diet-induced changes in body weight, lipid metabolism, and gut microbiota in mice. Physiol. Rep. 2020, 8, e14523. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Ahren, B.; Barnea, M.; Bar-Dayan, Y.; Froy, O. High-energy breakfast based on whey protein reduces body weight, postprandial glycemia and HbA1C in Type 2 diabetes. J. Nutr. Biochem. 2017, 49, 1–7. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef]
- Nabuco, H.C.; Tomeleri, C.M.; Sugihara Junior, P.; Fernandes, R.R.; Cavalcante, E.F.; Antunes, M.; Ribeiro, A.S.; Teixeira, D.C.; Silva, A.M.; Sardinha, L.B. Effects of whey protein supplementation pre-or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: A randomized clinical trial. Nutrients 2018, 10, 563. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Commean, P.K.; Reeds, D.N.; Klein, S.; Mittendorfer, B. Effect of protein supplementation during diet-induced weight loss on muscle mass and strength: A randomized controlled study. Obesity 2018, 26, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.J.; Santos, H.O.; Howell, S.L.; Pimentel, G.D. Whey protein in cancer therapy: A narrative review. Pharmacol. Res. 2019, 144, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak, K.; Fornal, E.; Waśko, A.; Gustaw, W. Effects of probiotic fermentation of selected milk and whey protein preparations on bioactive and technological properties. Ital. J. Food Sci. 2019, 31, 437–450. [Google Scholar]
- Wirunsawanya, K.; Upala, S.; Jaruvongvanich, V.; Sanguankeo, A. Whey Protein Supplementation Improves Body Composition and Cardiovascular Risk Factors in Overweight and Obese Patients: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Modler, W. Pioneer paper: Value-added components derived from whey. Am. Dairy Sci. Assoc. 2009, 1, 1–33. [Google Scholar]
- Jiang, B.; Wang, L.; Na, J.; Zhang, X.; Yuan, Y.; Liu, C.; Feng, Z. Environmentally-friendly strategy for separation of α-lactalbumin from whey by aqueous two phase flotation. Arab. J. Chem. 2020, 13, 3391–3402. [Google Scholar] [CrossRef]
- Heidebrecht, H.-J.; Kulozik, U. Fractionation of casein micelles and minor proteins by microfiltration in diafiltration mode. Study of the transmission and yield of the immunoglobulins IgG, IgA and IgM. Int. Dairy J. 2019, 93, 1–10. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Z.; Wusigale; Bakry, A.M.; Chen, Y.; Liang, L. Complexation of trans- and cis-resveratrol with bovine serum albumin, β-lactoglobulin or α-lactalbumin. Food Hydrocoll. 2018, 81, 242–252. [Google Scholar] [CrossRef]
- Sharma, R. Chapter 17—Whey proteins in functional foods. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: London, UK, 2019; pp. 637–663. [Google Scholar]
- Koh, B.-B.; Lee, E.-J.; Ramachandraiah, K.; Hong, G.-P. Characterization of bovine serum albumin hydrolysates prepared by subcritical water processing. Food Chem. 2019, 278, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Characteristics of bovine lactoferrin powders produced through spray and freeze drying processes. Int. J. Biol. Macromol. 2017, 95, 985–994. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.Q.; Kentish, S.E. Isolation of lactoferrin and immunoglobulins from dairy whey by an electrodialysis with filtration membrane process. Sep. Purif. Technol. 2020, 233, 115987. [Google Scholar] [CrossRef]
- Silva, E.; Oliveira, J.; Silva, Y.; Urbano, S.; Sales, D.; Moraes, E.; Rangel, A.; Anaya, K. Lactoperoxidase system in the dairy industry: Challenges and opportunities. Czech J. Food Sci. 2020, 38, 337–346. [Google Scholar] [CrossRef]
- Urtasun, N.; Baieli, M.F.; Hirsch, D.B.; Martínez-Ceron, M.C.; Cascone, O.; Wolman, F.J. Lactoperoxidase purification from whey by using dye affinity chromatography. Food Bioprod. Process. 2017, 103, 58–65. [Google Scholar] [CrossRef]
- Shokri, S.; Ehsani, A. Efficacy of whey protein coating incorporated with lactoperoxidase and α-tocopherol in shelf life extension of Pike-Perch fillets during refrigeration. LWT Food Sci. Technol. 2017, 85, 225–231. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, H.; Kumar, N.; Ranvir, S.; Jana, A.; Buttar, H.S.; Telessy, I.G.; Awuchi, C.G.; Okpala, C.O.R.; Korzeniowska, M.; et al. Whey proteins processing and emergent derivatives: An insight perspective from constituents, bioactivities, functionalities to therapeutic applications. J. Funct. Foods 2021, 87, 104760. [Google Scholar] [CrossRef]
- Hogenboom, J.A.; Rosi, V.; Monti, L. Effect of processing and storage conditions on the evolution of the proteose peptone content in pasteurized and extended shelf-life milk. Sci. E Tec. Latt. Casearia 2020, 70, 24–28. [Google Scholar] [CrossRef]
- Innocente, N.; Biasutti, M.; Blecker, C. HPLC profile and dynamic surface properties of the proteose–peptone fraction from bovine milk and from whey protein concentrate. Int. Dairy J. 2011, 21, 222–228. [Google Scholar] [CrossRef]
- Karamoko, G.; Renaville, R.; Blecker, C. Interfacial activities of milk total proteose-peptone: Contribution and miscibility of nonhydrophobic and hydrophobic fractions. Int. Dairy J. 2016, 61, 29–36. [Google Scholar] [CrossRef]
- Chungchunlam, S.M.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Effect of whey protein and glycomacropeptide on measures of satiety in normal-weight adult women. Appetite 2014, 78, 172–178. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, N.; O’Callaghan, J.; Buttò, L.F.; Kilcoyne, M.; Joshi, L.; Hickey, R.M. Bovine glycomacropeptide promotes the growth of Bifidobacterium longum ssp. infantis and modulates its gene expression. J. Dairy Sci. 2018, 101, 6730–6741. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Nicolás, J.L.; González-Olivares, L.G.; Vázquez-Rodríguez, G.A.; Lucho-Constatino, C.A.; Castañeda-Ovando, A.; Cruz-Guerrero, A.E. Valorization of whey using a biorefinery. Biofuels Bioprod. Biorefin. 2020, 14, 1010–1027. [Google Scholar] [CrossRef]
- Valdez Castillo, M.; Laxman Pachapur, V.; Brar, S.K.; Naghdi, M.; Arriaga, S.; Ávalos Ramirez, A. Yeast-driven whey biorefining to produce value-added aroma, flavor, and antioxidant compounds: Technologies, challenges, and alternatives. Crit. Rev. Biotechnol. 2020, 40, 930–950. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Sci. entific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Duan, F.; Zhao, R.; Yang, J.; Xiao, M.; Lu, L. Integrated Utilization of Dairy Whey in Probiotic β-Galactosidase Production and Enzymatic Synthesis of Galacto-Oligosaccharides. Catalysts 2021, 11, 658. [Google Scholar] [CrossRef]
- Duncan, P.I.; Aitio, O.; Heiskanen, A.; Niemelä, R.; Saarinen, J.; Helin, J.; Porta, N.; Fiaux, M.; Moënnoz, D.; Golliard, M.; et al. Structure and Function of Bovine Whey Derived Oligosaccharides Showing Synbiotic Epithelial Barrier Protective Properties. Nutrients 2020, 12, 2007. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Gong, Y.; Goddard, J.M.; Abbaspourrad, A. Synthesis and characterization of lactose fatty acid ester biosurfactants using free and immobilized lipases in organic solvents. Food Chem 2018, 266, 508–513. [Google Scholar] [CrossRef]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Mousavi Khaneghah, A.; Santana, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Chreptowicz, K.; Sternicka, M.K.; Kowalska, P.D.; Mierzejewska, J. Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett. Appl. Microbiol. 2018, 66, 153–160. [Google Scholar] [CrossRef]
- Izawa, N.; Kudo, M.; Nakamura, Y.; Mizukoshi, H.; Kitada, T.; Sone, T. Production of aroma compounds from whey using Wickerhamomyces pijperi. AMB Express 2015, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, D.; Cai, D.; Zhan, Y.; Wang, Q.; Chen, S. Identification and High-level Production of Pulcherrimin in Bacillus licheniformis DW2. Appl. Biochem. Biotechnol. 2017, 183, 1323–1335. [Google Scholar] [CrossRef]
- Türkel, S.; Korukluoğlu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef] [Green Version]
- Kolesovs, S.; Semjonovs, P. Production of bacterial cellulose from whey—Current state and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7723–7730. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Mann, B.; Athira, S.; Sharma, R.; Kumar, R.; Sarkar, P. Chapter 14—Bioactive Peptides from Whey Proteins. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: London, UK, 2019; pp. 519–547. [Google Scholar]
- Ahmed, M.E.; Hamdy, A.M.; Hammam, A.R. Therapeutic Benefits and Applications of Whey Protein. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 337–345. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods—Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Nasri, M. Chapter Four—Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits—A review. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: London, UK, 2017; Volume 81, pp. 109–159. [Google Scholar]
- Saadi, S.; Saari, N.; Anwar, F.; Abdul Hamid, A.; Ghazali, H.M. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef]
- Tavano, O.L. Protein hydrolysis using proteases: An important tool for food biotechnology. J. Mol. Catal. B Enzym. 2013, 90, 1–11. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; García-Cano, I.; Dabrowski, K.; Jiménez-Flores, R. Bacterial Diversity Analysis and Evaluation Proteins Hydrolysis During the Acid Whey and Fish Waste Fermentation. Microorganisms 2021, 9, 100. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H.; Park, B.-J.; Kim, S.-H.; Oh, D.-H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef]
- Alizadeh, O.; Aliakbarlu, J. Effects of ultrasound and ohmic heating pretreatments on hydrolysis, antioxidant and antibacterial activities of whey protein concentrate and its fractions. LWT 2020, 131, 109913. [Google Scholar] [CrossRef]
- Shen, X.; Shao, S.; Guo, M. Ultrasound-induced changes in physical and functional properties of whey proteins. Int. J. Food Sci. Technol. 2017, 52, 381–388. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Penha, F.M.; Cunha Petrus, J.C.; Rezzadori, K. Low purity enzymes and ultrasound pretreatment applied to partially hydrolyze whey protein. Food Biosci. 2020, 38, 100784. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Jia, J.; Kuang, C.; Yang, H. Effect of ultrasonic pretreatment on whey protein hydrolysis by alcalase: Thermodynamic parameters, physicochemical properties and bioactivities. Process Biochem. 2018, 67, 46–54. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Bettiol, M.d.R.; Vanden Braber, N.L.; Aminahuel, C.A.; Rossi, Y.E.; Petroselli, G.; Erra-Balsells, R.; Cavaglieri, L.R.; Montenegro, M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020, 319, 126472. [Google Scholar] [CrossRef]
- Zhao, C.; Ashaolu, T.J. Bioactivity and safety of whey peptides. LWT 2020, 134, 109935. [Google Scholar] [CrossRef]
- Sáez, L.; Murphy, E.; FitzGerald, R.J.; Kelly, P. Exploring the use of a modified High-Temperature, Short-Time Continuous Heat Exchanger with Extended Holding Time (HTST-EHT) for thermal inactivation of trypsin following selective enzymatic hydrolysis of the β-lactoglobulin fraction in whey protein isolate. Foods 2019, 8, 367. [Google Scholar]
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Olsen, J.V.; Ong, S.-E.; Mann, M. Trypsin Cleaves Exclusively C-terminal to Arginine and Lysine Residues. Mol. Cell. Proteom. 2004, 3, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Ambrosi, V.; Polenta, G.; Gonzalez, C.; Ferrari, G.; Maresca, P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov. Food Sci. Emerg. Technol. 2016, 38, 294–301. [Google Scholar] [CrossRef]
- Lestari, P. Antibacterial activity of hydrolysate protein from Etawa goat milk hydrolysed by crude extract bromelain. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012111. [Google Scholar]
- Silva, M.R.; Silvestre, M.P.; Silva, V.D.; Souza, M.W.; Lopes Junior, C.O.; Afonso, W.O.; Lana, F.C.; Rodrigues, D.F. Production of ACE-inhibitory whey protein concentrate hydrolysates: Use of pancreatin and papain. Int. J. Food Prop. 2014, 17, 1002–1012. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Mora-Cortes, W.G.; Leandro-Roldan, M.M.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Ramírez-Suarez, J.C.; González-Córdova, A.F.; Vallejo-Córdoba, B. Production of whey protein hydrolysates with angiotensin-converting enzyme-inhibitory activity using three new sources of plant proteases. Biocatal. Agric. Biotechnol. 2020, 28, 101724. [Google Scholar] [CrossRef]
- Onwulata, C.; Huth, P. Whey Processing, Functionality and Health Benefits; John Wiley & Sons: Ames, IA, USA, 2009; Volume 82. [Google Scholar]
- Liu, L.; Li, X.; Du, L.; Zhang, X.; Yang, W.; Zhang, H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT 2019, 109, 130–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.-H.; He, G.-Q. Effect of ultrasonic-ionic liquid pretreatment on the hydrolysis degree and antigenicity of enzymatic hydrolysates from whey protein. Ultrason. Sonochem. 2020, 63, 104926. [Google Scholar] [CrossRef]
- Cheison, S.C.; Leeb, E.; Toro-Sierra, J.; Kulozik, U. Influence of hydrolysis temperature and pH on the selective hydrolysis of whey proteins by trypsin and potential recovery of native alpha-lactalbumin. Int. Dairy J. 2011, 21, 166–171. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Cheison, S.C.; Kulozik, U. Impact of the environmental conditions and substrate pre-treatment on whey protein hydrolysis: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 418–453. [Google Scholar] [CrossRef]
- Anema, S.G. Chapter 9—The whey proteins in milk. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 325–384. [Google Scholar]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Antihypertensive peptides of animal origin: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 566–578. [Google Scholar] [CrossRef]
- Cracowski, J.L.; Boutouyrie, P. Chapitre 1—Inhibiteurs du système rénine-angiotensine. In Pharmacologie Cardiovasculaire et Respiratoire; Bellien, J., Cracowski, J.-L., Eds.; Elsevier Masson: Paris, France, 2016; pp. 3–14. [Google Scholar]
- Martínez-Medina, G.; Prado-Barragán, A.; Martínez-Hernández, J.; Ruíz, H.; Rodríguez, R.; Contreras-Esquivel, J.; Aguilar, C. Péptidos Bio-funcionales: Bioactividad, producción y aplicaciones. Rev. Cient. Univ. Autón. Coahuila 2019, 13, 1–7. [Google Scholar]
- Herrera-Ponce, A.L.; Alarcón-Rojo, A.D.; Salmeron, I.; Rodríguez-Figueroa, J.C. Efectos fisiológicos de los péptidos bioactivos derivados de las proteínas del lactosuero en la salud: Una revisión. Rev. Chil. Nutr. 2019, 46, 205–214. [Google Scholar] [CrossRef]
- Torruco-Uco, J.G.; Dominguez-Magaña, M.A.; Davila-Ortiz, G.; Martinez-Ayala, A.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Antihypertensive peptides, an alternative for treatment of natural origin: A review. Cienc. Tecnol. Aliment. 2008, 6, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-García, P. Péptidos Bioactivos Derivados de la Leche de Bovino y Sus Efectos Fisiológicos en Humanos: Generalidades y Aspectos Moleculares; CBG-IPN: Reynosa, Tamaulipas, Mexico, 2017; Volume 1, pp. 1–22. [Google Scholar]
- Tovar-Jiménez, X.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Mercado-Flores, Y.; Arana-Cuenca, A. Antioxidant and antihypertensive activity of bovine whey protein concentrate enzymatic hydrolysates. Biotecnia 2021, 23, 161–169. [Google Scholar] [CrossRef]
- Miralles, B.; Amigo, L.; Recio, I. Critical Review and Perspectives on Food-Derived Antihypertensive Peptides. J. Agric. Food Chem. 2018, 66, 9384–9390. [Google Scholar] [CrossRef]
- Hammam, A.; Tammam, A.; Elderwy, Y.; Hassan, A. Functional peptides in milk whey: An overview. Assiut J. Agric. Sci. 2017, 48, 77–91. [Google Scholar] [CrossRef]
- Jahandideh, F.; Wu, J. Perspectives on the potential benefits of antihypertensive peptides towards metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2192. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [Green Version]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of Use of Antiviral Peptides against Influenza Virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.B.; Cheung, R.C.F.; Wong, J.H.; Wang, Y.; Ip, D.T.M.; Wan, D.C.C.; Xia, J. Antiviral activities of whey proteins. Appl. Microbiol. Biotechnol. 2015, 99, 6997–7008. [Google Scholar] [CrossRef]
- Garg, L.; Kumar, K. Industrial applications of whey. Pharma 2021, 2, 387–390. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O. Bioactivity of Whey Peptides. Int. J. Sci. Adv. 2020, 1, 10–13. [Google Scholar] [CrossRef]
- Sitohy, M.; Taha, S.; Osman, A.; Abdel-Hamid, M.; Hamed, A.; Abdelbacki, A. Antiviral action of native and methylated lactoferrin and β-Lactoglobulin against potato virus Y (PVY) infected into potato plants grown in an open field. Antibiotics 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Isolation and identification of anti-proliferative peptides from Spirulina platensis using three-step hydrolysis. J. Sci. Food Agric. 2017, 97, 918–922. [Google Scholar] [CrossRef]
- Kamal, H.; Jafar, S.; Mudgil, P.; Murali, C.; Amin, A.; Maqsood, S. Inhibitory properties of camel whey protein hydrolysates toward liver cancer cells, dipeptidyl peptidase-IV, and inflammation. J. Dairy Sci. 2018, 101, 8711–8720. [Google Scholar] [CrossRef] [Green Version]
- Carrillo Pérez, C.; Cavia Camarero, M.d.M.; Alonso de la Torre, S. Antitumor effect of oleic acid; mechanisms of action. A review. Nutr. Hosp. 2012, 27, 1860–1865. [Google Scholar]
- Permyakov, E.A.; Berliner, L.J. α-Lactalbumin: Structure and function. FEBS Lett. 2000, 473, 269–274. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta Gen. Subj. 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Cross, M.L.; Gill, H.S. Immunomodulatory properties of milk. Br. J. Nutr. 2000, 84 (Suppl. 1), S81–S89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yuan, S.; Huang, M.; Gao, J.; Wu, Z.; Tong, P.; Yang, A.; Chen, H. Identification of IgE and IgG epitopes on native Bos d 4 allergen specific to allergic children. Food Funct. 2016, 7, 2996–3005. [Google Scholar] [CrossRef]
- Willison, L.N.; Zhang, Q.; Su, M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Conformational epitope mapping of Pru du 6, a major allergen from almond nut. Mol. Immunol. 2013, 55, 253–263. [Google Scholar] [CrossRef]
- Pomés, A. Relevant B Cell Epitopes in Allergic Disease. Int. Arch. Allergy Immunol. 2010, 152, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Keppler, J.; Koudelka, T.; Palani, K.; Tholey, A.; Schwarz, K. Interaction of β-Lactoglobulin with Small Hydrophobic Ligands—Influence of Covalent AITC Modification on β-LG Tryptic Cleavage. Food Biophys. 2014, 9, 349–358. [Google Scholar] [CrossRef]
- Keppler, J.K.; Martin, D.; Garamus, V.M.; Berton-Carabin, C.; Nipoti, E.; Coenye, T.; Schwarz, K. Functionality of whey proteins covalently modified by allyl isothiocyanate. Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocoll. 2017, 65, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Pérez, V.; Bumke-Vogt, C.; Schreiner, M.; Mewis, I.; Borchert, A.; Pfeiffer, A.F.H. Benzylglucosinolate Derived Isothiocyanate from Tropaeolum majus Reduces Gluconeogenic Gene and Protein Expression in Human Cells. PLoS ONE 2016, 11, e0162397. [Google Scholar] [CrossRef]
- Spöttel, J.; Brockelt, J.; Badekow, S.; Rohn, S. Immunological Analysis of Isothiocyanate-Modified α-Lactalbumin Using High-Performance Thin Layer Chromatography. Mol. 2021, 26, 1842. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino Acid Composition and Antioxidant Properties of Pea Seed (Pisum sativum L.) Enzymatic Protein Hydrolysate Fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Vivas, Y.A.; Morales, A.J.; Otálvaro, Á.M. Aprovechamiento de lactosuero para el desarrollo de una bebida refrescante con antioxidantes naturales. Utilization of whey in the development of a refreshing beverage with natural antioxidants. Aliment. Hoy 2016, 24, 185–199. [Google Scholar]
- Guimarães, J.T.; Silva, E.K.; Ranadheera, C.S.; Moraes, J.; Raices, R.S.L.; Silva, M.C.; Ferreira, M.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Effect of high-intensity ultrasound on the nutritional profile and volatile compounds of a prebiotic soursop whey beverage. Ultrason. Sonochem. 2019, 55, 157–164. [Google Scholar] [CrossRef]
- Ferreira, M.V.S.; Cappato, L.P.; Silva, R.; Rocha, R.S.; Guimarães, J.T.; Balthazar, C.F.; Esmerino, E.A.; Freitas, M.Q.; Rodrigues, F.N.; Granato, D.; et al. Ohmic heating for processing of whey-raspberry flavored beverage. Food Chem. 2019, 297, 125018. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, L.; Silva, L.P.; Prates, M.V.; Bloch, C.; Takeiti, C.Y.; Gomes, D.M.; da Silva-Santos, J.E.; Deliza, R.; Brígida, A.I.S.; Furtado, A.; et al. Whey hydrolysate-based ingredient with dual functionality: From production to consumer’s evaluation. Food Res. Int. 2019, 122, 123–128. [Google Scholar] [CrossRef]
- Jeewanthi, R.K.C.; Lee, N.-K.; Paik, H.-D. Improved Functional Characteristics of Whey Protein Hydrolysates in Food Industry. Korean J. Food Sci. Anim. Resour. 2015, 35, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Skrzypczak, K.; Gustaw, W.; Fornal, E.; Kononiuk, A.; Michalak-Majewska, M.; Radzki, W.; Waśko, A. Functional and Technological Potential of Whey Protein Isolate in Production of Milk Beverages Fermented by New Strains of Lactobacillus helveticus. Appl. Sci. 2020, 10, 7089. [Google Scholar] [CrossRef]
- Scalone, G.L.L.; Ioannidis, A.G.; Lamichhane, P.; Devlieghere, F.; De Kimpe, N.; Cadwallader, K.; De Meulenaer, B. Impact of whey protein hydrolysates on the formation of 2,5-dimethylpyrazine in baked food products. Food Res. Int. 2020, 132, 109089. [Google Scholar] [CrossRef]
- Jrad, Z.; Oussaief, O.; Khorchani, T.; El-Hatmi, H. Microbial and enzymatic hydrolysis of dromedary whey proteins and caseins: Techno-functional, radical scavenging, antimicrobial properties and incorporation in beverage formulation. J. Food Meas. Charact. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Mann, B.; Kumari, A.; Kumar, R.; Sharma, R.; Prajapati, K.; Mahboob, S.; Athira, S. Antioxidant activity of whey protein hydrolysates in milk beverage system. J. Food Sci. Technol. 2015, 52, 3235–3241. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Corrochano, A.R.; Shanahan, C.; Villalva, M.; Jaime, L.; Santoyo, S.; Callanan, M.J.; Murphy, E.; Giblin, L. Antioxidant activity and characterization of whey protein-based beverages: Effect of shelf life and gastrointestinal transit on bioactivity. Innov. Food Sci. Emerg. Technol. 2019, 57, 102209. [Google Scholar] [CrossRef]
- Goudarzi, M.; Madadlou, A.; Mousavi, M.E.; Emam-Djomeh, Z. Formulation of apple juice beverages containing whey protein isolate or whey protein hydrolysate based on sensory and physicochemical analysis. Int. J. Dairy Technol. 2015, 68, 70–78. [Google Scholar] [CrossRef]
- Kabašinskienė, A.; Liutkevičius, A.; Sekmokienė, D.; Zaborskienė, G.; Šlapkauskaitė, J. Evaluation of the Physicochemical Parameters of Functional Whey Beverages. Food Technol. Biotechnol. 2015, 53, 110–115. [Google Scholar] [CrossRef]
- Jelen, P. Whey-based functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2009; pp. 259–280. [Google Scholar]
- Ali, A.; Lee, S.-J.; Rutherfurd-Markwick, K.J. Chapter 16—Sports and exercise supplements. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: London, UK, 2019; pp. 579–635. [Google Scholar]
- Huecker, M.; Sarav, M.; Pearlman, M.; Laster, J. Protein Supplementation in Sport: Source, Timing, and Intended Benefits. Curr. Nutr. Rep. 2019, 8, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Nakayama, K.; Fukasawa, T.; Koga, J.; Kanegae, M.; Kawanaka, K.; Higuchi, M. Post-exercise whey protein hydrolysate supplementation induces a greater increase in muscle protein synthesis than its constituent amino acid content. Br. J. Nutr. 2013, 110, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Fassina, P.; Nunes, G.Q.; Adami, F.S.; Goettert, M.I.; Volken de Souza, C.F. Importance of Cheese Whey Processing: Supplements for Sports Activities—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 83–99. [Google Scholar] [CrossRef]
- Lockwood, C.M.; Roberts, M.D.; Dalbo, V.J.; Smith-Ryan, A.E.; Kendall, K.L.; Moon, J.R.; Stout, J.R. Effects of Hydrolyzed Whey versus Other Whey Protein Supplements on the Physiological Response to 8 Weeks of Resistance Exercise in College-Aged Males. J. Am. Coll. Nutr. 2017, 36, 16–27. [Google Scholar] [CrossRef]
- Hansen, M.; Oxfeldt, M.; Larsen, A.E.; Thomsen, L.S.; Rokkedal-Lausch, T.; Christensen, B.; Rittig, N.; De Paoli, F.V.; Bangsbo, J.; Ørtenblad, N.; et al. Supplement with whey protein hydrolysate in contrast to carbohydrate supports mitochondrial adaptations in trained runners. J. Int. Soc. Sports Nutr. 2020, 17, 46. [Google Scholar] [CrossRef]
- Weisgarber, K.D.; Candow, D.G.; Farthing, J.P. Whey protein and high-volume resistance training in postmenopausal women. J. Nutr. Health Aging 2015, 19, 511–517. [Google Scholar] [CrossRef]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Whey protein hydrolysate supplementation accelerates recovery from exercise-induced muscle damage in females. Appl. Physiol. Nutr. Metab. 2018, 43, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Krause, M.; Newsholme, P. Amino acid supplementation and impact on immune function in the context of exercise. J. Int. Soc. Sports Nutr. 2014, 11, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | Sweet Whey | Acid Whey |
|---|---|---|
| pH | >5.6 | <5.6 |
| Water | 93–94% | 94–95% |
| Protein (g/L) | 6–10 | 6–8 |
| Lactose (g/L) | 46–52 | 44–46 |
| Minerals (g/L) | 2.5–4.7 | 4.3–7.2 |
| Obtained by | Enzymatic action | Organic acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-López, A.; Pérez-Marroquín, X.A.; Estrada-Fernández, A.G.; Campos-Lozada, G.; Morales-Peñaloza, A.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications. Polymers 2022, 14, 1258. https://doi.org/10.3390/polym14061258
León-López A, Pérez-Marroquín XA, Estrada-Fernández AG, Campos-Lozada G, Morales-Peñaloza A, Campos-Montiel RG, Aguirre-Álvarez G. Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications. Polymers. 2022; 14(6):1258. https://doi.org/10.3390/polym14061258
Chicago/Turabian StyleLeón-López, Arely, Xóchitl Alejandra Pérez-Marroquín, Ana Guadalupe Estrada-Fernández, Gieraldin Campos-Lozada, Alejandro Morales-Peñaloza, Rafael G. Campos-Montiel, and Gabriel Aguirre-Álvarez. 2022. "Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications" Polymers 14, no. 6: 1258. https://doi.org/10.3390/polym14061258
APA StyleLeón-López, A., Pérez-Marroquín, X. A., Estrada-Fernández, A. G., Campos-Lozada, G., Morales-Peñaloza, A., Campos-Montiel, R. G., & Aguirre-Álvarez, G. (2022). Milk Whey Hydrolysates as High Value-Added Natural Polymers: Functional Properties and Applications. Polymers, 14(6), 1258. https://doi.org/10.3390/polym14061258