Infrared Linear Dichroism for the Analysis of Molecular Orientation in Polymers and in Polymer Composites
Abstract
1. Introduction
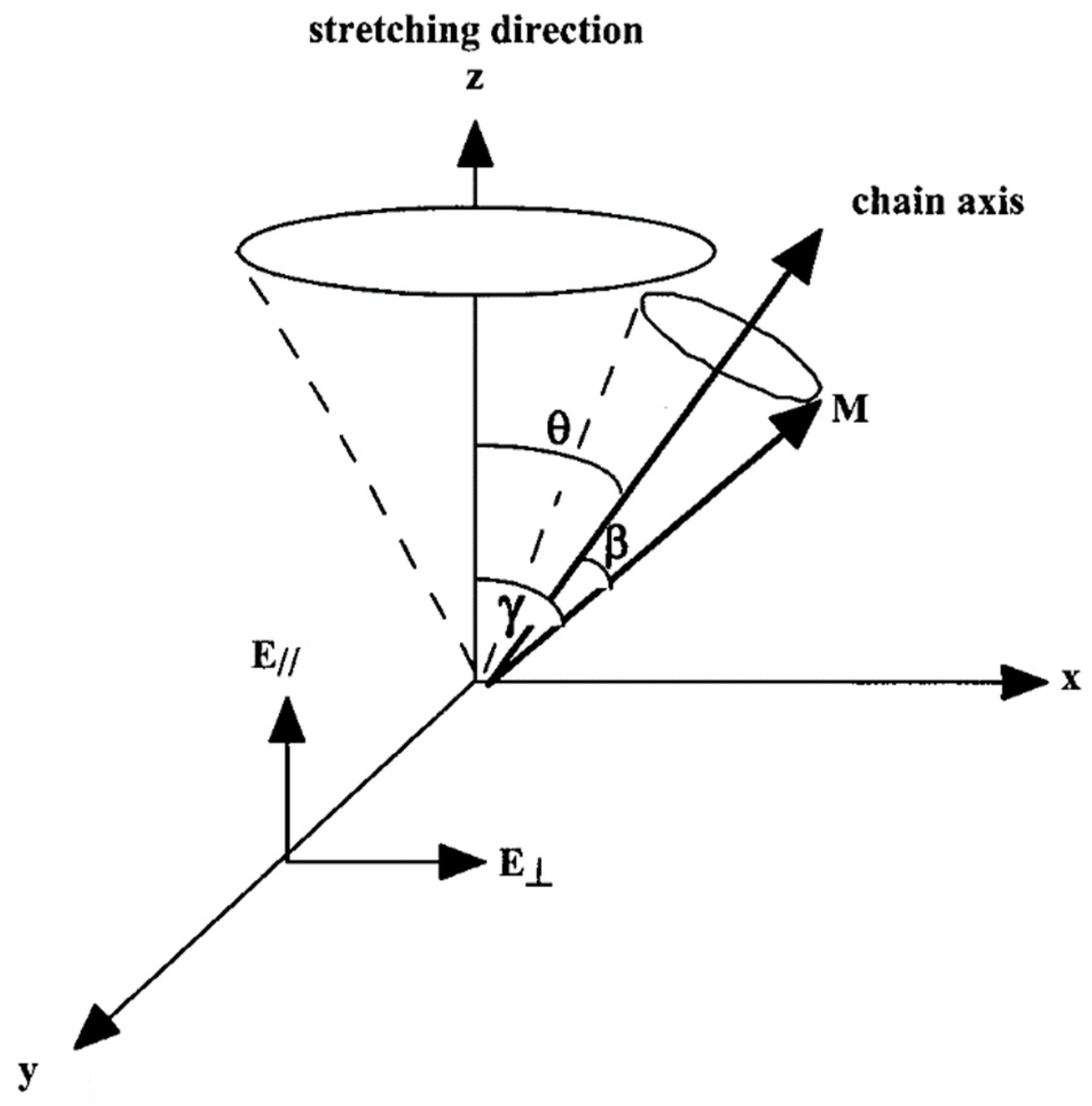
2. Definition of Orientation and Orientation Functions
3. Orientation of Homopolymers, Semi-Crystalline Polymers, Copolymers, Polymer Blends
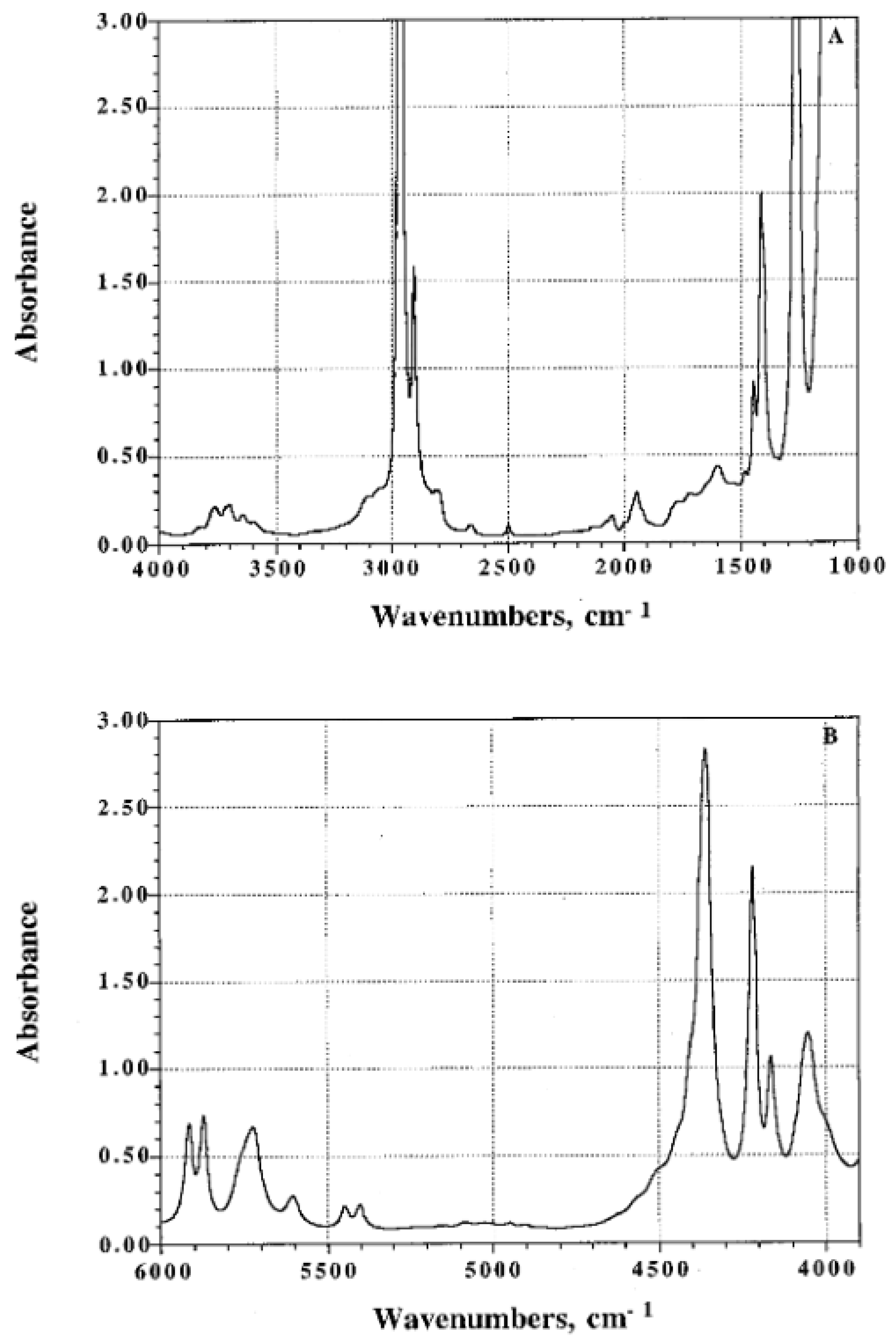
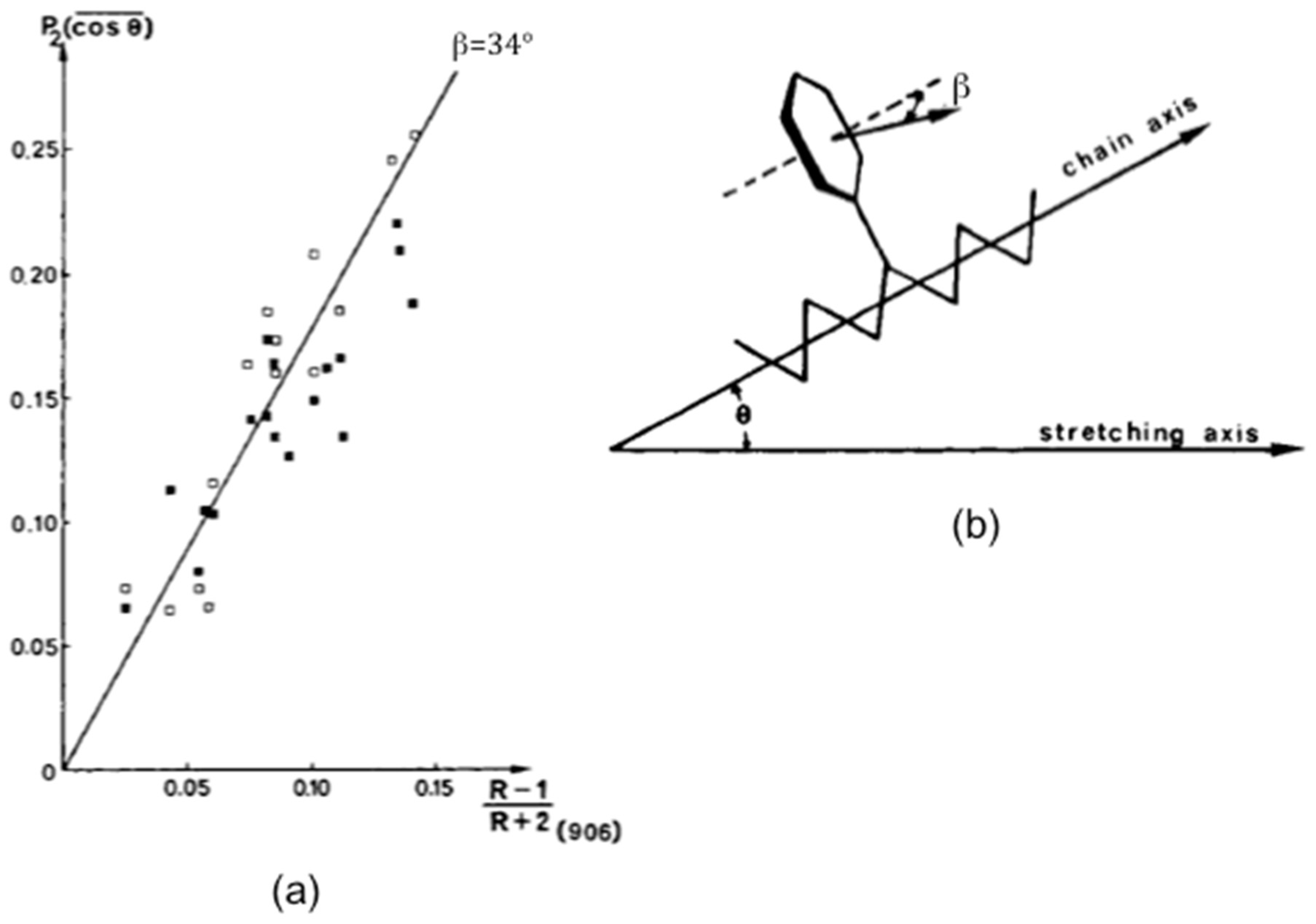
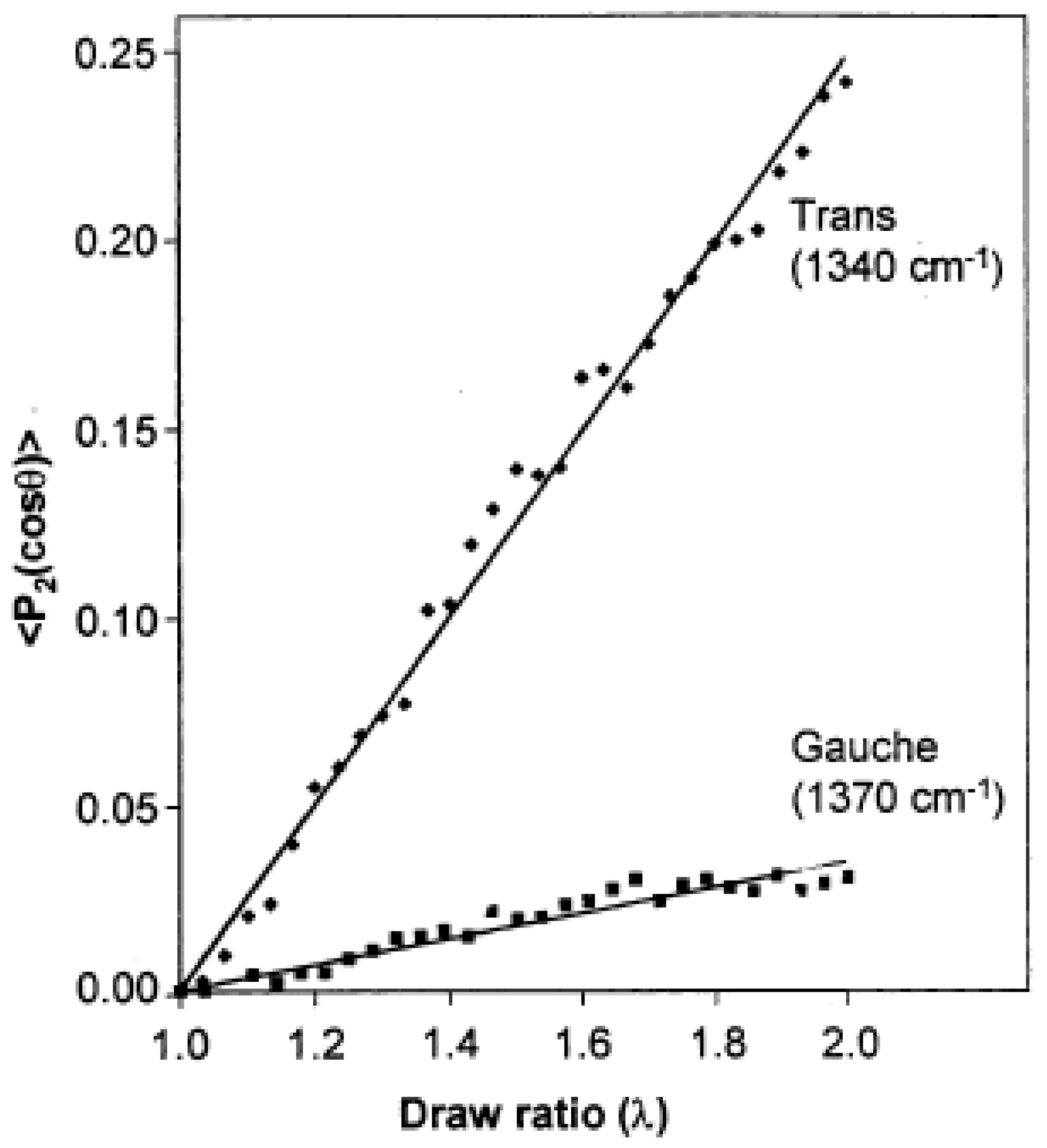
3.1. Semi-Crystalline Polymers
3.2. Copolymers
3.3. Polymer Blends
4. Orientation of Polymer Composites
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jasse, B.; Koenig, J.L. Orientation measurements in polymers using vibrational spectroscopy. J. Macromol. Sci. Part C 1979, 17, 61–135. [Google Scholar] [CrossRef]
- Lefebvre, D.; Jasse, B.; Monnerie, L. Orientation and relaxation in uniaxially stretched atactic polystyrene. Polymer 1983, 24, 1240–1244. [Google Scholar] [CrossRef]
- Kissin, Y.V. Orientation of isotactic polypropylene in crystalline and amorphous phases: IR methods. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 2085–2096. [Google Scholar] [CrossRef]
- Unwin, A.P.; Bower, D.I.; Ward, I.M. The determination of molecular orientation in oriented polypropylene by wide angle X-ray diffraction polarized fluorescence and refractive index measurements. Polymer 1985, 26, 1605–1610. [Google Scholar] [CrossRef]
- Jasse, B.; Tassin, J.F.; Monnerie, L. Orientation and chain relaxation of amorphous polymers and compatible polymer blends. Prog. Colloid Polym. Sci. 1993, 92, 8–22. [Google Scholar]
- Oultache, A.K.; Kong, X.; Pellerin, C.; Brisson, J.; Pézolet, M.; Prud’homme, R.E. Orientation and relaxation of orientation of amorphous poly(ethylene terephthalate). Polymer 2001, 42, 9051–9058. [Google Scholar] [CrossRef]
- Pellerin, C.; Prud’homme, R.E.; Pézolet, M. Effect of thermal history on the molecular orientation in polystyrene/poly(vinyl methyl ether) blends. Polymer 2003, 44, 3291–3297. [Google Scholar] [CrossRef]
- Song, Y.; Nitta, K.-H.; Nemoto, N. Molecular orientations and true stress-strain relationship in isotactic polypropylene film. Macromolecules 2003, 36, 8066–8073. [Google Scholar] [CrossRef]
- Ward, I.M. Determination of molecular orientation by spectroscopic techniques. Adv. Polym. Sci. 1985, 66, 81–115. [Google Scholar]
- Spiess, H.W. Deuteron NMR—A new tool for studying chain mobility and orientation in polymers. Adv. Polym. Sci. 1985, 66, 23–58. [Google Scholar]
- Kimura, H.; Dohi, H.; Kotani, M.; Matsunaga, T.; Yamauchi, K.; Kaji, H.; Kurosu, H.; Asakura, T. Molecular dynamics and orientation of stretched rubber by solid-state 13C NMR. Polym. J. 2010, 42, 25–30. [Google Scholar] [CrossRef]
- Jarry, J.P.; Monnerie, L. Orientation and molecular dynamics in uniaxial polymers. I. Theory of fluorescence polarization. J. Polym. Sci. Polym. Phys. Ed. 1978, 16, 443–455. [Google Scholar] [CrossRef]
- Nobbs, J.H.; Bower, D.I.; Ward, I.M. Comparison of polarized fluorescence with polarized Raman and infrared dichroism measures of orientation in uniaxially drawn poly (ethylene terephthalate). J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 259–272. [Google Scholar] [CrossRef]
- Lafrance, C.-P.; Chabot, P.; Pigeon, M.; Prud’homme, R.E.; Pézolet, M. Study of the distribution of the molecular orientation in thick polyethylene samples by X-ray diffraction, infra-red dichroism and Raman spectroscopy. Polymer 1993, 34, 5029–5037. [Google Scholar] [CrossRef]
- Tanaka, M.; Young, R.J. Review polarized Raman spectroscopy for the study of molecular orientation distributions in polymers. J. Mater. Sci. 2006, 41, 963–991. [Google Scholar] [CrossRef]
- Bokobza, L. Molecular Orientation Studies of Polymers by Infrared Spectroscopy in Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Buffeteau, T.; Pézolet, M. Linear dichroism in infrared spectroscopy. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Buffeteau, T.; Desbat, B.; Turlet, J.M. Polarization modulation FT-IR spectroscopy of surfaces and ultra-thin films: Experimental procedure and quantitative analysis. Appl. Spectrosc. 1991, 45, 380–389. [Google Scholar] [CrossRef]
- Looijmans, S.F.S.P.; Carmeli, E.; Puskar, L.; Ellis, G.; Cavallo, D.; Anderson, P.D.; van Breemen, L.C.A. Polarisation modulated infrared spectroscopy: A pragmatic tool for polymer science and engineering. Polym. Cryst. 2020, 3, e10138. [Google Scholar] [CrossRef]
- Buffeteau, T.; Desbat, B.; Besbes, S.; Nafati, M.; Bokobza, L. Molecular orientation studies in polymer films by polarization modulation FTi.r. spectroscopy. Polymer 1994, 35, 2538–2541. [Google Scholar] [CrossRef]
- Miller, C.E. Near-infrared spectroscopy of synthetic polymers. Appl. Spectrosc. Rev. 1991, 26, 277–339. [Google Scholar] [CrossRef]
- Bokobza, L. Near infrared spectroscopy. J. Near Infrared Spectrosc. 1998, 6, 3–17. [Google Scholar] [CrossRef]
- Workman, J.J., Jr. Near-infrared spectroscopy of polymers and rubbers. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Heigl, N.; Petter, C.H.; Rainer, M.; Najam-ul-Haq, M.; Vallant, R.M.; Bakry, R.; Bonn, G.K.; Huck, C.W. Near infrared spectroscopy for polymer research, quality control and reaction monitoring. J. Near Infrared Spectrosc. 2007, 15, 269–282. [Google Scholar] [CrossRef]
- Bokobza, L.; Buffeteau, T.; Desbat, B. Mid- and near-infrared investigation of molecular orientation investigation of molecular orientation in elastomeric networks. Appl. Spectrosc. 2000, 54, 360–365. [Google Scholar] [CrossRef]
- Mizushima, M.; Kawamura, T.; Takahashi, K.; Nitta, K.-H. Characterization of molecular orientation under tensile deformation by near infrared spectroscopy. E-Polymers 2012, 12, 68. [Google Scholar] [CrossRef]
- Jasse, B.; Koenig, J.L. Fourier transform infrared study of uniaxially oriented atactic polystyrene. J. Polym. Sci. Polym. Phys. Ed. 1979, 17, 799–810. [Google Scholar] [CrossRef]
- Rault, J.; Marchal, J.; Judeinstein, P.; Albouy, P.A. Stress-induced crystallization and reinforcement in filled natural rubbers: 2H NMR study. Macromolecules 2006, 39, 8356–8368. [Google Scholar] [CrossRef]
- Huneau, B. Strain-induced crystallization of natural rubber: A review of X-ray diffraction investigations. Rubber Chem. Technol. 2011, 84, 425–452. [Google Scholar] [CrossRef]
- Sotta, P.; Albouy, P.-A. Stain-induced crystallization in natural rubber: Flory’s theory revisited. Macromolecules 2020, 53, 3097–3109. [Google Scholar] [CrossRef]
- Loos, K.; Aydogdu, A.B.; Lion, A.; Johlitz, M.; Calipel, J. Strain-induced crystallization in natural rubber: A thermodynamic consistent model of the material behaviour using a serial connection of phases. Contin. Mech. Thermodyn. 2021, 33, 1107–1140. [Google Scholar] [CrossRef]
- Siesler, H.W. Rheo-optical Fourier-transforminfrared spectroscopy: Vibrational spectra and mechanical properties of polymers. Adv. Polym. Sci. 1984, 65, 1–77. [Google Scholar]
- Amram, B.; Bokobza, L.; Queslel, J.P.; Monnerie, L. Fourier-transform infra-red study of molecular orientation in synthetic high cis-1,4-polyisoprene and in natural rubber. Polymer 1986, 27, 877–882. [Google Scholar] [CrossRef]
- Cole, K.C.; Guèvremont, J.; Ajji, A.; Dumoulin, M.M. Characterization of surface orientation in poly(ethylene terephthalate) by front-surface reflection infrared spectroscopy. Appl. Spectrosc. 1994, 48, 1513–1521. [Google Scholar] [CrossRef]
- Cole, K.C.; Guèvremont, J.; Ajji, A.; Dumoulin, M.M. Orientation and conformation in PET: New information from specular reflection FT-IR. In Progress in Fourier Transform SpectroscopyMikrochimica; Springer: Berlin/Heidelberg, Germany, 1997; pp. 403–405. [Google Scholar]
- Duchesne, C.; Kong, X.; Brisson, J.; Pézolet, M.; Prud’homme, R.E. Molecular orientation and relaxation of poly(ethylene terephthalate) by polarization modulation infrared spectroscopy. Macromolecules 2002, 35, 8768–8773. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. The thermal analysis of poly(ethylene terephthalate) by FTIR spectroscopy. Thermichimica Acta 2013, 552, 123–130. [Google Scholar] [CrossRef]
- Tassin, J.F.; Monnerie, L.; Fetters, L.J. Infra-red dichroism investigation of molecular viscoelasticity using isotopically labelled block copolymers. Polym. Bull. 1986, 15, 165–171. [Google Scholar] [CrossRef]
- Sakurai, S.; Sakamoto, J.; Shibayama, M.; Nomura, S. Effects of microdomain structures on the molecular orientation of poly(styrene-block-butadiene-block-styrene) triblock copolymer. Macromolecules 1993, 26, 3351–3356. [Google Scholar] [CrossRef]
- Erman, B.; Monnerie, L. Theory of segmental orientation in amorphous polymer networks. Macromolecules 1985, 18, 1985–1991. [Google Scholar] [CrossRef]
- Erman, B.; Haliloglu, T.; Bahar, I.; Mark, J.E. Segmental orientation in uniaxially deformed networks: A higher order aproximation for finite chains and large deformations. Macromolecules 1991, 24, 901–907. [Google Scholar] [CrossRef]
- Besbes, S.; Cermelli, I.; Bokobza, L.; Monnerie, L.; Bahar, I.; Erman, B.; Herz, J. Segmental orientation in model networks of poly(dimethylsiloxane): Fourier transform infrared dichroism measurements and theoretical interpretation. Macromolecules 1992, 25, 1949–1954. [Google Scholar] [CrossRef]
- Bokobza, L. Styrene-butadiene copolymers: Mechanical, swelling and orientational properties. Polym. Int. 2000, 49, 743–750. [Google Scholar] [CrossRef]
- Bokobza, L.; Lapra, A. Styrene-butadiene copolymers with different butadiene microstructure: Mechanical, swelling and orientational properties. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2449–2456. [Google Scholar] [CrossRef]
- Abtal, E.; Prud’homme, R.E. Orientation of miscible and immiscible polymer blends. Polym. Eng. Sci. 1992, 32, 1857–1862. [Google Scholar] [CrossRef]
- Pézolet, M.; Pellerin, C.; Pru’homme, R.E.; Buffeteau, T. Study of polymer orientation and relaxation by polarization modulation and 2D-FTIR spectroscopy. Vib. Spectrosc. 1998, 18, 103–110. [Google Scholar] [CrossRef]
- Kalkar, A.K.; Siesler, H.W.; Pfeifer, F.; Wadekar, S.A. Molecular orientation and relaxation in poly(butylene terephthalate)/polycarbonate blends. Polymer 2003, 44, 7251–7264. [Google Scholar] [CrossRef]
- Bokobza, L. The reinforcement of elastomeric by fillers. Macromol. Mater. Eng. 2004, 289, 607–621. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, V.; Musto, S.; Cioppa, S.; Peli, G.; Mauro, M.; Gaetano, G.; Agnelli, S.; Theonis, R.; Kumar, V. Recent advancements in rubber nanocomposites. Rubber Chem. Technol. 2014, 87, 417–442. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Ponnamma, D.; Thomas, S.; Grohens, Y. Evolution from graphite to graphene elastomer composites. Prog. Polym. Sci. 2014, 39, 749–780. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, H.; Zhang, Z.; Chen, Y. Polymer-filler interaction of fumed silica filled polydimethylsiloxane investigated by bound rubber. Compos. Sci. Technol. 2013, 86, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Zong, G.; He, L.; Zhang, Y.; Liu, C.; Wang, L. Effects of fumed and mesoporous silica nanoparticules on the properties of Sylgard 184 polydimethylsiloxane. Micromachines 2015, 6, 855–864. [Google Scholar] [CrossRef]
- Eslami, H.; Rahimi, M.; Müller-Plathe, F. Molecular dynamics simulation of a silica nanoparticle in oligomeric poly(methyl methacrylate): A model system for studying the interphase thickness in a polymer-nanocomposite via different properties. Macromolecules 2013, 46, 8680–8692. [Google Scholar] [CrossRef]
- Mortazavian, H.; Fennell, C.J.; Blum, F.D. Surface bonding is stronger for poly(methyl methacrylate) than for poly(vinyl acetate). Macromolecules 2016, 49, 4211–4219. [Google Scholar] [CrossRef]
- Mortazavian, H.; Fennell, C.J.; Blum, F.D. Structure of the interfacial region in adsorbed poly(vinyl acetate) on silica. Macromolecules 2016, 49, 298–307. [Google Scholar] [CrossRef]
- Higuchi, C.; Tanaka, H.; Yoshizawa, K. Molecular understanding of the adhesive interactions between silica surface and epoxy resin: Effects of interfacial water. J. Comput. Chem. 2019, 40, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Clément, F. Etude des Mécanismes de Renforcement Dans les Réseaux de Polydiméthylsiloxane Chargés Silice. Ph.D. Thesis, University of Paris VI, Paris, France, 1999. [Google Scholar]
- Bokobza, L. Filled elastomers: A new approach based on measurement of chain orientation. Polymer 2001, 42, 5415–5423. [Google Scholar] [CrossRef]
- Bokobza, L.; Ladouce, L.; Bomal, Y.; Amram, B. Infrared dichroism and birefringence studies of silica-filled styrene-butadiene rubbers. J. Appl. Polym. Sci. 2001, 82, 1006–1012. [Google Scholar] [CrossRef]
- Xu, K.; Gao, C.; Chen, G.; Qiu, D. Direct evidence of molecular orientation on thermoelectric performance of organic polymer materials by infrared dichroism. Org. Electron. 2016, 31, 41–47. [Google Scholar] [CrossRef]
- Bokobza, L.; Chauvin, J.P. Reinforcement of natural rubber: Use of in situ generated silicas and nanofibres of sepiolite. Polymer 2005, 46, 4144–4151. [Google Scholar] [CrossRef]
- Bokobza, L. Infrared analysis of elastomeric composites under uniaxial extension. Macromol. Symp. 2005, 220, 45–59. [Google Scholar] [CrossRef]
- Mooney, M. A theory of large elastic deformation. J. Appl. Phys. 1940, 11, 582–592. [Google Scholar] [CrossRef]
- Rivlin, R.S.; Saunders, D.W. Large elastic deformations of isotropic materials. VII. Experiments on the deformation of rubber. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 1951, 243, 251–288. [Google Scholar]
- Satyanarayana, M.S.; Sreenath, P.R.; Bhowmick, A.K.; Kumar, K.D. Selective orientation of needlelike sepiolite nanoclay in polymer blend for controlled properties. ACS Omega 2018, 3, 11691–11702. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Maiti, M.; Bhowmick, A.K. Influence of different nanofillers and their dispersion methods on the properties of natural rubber nanocomposites. Rubber Chem. Technol. 2008, 81, 782–808. [Google Scholar] [CrossRef]
- Di Credico, B.; Tagliaro, I.; Cobani, E.; Conzatti, L.; D’Arienzo, M.; Giannini, L.; Mascotto, S.; Scotti, R.; Stagnaro, P.; Tadiello, L. A green approach for preparing high-loaded sepiolite/polymer biocomposites. Nanomaterials 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Cobani, E.; Tagliaro, I.; Geppi, M.; Giannini, L.; Leclère, P.; Martini, F.; Nguyen, T.C.; Lazzaroni, R.; Scotti, R.; Tadiello, L.; et al. Hybrid interface in sepiolite rubber nanocomposites: Role of self-assembled nanostructure in controlling dissipative phenomena. Nanomaterials 2019, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Hayasaki, T.; Almarasy, A.A.; Yabu, H.; Tokita, M.; Fujimori, A. Influence of uniaxial orientation of fluorinated polymer/phosphonate-modified needle-like nanofiller composite by drawing. Polym. Compos. 2020, 41, 3062–3073. [Google Scholar] [CrossRef]
- Bokobza, L.; Garnaud, G.; Beaunier, P.; Bruneel, J.-L. Vibrational and electrical investigations of a uniaxially/carbon nanotube composite. Vib. Spectrosc. 2013, 67, 6–13. [Google Scholar] [CrossRef]
- Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C J. Carbon Res. 2019, 5, 84. [Google Scholar] [CrossRef]
- Stevenson, W.T.K.; Garton, A. Infrared spectroscopy of carbon-filled polymers. J. Mater. Sci. Lett. 1987, 6, 580–582. [Google Scholar] [CrossRef]
- Delor, F.; Barrois-Oudin, N.; Duteurtre, X.; Cardinet, C.; Lemaire, J.; Lacoste, J. Oxidation of rubbers analysed by HATR/IR spectroscopy. Polym. Degrad. Stab. 1998, 62, 395–401. [Google Scholar] [CrossRef]
- Do, T.-T.; Celina, M.; Fredericks, P.M. Attenuated total reflectance infrared microspectroscopy of aged carbon-filled rubbers. Polym. Degrad. Stab. 2002, 77, 417–422. [Google Scholar] [CrossRef][Green Version]
- Matsuda, S.-I.; Ando, S. Molecular orientation of rigid-rod polyimide films characterized by polarized attenuated total reflection/Fourier transform infrared spectroscopy. J. Polym. Scence Part B Polym. Phys. 2003, 41, 418–428. [Google Scholar] [CrossRef]
- Everall, N.J. Measurement of orientation and crystallinity in uniaxially drawn poly(ethylene terephthalate using polarized confocal Raman spectroscopy. Appl. Spectrosc. 1998, 52, 1498–1504. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Wilson, N.R.; Kinloch, I.A.; Vallés, C.; Li, Z. Effect of orientation of graphene-based nanoplatelets upon the Young’s modulus of nanocomposites. Compos. Sci. Technol. 2016, 123, 125–133. [Google Scholar] [CrossRef]
- Alexiou, V.F.; Mathioudakis, G.N.; Andrikopoulos, K.S.; Beobide, A.S.; Voyiatzis, G.A. Poly(ethylene terephthalate) carbon-based nanocomposites: A crystallization and molecular orientation study. Polymers 2020, 12, 2626. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, Z.; Wang, L.; Liu, J.; Wu, Y.; Zhang, L. Dispersion and shear-induced orientation of anisotropic nanoparticle filled polymer nanocomposites: Insights from molecular dynamics simulation. Nanotechnology 2016, 27, 265704. [Google Scholar] [CrossRef]
- Azimi, M.; Mirjavadi, S.S.; Hamouda, A.M.S.; Makki, H. Heterogeneities in polymer structural and dynamic properties in graphene and graphene oxide nanocomposites: Molecular dynamic simulations. Macromol. Theory Simul. 2017, 26, 1600086. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokobza, L. Infrared Linear Dichroism for the Analysis of Molecular Orientation in Polymers and in Polymer Composites. Polymers 2022, 14, 1257. https://doi.org/10.3390/polym14061257
Bokobza L. Infrared Linear Dichroism for the Analysis of Molecular Orientation in Polymers and in Polymer Composites. Polymers. 2022; 14(6):1257. https://doi.org/10.3390/polym14061257
Chicago/Turabian StyleBokobza, Liliane. 2022. "Infrared Linear Dichroism for the Analysis of Molecular Orientation in Polymers and in Polymer Composites" Polymers 14, no. 6: 1257. https://doi.org/10.3390/polym14061257
APA StyleBokobza, L. (2022). Infrared Linear Dichroism for the Analysis of Molecular Orientation in Polymers and in Polymer Composites. Polymers, 14(6), 1257. https://doi.org/10.3390/polym14061257





