Self-Photopolymerizable Hydrogel–Ceramic Composites with Scavenger Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymeric–Ceramic Composition Formulation and Fabrication of Composites
2.3. Physicochemical Characterization of Ceramic Powders and Composites
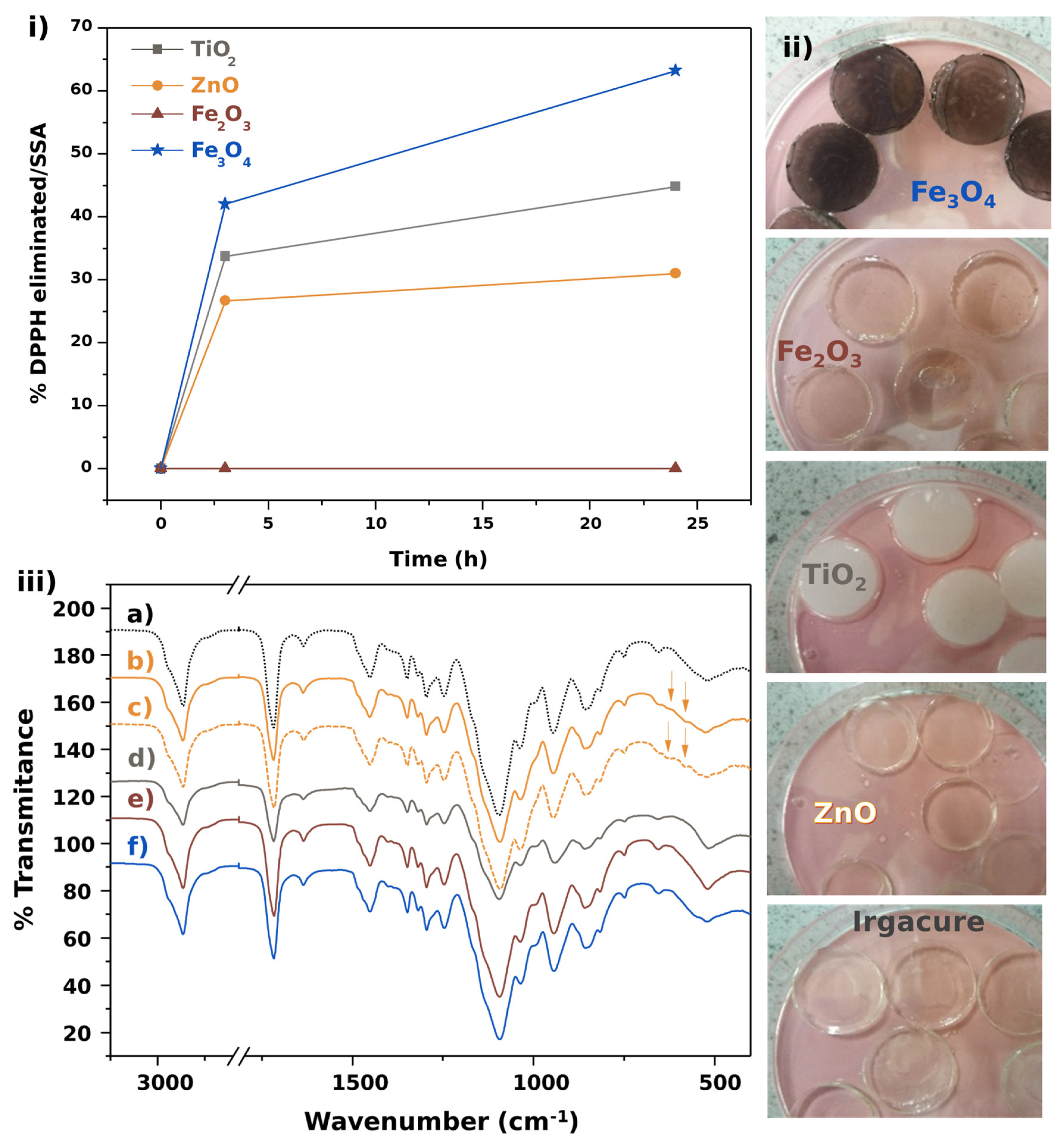
2.4. Scavenging Activity Assays
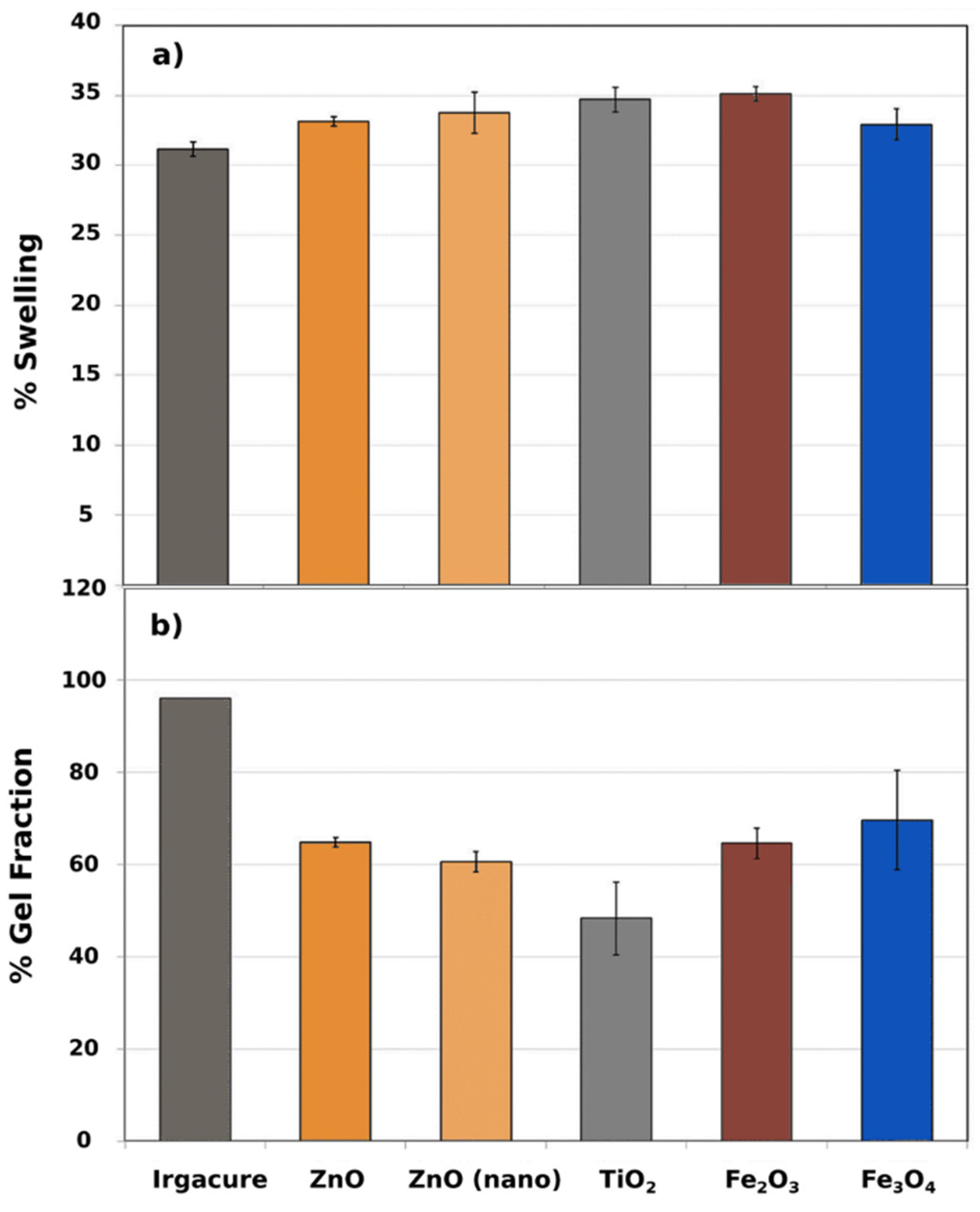
2.5. Swelling and Gel Fraction
3. Results and Discussion
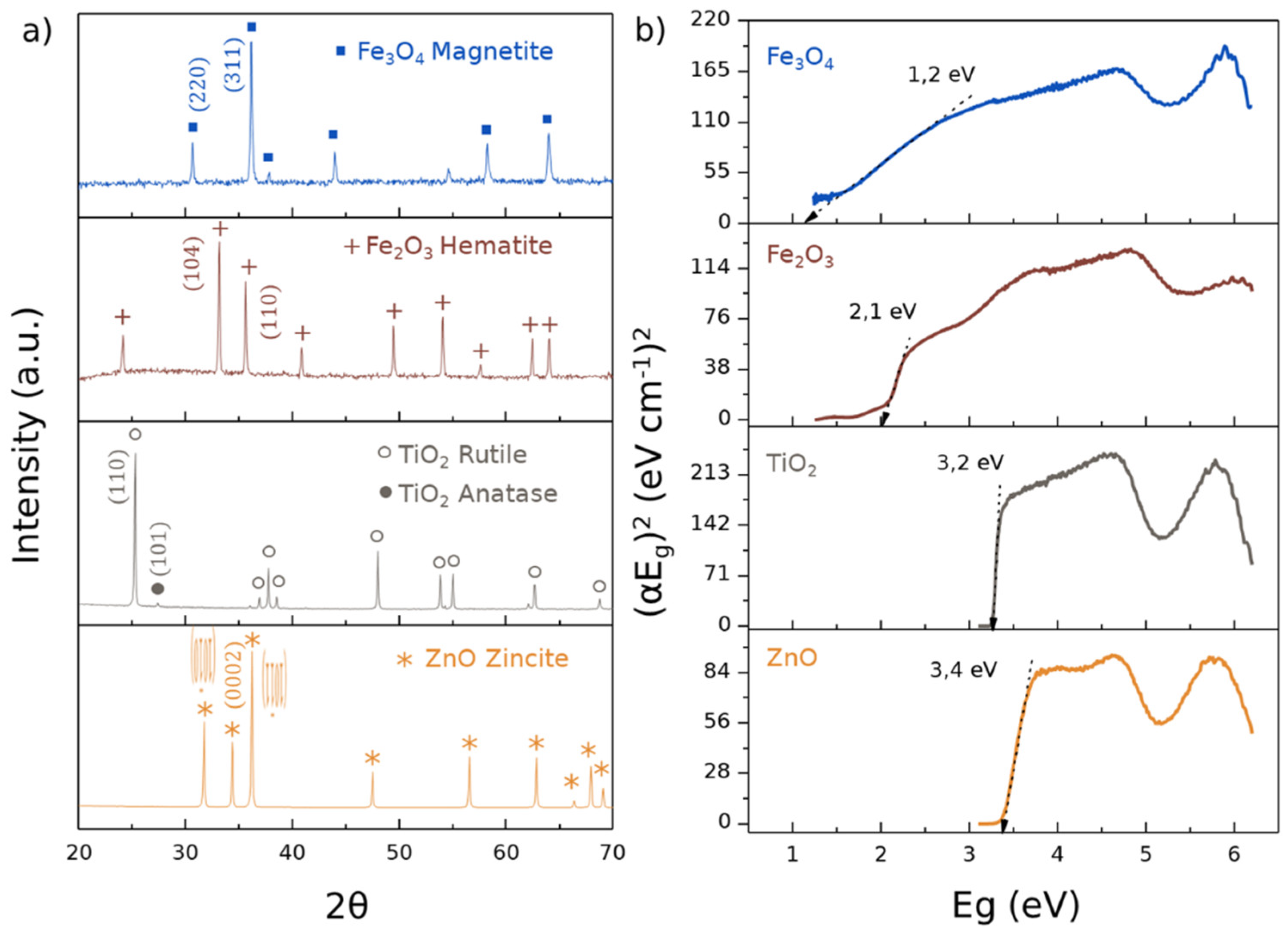
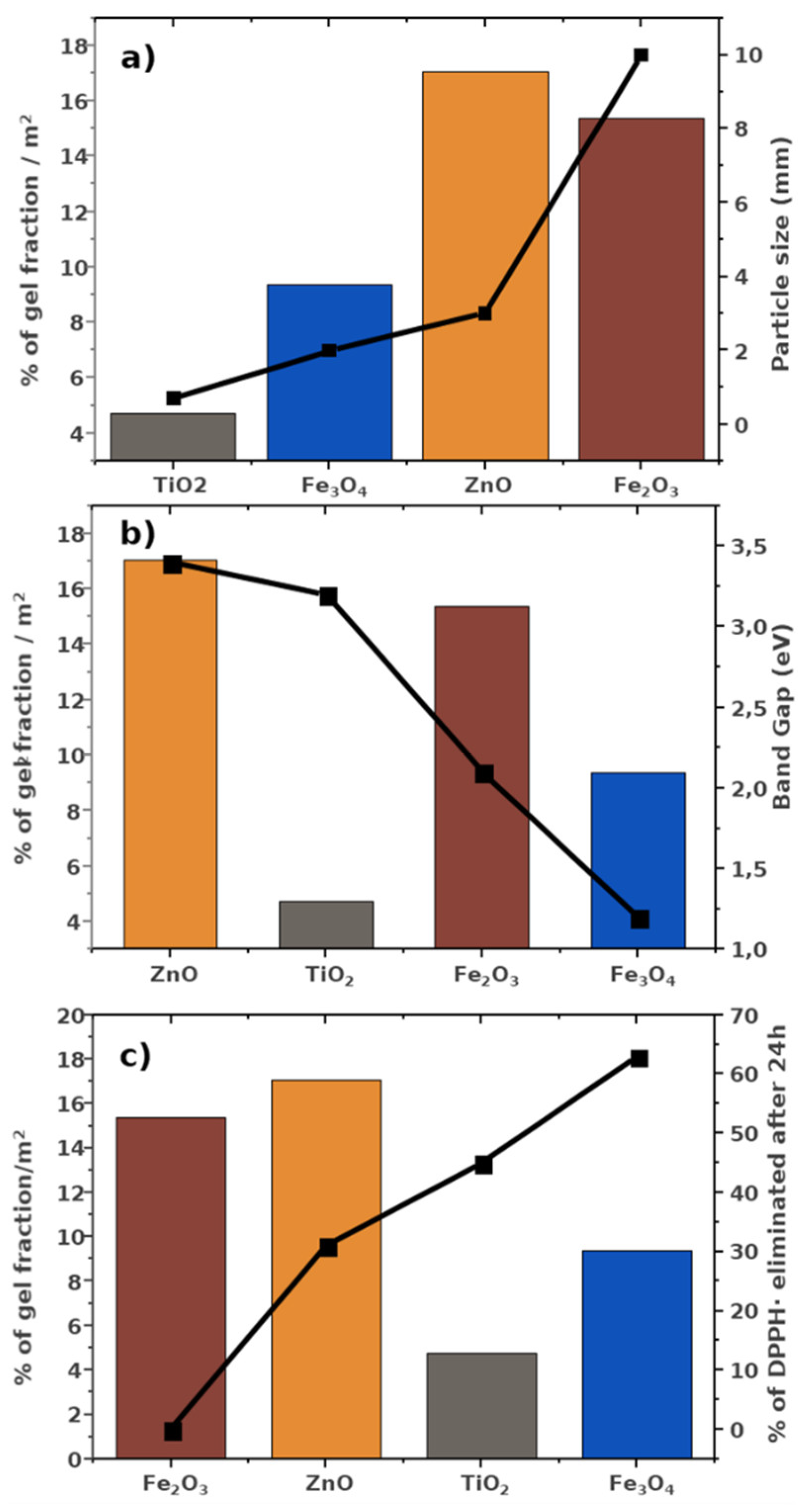
3.1. Physicochemical Characterization of Ceramic Powders
3.2. Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Band Assignment | Wavenumber (cm−1) |
|---|---|
| OH stretching | 3569 |
| CH stretching | 2867 |
| C=C bending | 1721 and 1638 |
| CCH2 bending | 1452 |
| CH vibration of CH3 groups | 1349 |
| OR stretching | 1093 |
References
- De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Suzuki, R.; Muyco, J.; McKittrick, J.; Frangos, J.A. Reactive oxygen species inhibited by titanium oxide coatings. J. Biomed. Mater. Res. 2003, 66, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, H.; Contreras, R.; Gaskill, D.F.; Bjursten, L.M.; Frangos, J.A. Anti-inflammatory properties of micropatterned titanium coatings. J. Biomed. Mater. Res. Part A 2006, 77, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Rajh, T.; Dimitrijevic, N.M.; Bissonnette, M.; Koritarov, T.; Konda, V. Titanium Dioxide in the Service of the Biomedical Revolution. Chem. Rev. 2014, 114, 10177–10216. [Google Scholar] [CrossRef]
- Canillas, M.; Chinarro, E.; Freitas, M.; Pêgo, A.P.; Moreno, B. Titanium dioxide catalytic activity contributes to the process of free radical scavenging. J. Catal. 2020, 381, 186–192. [Google Scholar] [CrossRef]
- Canillas, M.; Chinarro, E.; Pêgo, A.P.; Moreno, B. Scavenging activity of Magnéli phases as a function of Ti 4+ /Ti 3+ ratios. Chem. Commun. 2017, 53, 10580–10583. [Google Scholar] [CrossRef] [Green Version]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability In vivo in Mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- Damm, C.; Völtzke, D.; Abicht, H.-P.; Israel, G. Influence of the properties of TiO2 particles on a photocatalytic acrylate polymerisation. J. Photochem. Photobiol. A Chem. 2005, 174, 171–179. [Google Scholar] [CrossRef]
- Stroyuk, A.L.; Sobran, I.V.; Kuchmiy, S.Y. Photoinitiation of acrylamide polymerization by Fe2O3 nanoparticles. J. Photochem. Photobiol. A Chem. 2007, 192, 98–104. [Google Scholar] [CrossRef]
- Ulku, I.; Morlet-Savary, F.; Lalevée, J.; Yagci Acar, H. Homogenous photopolymerization of acrylic monomers initiated with ZnO-methacrylate in non-aqueous medium and production of luminescent nanocomposites. Polym. Chem. 2018, 9, 828–833. [Google Scholar] [CrossRef]
- Moskvin, M.; Horák, D. Carbohydrate-Modified Magnetic Nanoparticles for Radical Scavenging. Physiol. Res. 2016, 65, S243–S251. [Google Scholar] [CrossRef] [PubMed]
- Marin-Flores, C.A.; Rodríguez-Nava, O.; García-Hernández, M.; Ruiz-Guerrero, R.; Juárez-López, F.; Morales-Ramírez, A.d.J. Free-radical scavenging activity properties of ZnO sub-micron particles: Size effect and kinetics. J. Mater. Res. Technol. 2021, 13, 1665–1675. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Yar, Y.; Yagci Acar, H.; Yagci, Y. Magnetic iron oxide nanoparticles as long wavelength photoinitiators for free radical polymerization. Polym. Chem. 2015, 6, 1918–1922. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes eliminate intracellular reactive oxygen species in Magnetospirillum gryphiswaldense MSR-1. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef]
- Schmitt, M. ZnO Nanoparticle Induced Photo-Kolbe Reaction, Fragment Stabilization and Effect on Photopolymerization Monitored by Raman-UV-Vis Measurements. Macromol. Chem. Phys. 2012, 213, 1953–1962. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Ghosh, H.N. Effect of Particle Size on the Reactivity of Quantum Size ZnO Nanoparticles and Charge-Transfer Dynamics with Adsorbed Catechols. Langmuir 2003, 19, 3006–3012. [Google Scholar] [CrossRef]
- Goourey, G.G.; de Sainte Claire, P.; Balan, L.; Israëli, Y. Acrylate photopolymer doped with ZnO nanoparticles: An interesting candidate for photo-patterning applications. J. Mater. Chem. C 2013, 1, 3430. [Google Scholar] [CrossRef]
- Brezová, V.; Dvoranová, D.; Staško, A. Characterization of titanium dioxide photoactivity following the formation of radicals by EPR spectroscopy. Res. Chem. Intermed. 2007, 33, 251–268. [Google Scholar] [CrossRef]
- Yulizar, Y.; Kusrini, E.; Apriandanu, D.O.B.; Nurdini, N. Datura metel L. Leaves extract mediated CeO2 nanoparticles: Synthesis, characterizations, and degradation activity of DPPH radical. Surf. Interfaces 2020, 19, 100437. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Rose, N.R.; Mackay, I.R. The Autoimmune Diseases; Academic Press: Cambridge, MA, USA, 2006; ISBN 9780125959612. [Google Scholar]
- Rawla, P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia/Rheumatology 2019, 57, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjigogos, K. The role of free radicals in the pathogenesis of rheumatoid arthritis. Panminerva Med. 2003, 45, 7–13. [Google Scholar] [PubMed]
- Laires, P.A.; Laíns, J.; Miranda, L.C.; Cernadas, R.; Rajagopalan, S.; Taylor, S.D.; Silva, J.C. Inadequate pain relief among patients with primary knee osteoarthritis. Rev. Bras. Reumatol. 2017, 57, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Killion, J.A.; Geever, L.M.; Devine, D.M.; Farrell, H.; Higginbotham, C.L. Compressive Strength and Bioactivity Properties of Photopolymerizable Hybrid Composite Hydrogels for Bone Tissue Engineering. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 641–650. [Google Scholar] [CrossRef]
- Van Leeuwen, R.A.; Hung, C.-J.; Kammler, D.R.; Switzer, J.A. Optical and Electronic Transport Properties of Electrodeposited Thallium(III) Oxide Films. J. Phys. Chem. 1995, 99, 15247–15252. [Google Scholar] [CrossRef]
- Suzuki, R.; Frangos, J.A. Inhibition of Inflammatory Species by Titanium Surfaces. Clin. Orthop. Relat. Res. 2000, 372, 280–289. [Google Scholar] [CrossRef]
- de Lima, G.G.; Campos, L.; Junqueira, A.; Devine, D.M.; Nugent, M.J.D. A novel pH-sensitive ceramic-hydrogel for biomedical applications. Polym. Adv. Technol. 2015, 26, 1439–1446. [Google Scholar] [CrossRef]
- Diebold, U. Structure and properties of TiO2 surfaces: A brief review. Appl. Phys. A Mater. Sci. Process. 2003, 76, 681–687. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.M.; Cheng, H.-M. On the True Photoreactivity Order of {001}, {010}, and {101} Facets of Anatase TiO2 Crystals. Angew. Chemie Int. Ed. 2011, 50, 2133–2137. [Google Scholar] [CrossRef]
- Futera, Z.; English, N.J. Exploring Rutile (110) and Anatase (101) TiO 2 Water Interfaces by Reactive Force-Field Simulations. J. Phys. Chem. C 2017, 121, 6701–6711. [Google Scholar] [CrossRef]
- Liu, X.; Ye, L.; Liu, S.; Li, Y.; Ji, X. Photocatalytic Reduction of CO2 by ZnO Micro/nanomaterials with Different Morphologies and Ratios of {0001} Facets. Sci. Rep. 2016, 6, 38474. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, G.; Lau, T.-C. Effects of morphology and exposed facets of α-Fe2O3 nanocrystals on photocatalytic water oxidation. RSC Adv. 2015, 5, 52210–52216. [Google Scholar] [CrossRef]
- Gamba, O.; Hulva, J.; Pavelec, J.; Bliem, R.; Schmid, M.; Diebold, U.; Parkinson, G.S. The Role of Surface Defects in the Adsorption of Methanol on Fe3O4(001). Top. Catal. 2017, 60, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, G.G.; Elter, J.K.; Chee, B.S.; Magalhães, W.L.E.; Devine, D.M.; Nugent, M.J.D.; de Sá, M.J.C. A tough and novel dual-response PAA/P(NiPAAM-co-PEGDMA) IPN hydrogels with ceramics by photopolymerization for consolidation of bone fragments following fracture. Biomed. Mater. 2019, 14, 054101. [Google Scholar] [CrossRef]
| Materials | Particle Size (µm) | Specific Surface Area (m2/g) |
|---|---|---|
| ZnO | 3 | 3.8 |
| TiO2 | 0.7 | 10.2 |
| Fe2O3 | 10 | 4.2 |
| Fe3O4 | 2 | 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canillas, M.; de Lima, G.G.; de Sá, M.J.C.; Nugent, M.J.D.; Rodríguez, M.A.; Devine, D.M. Self-Photopolymerizable Hydrogel–Ceramic Composites with Scavenger Properties. Polymers 2022, 14, 1261. https://doi.org/10.3390/polym14061261
Canillas M, de Lima GG, de Sá MJC, Nugent MJD, Rodríguez MA, Devine DM. Self-Photopolymerizable Hydrogel–Ceramic Composites with Scavenger Properties. Polymers. 2022; 14(6):1261. https://doi.org/10.3390/polym14061261
Chicago/Turabian StyleCanillas, Maria, Gabriel Goetten de Lima, Marcelo J. C. de Sá, Michael J. D. Nugent, Miguel A. Rodríguez, and Declan M. Devine. 2022. "Self-Photopolymerizable Hydrogel–Ceramic Composites with Scavenger Properties" Polymers 14, no. 6: 1261. https://doi.org/10.3390/polym14061261
APA StyleCanillas, M., de Lima, G. G., de Sá, M. J. C., Nugent, M. J. D., Rodríguez, M. A., & Devine, D. M. (2022). Self-Photopolymerizable Hydrogel–Ceramic Composites with Scavenger Properties. Polymers, 14(6), 1261. https://doi.org/10.3390/polym14061261








