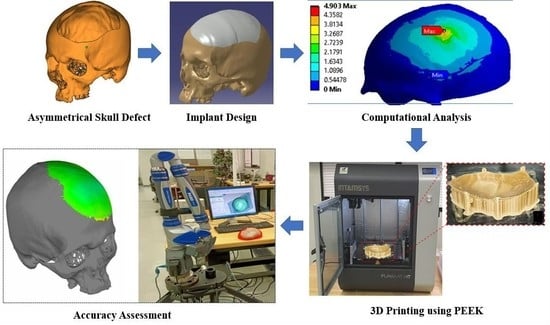
Adaptive Mechanism for Designing a Personalized Cranial Implant and Its 3D Printing Using PEEK
Abstract
:1. Introduction
- Mirrored reconstruction design technique: This is based on the symmetrical nature of bones, and is more suitable for the treatment of unilateral skull damage [4,5,6,7]. The unhealthy or tumorous region is removed and replaced by the healthy opposite side in this technique. The precise execution of the mirroring approach is not simple, as it necessitates many manual operations, including the determination of the symmetry plane, the segregation of the healthier portion relating to the lesion, and the tweaking of the generated patch on the deficient region of the skull [8]. One of the major drawbacks of mirror-image reconstruction technique is that it cannot be used in asymmetrical body regions. If there is tumor in the center of the skull, the mirror-image design technique cannot provide the healthy opposite side.
- Anatomical reconstruction design approach: This is a curve-based surface manipulation and modification method in which two end curves of the resected regions are chosen and a guiding curve is employed for anatomical bone regeneration [9]. The fundamental benefit of the anatomical reconstruction technique is that it could be employed in both symmetrical and asymmetrical regions; nevertheless, in highly contoured parts, it requires technical competence. According to Moiduddin et al. [10], in mandible reconstruction, the anatomical reconstruction technique is more efficacious than the mirroring technique, resulting in less variation from the reference bone.
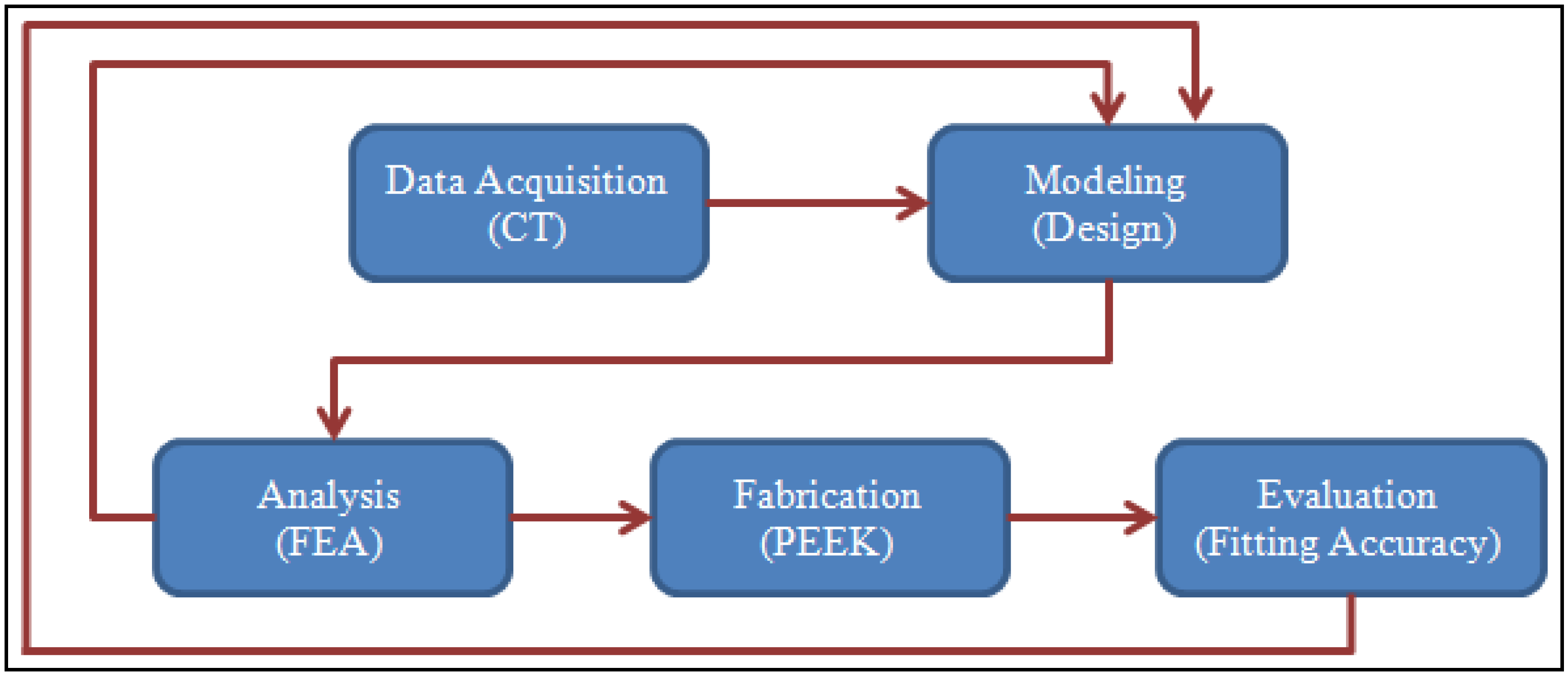
2. Proposed Methodology
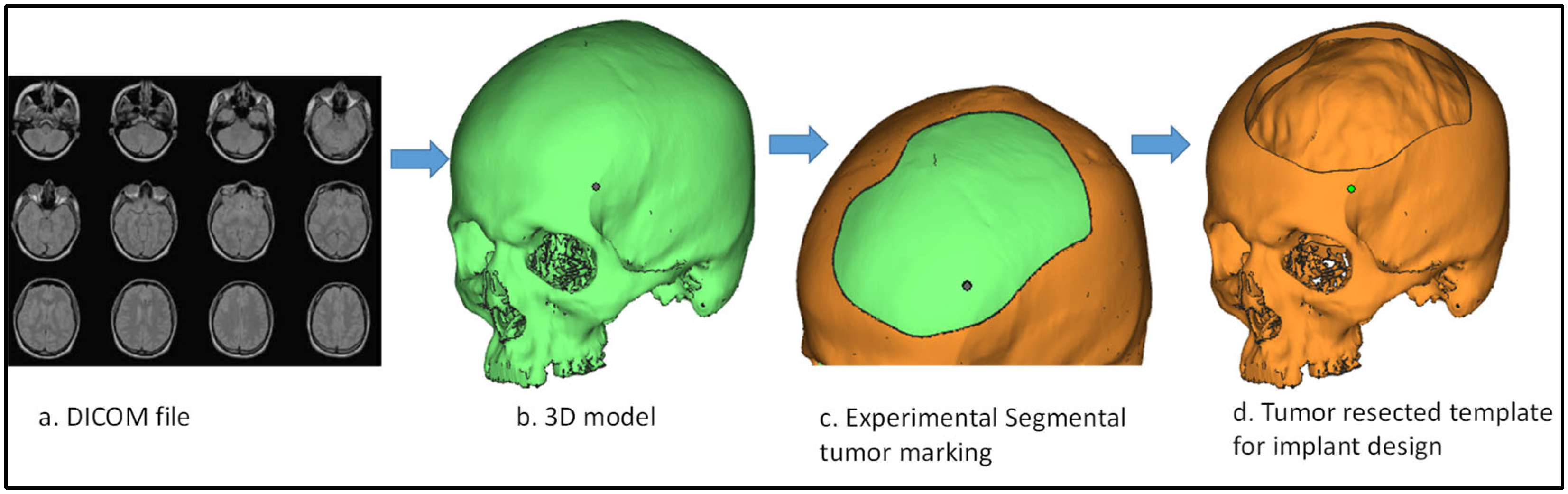
2.1. Design and Modeling
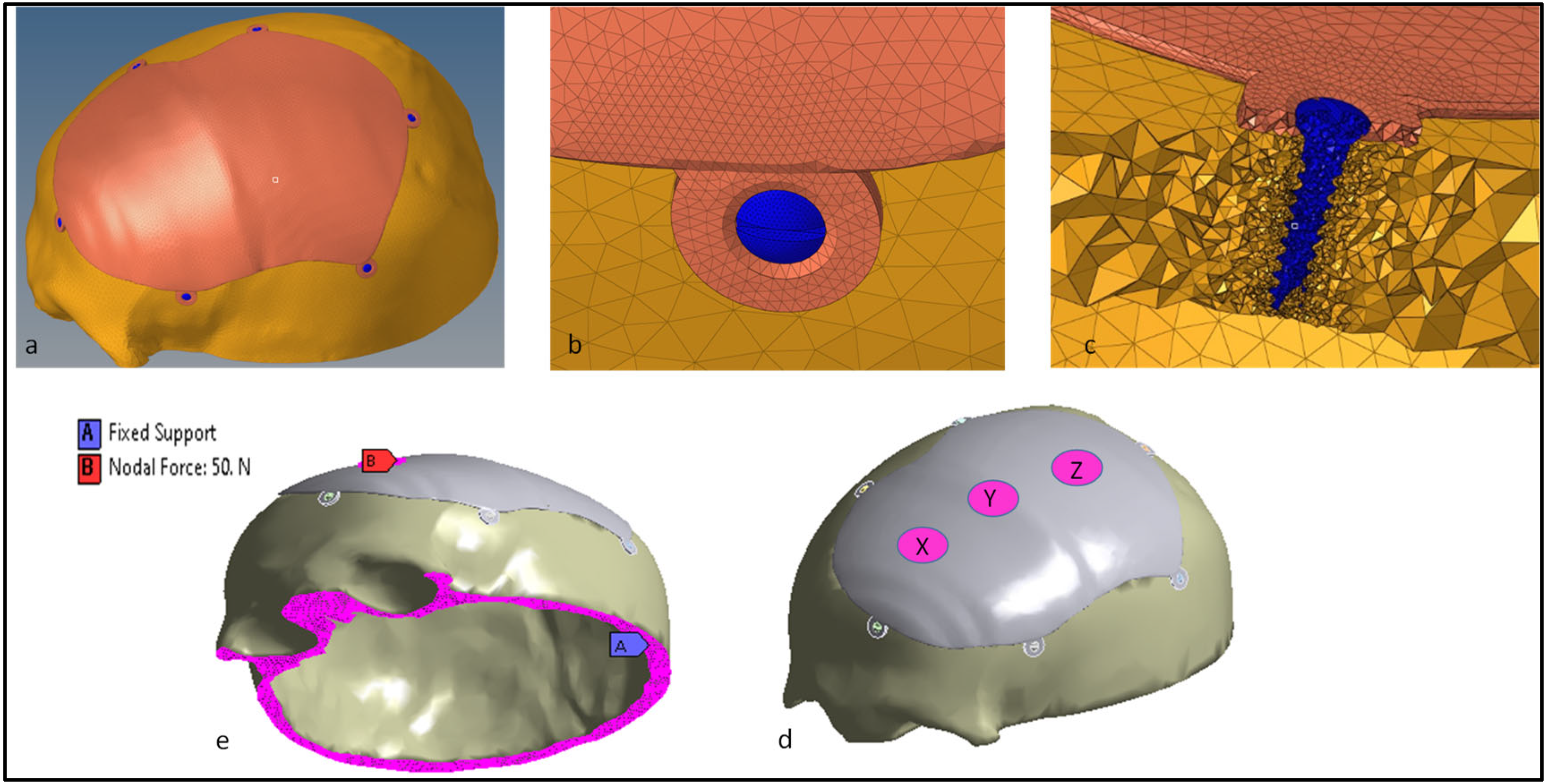
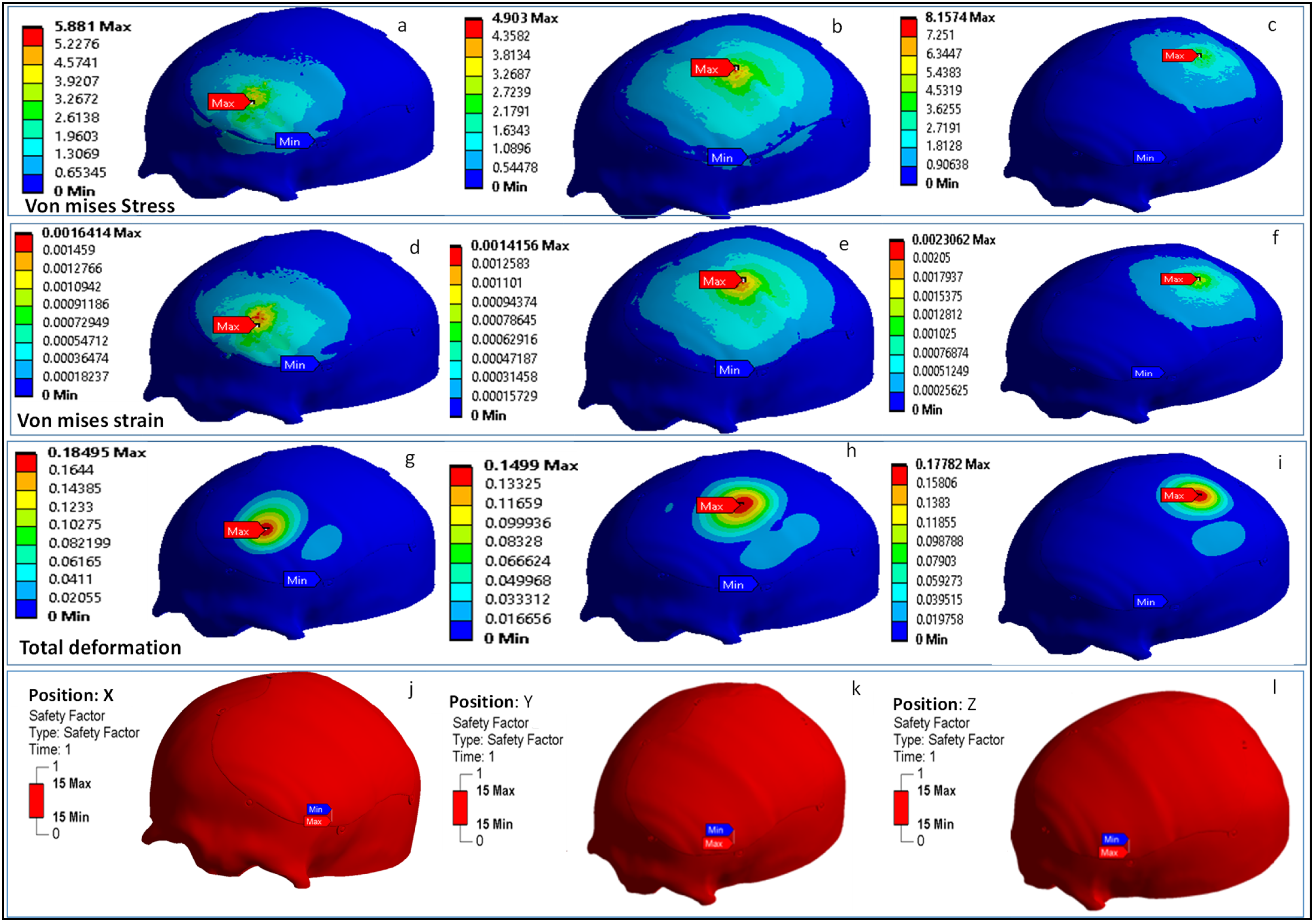
2.2. Computational Model and Analysis
2.3. Fabrication of the Skull Model and Cranial Implant
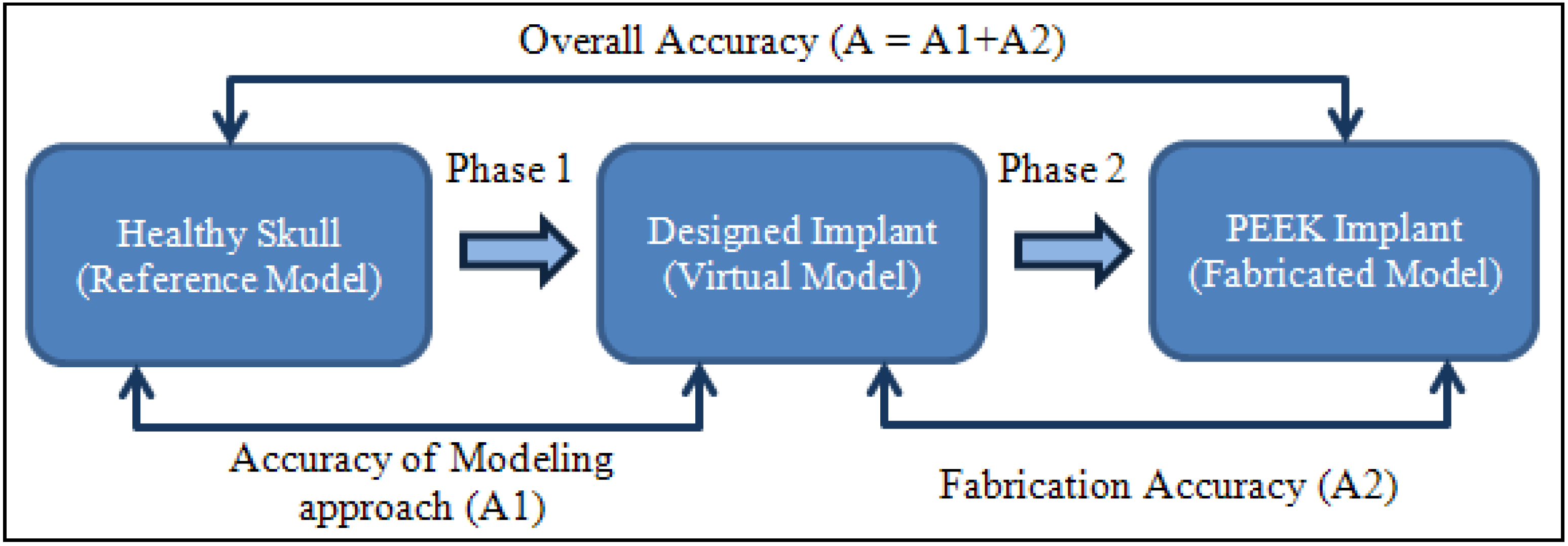
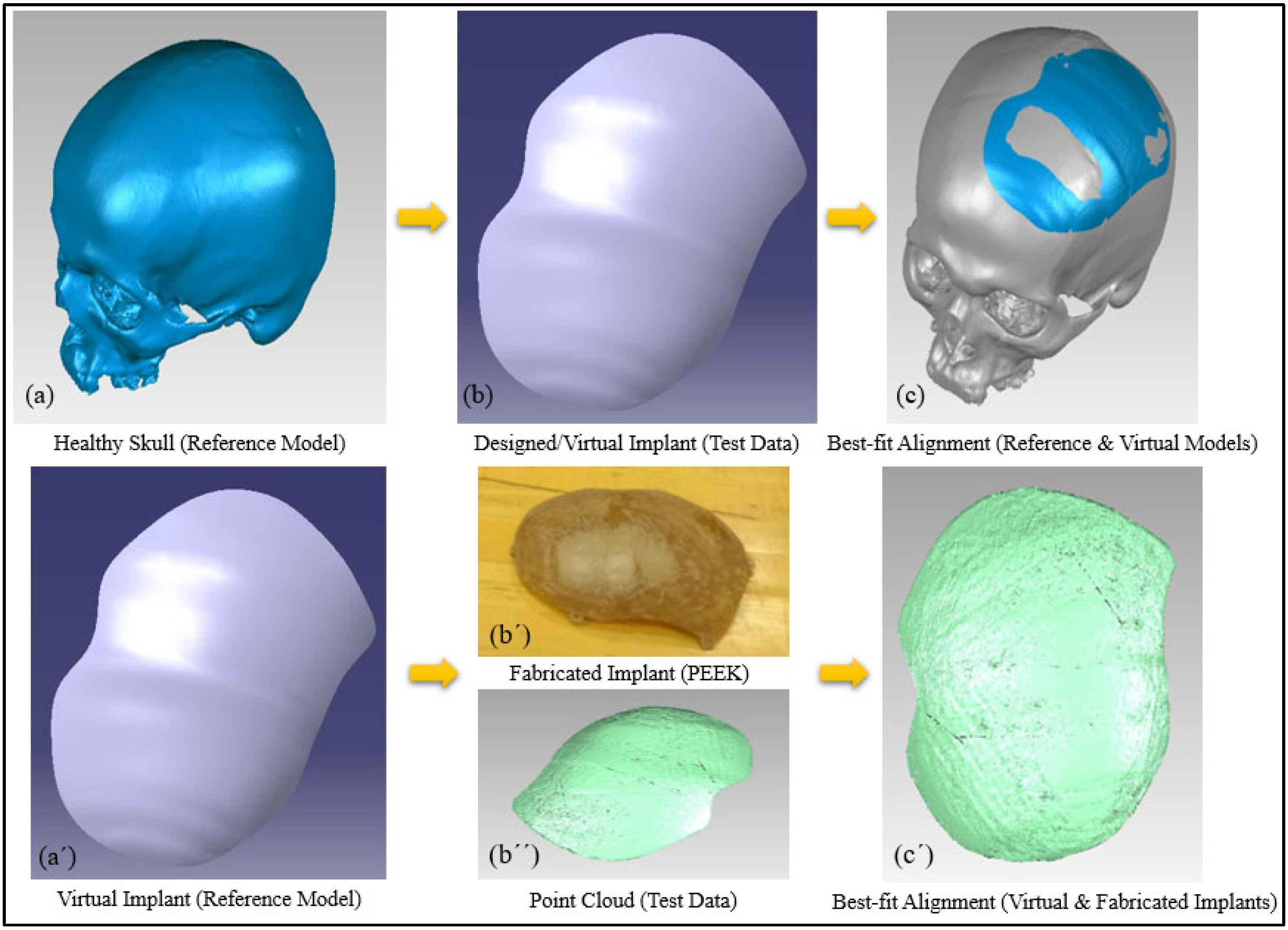
2.4. Accuracy Assessment
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameen, W.; Al-Ahmari, A.; Mohammed, M.K.; Abdulhameed, O.; Umer, U.; Moiduddin, K. Design, Finite Element Analysis (FEA), and Fabrication of Custom Titanium Alloy Cranial Implant Using Electron Beam Melting Additive Manufacturing. Adv. Prod. Eng. Manag. 2018, 13, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Mandolini, M.; Brunzini, A.; Serrani, E.B.; Pagnoni, M.; Mazzoli, A.; Germani, M. Design of a Custom-Made Cranial Implant in Patients Suffering from Apert Syndrome. Proc. Des. Soc. Int. Conf. Eng. Des. 2019, 1, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Park, D.K.; Song, I.; Lee, J.H.; You, Y.J. Forehead Augmentation with a Methyl Methacrylate Onlay Implant Using an Injection-Molding Technique. Arch. Plast Surg 2013, 40, 597–602. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xu, L.; Li, X.; Egger, J. Computer-Aided Implant Design for the Restoration of Cranial Defects. Sci. Rep. 2017, 7, 4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, W.-M.; Tzeng, I.-S.; Lin, M.-S. Three-Dimensional CAD in Skull Reconstruction: A Narrative Review with Focus on Cranioplasty and Its Potential Relevance to Brain Sciences. Appl. Sci. 2020, 10, 1847. [Google Scholar] [CrossRef] [Green Version]
- Brunzini, A.; Mandolini, M.; Manieri, S.; Germani, M.; Mazzoli, A.; Pagnoni, M.; Iannetti, G.; Modugno, A. Orbital Wall Reconstruction by Selective Laser Sintered Mould. In Proceedings of the 2017 13th IASTED International Conference on Biomedical Engineering (BioMed), Innsbruck, Austria, 20–21 February 2017; pp. 260–264. [Google Scholar]
- Igor, D.; Hren, N.; Strojnik, T.; Brajlih, T.; Valentan, B.; Pogačar, V.; Hartner, T. Applications of Rapid Prototyping in Cranio- Maxilofacial Surgery Procedures. Int. J. Biol. Biomed. Eng. 2008, 1, 29–38. [Google Scholar]
- Buonamici, F.; Furferi, R.; Genitori, L.; Governi, L.; Marzola, A.; Mussa, F.; Volpe, Y. Reverse Engineering Techniques for Virtual Reconstruction of Defective Skulls: An Overview of Existing Approaches. Comput. -Aided Des. Appl. 2019, 16, 103–112. [Google Scholar] [CrossRef]
- Harrysson, O.L.; Hosni, Y.A.; Nayfeh, J.F. Custom-Designed Orthopedic Implants Evaluated Using Finite Element Analysis of Patient-Specific Computed Tomography Data: Femoral-Component Case Study. BMC Musculoskelet. Disord. 2007, 8, 91. [Google Scholar] [CrossRef] [Green Version]
- Moiduddin, K.; Al-Ahmari, A.; Nasr, E.S.A.; Mian, S.H.; Al Kindi, M. A Comparison Study on the Design of Mirror and Anatomy Reconstruction Technique in Maxillofacial Region. Technol. Health Care 2016, 24, 377–389. [Google Scholar] [CrossRef]
- Chee Kai, C.; Siaw Meng, C.; Sin Ching, L.; Seng Teik, L.; Chit Aung, S. Facial Prosthetic Model Fabrication Using Rapid Prototyping Tools. Integr. Mfg Syst. 2000, 11, 42–53. [Google Scholar] [CrossRef]
- Bhargava, D.; Bartlett, P.; Russell, J.; Liddington, M.; Tyagi, A.; Chumas, P. Construction of Titanium Cranioplasty Plate Using Craniectomy Bone Flap as Template. Acta Neurochir 2010, 152, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Dumbrigue, H.B.; Arcuri, M.R.; LaVelle, W.E.; Ceynar, K.J. Fabrication Procedure for Cranial Prostheses. J. Prosthet. Dent. 1998, 79, 229–231. [Google Scholar] [CrossRef]
- Abdel-Haleem, A.K.; Nouby, R.; Taghian, M. The Use of the Rib Grafts in Head and Neck Reconstruction. Egypt. J. Ear Nose Throat Allied Sci. 2011, 12, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery 2015, 77, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, A.; Gibson, I.; Veetil, J.K.; Ghasemi, A.H. A Review of Technological Improvements in Laser-Based Powder Bed Fusion of Metal Printers. Int. J. Adv. Manuf Technol. 2020, 108, 191–209. [Google Scholar] [CrossRef]
- Ali, M.d.H.; Issayev, G.; Shehab, E.; Sarfraz, S. A Critical Review of 3D Printing and Digital Manufacturing in Construction Engineering. Rapid Prototyp. J. 2022, ahead-of-print. [Google Scholar] [CrossRef]
- Mian, S.H.; Moiduddin, K.; Abdo, B.M.A.; Sayeed, A.; Alkhalefah, H. Modelling and Evaluation of Meshed Implant for Cranial Reconstruction. Int. J. Adv. Manuf Technol. 2022, 118, 1967–1985. [Google Scholar] [CrossRef]
- Rukskul, P.; Suvannapruk, W.; Suwanprateeb, J. Cranial Reconstruction Using Prefabricated Direct 3DP Porous Polyethylene. Rapid Prototyp. J. 2019, 26, 278–287. [Google Scholar] [CrossRef]
- Yadla, S.; Campbell, P.G.; Chitale, R.; Maltenfort, M.G.; Jabbour, P.; Sharan, A.D. Effect of Early Surgery, Material, and Method of Flap Preservation on Cranioplasty Infections: A Systematic Review. Neurosurgery 2011, 68, 1124–1130. [Google Scholar] [CrossRef]
- Marchac, D.; Greensmith, A. Long-Term Experience with Methylmethacrylate Cranioplasty in Craniofacial Surgery. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 744–752. [Google Scholar] [CrossRef]
- Rupprecht, S.; Merten, H.-A.; Kessler, P.; Wiltfang, J. Hydroxyapatite Cement (BoneSourceTM) for Repair of Critical Sized Calvarian Defects—an Experimental Study. J. Cranio-Maxillofac. Surg. 2003, 31, 149–153. [Google Scholar] [CrossRef]
- Pang, D.; Tse, H.H.; Zwienenberg-Lee, M.; Smith, M.; Zovickian, J. The Combined Use of Hydroxyapatite and Bioresorbable Plates to Repair Cranial Defects in Children. J. Neurosurg. 2005, 102, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Nakatsuka, T.; Saijo, H.; Fujihara, Y.; Atsuhiko, H.; Chung, U.; Takato, T.; Hoshi, K. Computed Tomographic Evaluation of Novel Custom-Made Artificial Bones, “CT-Bone”, Applied for Maxillofacial Reconstruction. Regen. Ther. 2016, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials; InTechOpen: London, UK, 2013. [Google Scholar]
- Trevisan, F.; Calignano, F.; Aversa, A.; Marchese, G.; Lombardi, M.; Biamino, S.; Ugues, D.; Manfredi, D. Additive Manufacturing of Titanium Alloys in the Biomedical Field: Processes, Properties and Applications. J. Appl. Biomater. Funct. Mater. 2018, 16, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P.; Lundström, I. Physico-Chemical Considerations of Titanium as a Biomaterial. Clin. Mater. 1992, 9, 115–134. [Google Scholar] [CrossRef]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of Mechanical Forces on Maintenance and Adaptation of Form in Trabecular Bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-T.; Koak, J.-Y.; Lim, Y.-J.; Kim, S.-K.; Kwon, H.-B.; Kim, M.-J. Stress Shielding and Fatigue Limits of Poly-Ether-Ether-Ketone Dental Implants. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1044–1052. [Google Scholar] [CrossRef]
- Agapovichev, A.; Sotov, A.; Kokareva, V.; Smelov, V. Possibilities and Limitations of Titanium Alloy Additive Manufacturing. MATEC Web Conf. 2018, 224, 01064. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Dondi, M.; Ercolani, G.; Marsigli, M.; Melandri, C.; Mingazzini, C. The Chemical Composition of Porcelain Stoneware Tiles and Its Influence on Microstructure and Mechanical Properties. InterCeram: Int. Ceram. Rev. 1999, 48, 75–83. [Google Scholar]
- Brockett, C.L.; Carbone, S.; Fisher, J.; Jennings, L.M. PEEK and CFR-PEEK as Alternative Bearing Materials to UHMWPE in a Fixed Bearing Total Knee Replacement: An Experimental Wear Study. Wear 2017, 374–375, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Grimal, Q.; Laugier, P. Quantitative Ultrasound Assessment of Cortical Bone Properties Beyond Bone Mineral Density. IRBM 2019, 40, 16–24. [Google Scholar] [CrossRef]
- Zioupos, P.; Cook, R.B.; Hutchinson, J.R. Some Basic Relationships between Density Values in Cancellous and Cortical Bone. J. Biomech. 2008, 41, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Gradl, R.; Zanette, I.; Ruiz-Yaniz, M.; Dierolf, M.; Rack, A.; Zaslansky, P.; Pfeiffer, F. Mass Density Measurement of Mineralized Tissue with Grating-Based X-Ray Phase Tomography. PLoS ONE 2016, 11, e0167797. [Google Scholar] [CrossRef]
- Lancaster, P.; Brettle, D.; Carmichael, F.; Clerehugh, V. In-Vitro Thermal Maps to Characterize Human Dental Enamel and Dentin. Front. Physiol. 2017, 8, 461. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, A.; Wili, P.; Maquer, G.; Zysset, P. The Thermal Conductivity of Cortical and Cancellous Bone. Eur Cell Mater. 2018, 35, 25–33. [Google Scholar] [CrossRef]
- Rivière, L.; Caussé, N.; Lonjon, A.; Dantras, É.; Lacabanne, C. Specific Heat Capacity and Thermal Conductivity of PEEK/Ag Nanoparticles Composites Determined by Modulated-Temperature Differential Scanning Calorimetry. Polym. Degrad. Stab. 2016, 127, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Properties: Zirconia—ZrO2, Zirconium Dioxide. Available online: https://www.azom.com/properties.aspx?ArticleID=133 (accessed on 24 January 2022).
- Gowda, E.M.; Iyer, S.R.; Verma, K.; Murali Mohan, S. Evaluation of PEEK Composite Dental Implants: A Comparison of Two Different Loading Protocols. J. Dent. Res. Rep. 2018, 1. [Google Scholar] [CrossRef]
- Sharma, N.; Aghlmandi, S.; Dalcanale, F.; Seiler, D.; Zeilhofer, H.-F.; Honigmann, P.; Thieringer, F.M. Quantitative Assessment of Point-of-Care 3D-Printed Patient-Specific Polyetheretherketone (PEEK) Cranial Implants. Int. J. Mol. Sci. 2021, 22, 8521. [Google Scholar] [CrossRef]
- Skirbutis, G.; Dzingutė, A.; Masiliūnaitė, V.; Šulcaitė, G.; Žilinskas, J. A Review of PEEK Polymer’s Properties and Its Use in Prosthodontics. Stomatologija 2017, 19, 19–23. [Google Scholar]
- Mishra, S.; Chowdhary, R. PEEK Materials as an Alternative to Titanium in Dental Implants: A Systematic Review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyetheretherketone (PEEK): MakeItFrom.com. Available online: https://www.makeitfrom.com/material-properties/Polyetheretherketone-PEEK (accessed on 15 February 2022).
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The Use of PEEK in Digital Prosthodontics: A Narrative Review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Suonan, A.; Zhou, J.; Yuan, Q.; Liu, L.; Zhao, X.; Lou, X.; Yang, C.; Li, D.; Zhang, Y. PEEK (Polyether-Ether-Ketone) and Its Composite Materials in Orthopedic Implantation. Arab. J. Chem. 2021, 14, 102977. [Google Scholar] [CrossRef]
- Garrido, B.; Albaladejo-Fuentes, V.; Cano, I.G.; Dosta, S. Development of Bioglass/PEEK Composite Coating by Cold Gas Spray for Orthopedic Implants. J. Spray Tech. 2022, 31, 186–196. [Google Scholar] [CrossRef]
- Yadav, D.; Garg, R.K.; Ahlawat, A.; Chhabra, D. 3D Printable Biomaterials for Orthopedic Implants: Solution for Sustainable and Circular Economy. Resour. Policy 2020, 68, 101767. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Rashid Habib, S.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An Emerging Biomaterial for Oral Implants and Dental Prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H.K. Review on Development and Dental Applications of Polyetheretherketone-Based Biomaterials and Restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- Alexakou, E.; Damanaki, M.; Zoidis, P.; Bakiri, E.; Mouzis, N.; Smidt, G.; Kourtis, S. PEEK High Performance Polymers: A Review of Properties and Clinical Applications in Prosthodontics and Restorative Dentistry. Eur. J. Prosthodont. Restor. Dent. 2019, 27. [Google Scholar] [CrossRef]
- Bathala, L.; Majeti, V.; Rachuri, N.; Singh, N.; Gedela, S. The Role of Polyether Ether Ketone (Peek) in Dentistry—A Review. J. Med. Life 2019, 12, 5–9. [Google Scholar] [CrossRef]
- Van de Vijfeijken, S.E.C.M.; Münker, T.J.A.G.; Spijker, R.; Karssemakers, L.H.E.; Vandertop, W.P.; Becking, A.G.; Ubbink, D.T. CranioSafe Group Autologous Bone Is Inferior to Alloplastic Cranioplasties: Safety of Autograft and Allograft Materials for Cranioplasties, a Systematic Review. World Neurosurg. 2018, 117, 443–452.e8. [Google Scholar] [CrossRef] [PubMed]
- Binhammer, A.; Jakubowski, J.; Antonyshyn, O.; Binhammer, P. Comparative Cost-Effectiveness of Cranioplasty Implants. Plast Surg 2020, 28, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kinsman, M.; Aljuboori, Z.; Ball, T.; Nauta, H.; Boakye, M. Rapid High-Fidelity Contour Shaping of Titanium Mesh Implants for Cranioplasty Defects Using Patient-Specific Molds Created with Low-Cost 3D Printing: A Case Series. Surg Neurol. Int. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- MedCAD | PEEK Cost and Price Comparison. Available online: https://medcad.com/peek-cost-and-price-comparison/ (accessed on 15 February 2022).
- Thien, A.; King, N.K.K.; Ang, B.T.; Wang, E.; Ng, I. Comparison of Polyetheretherketone and Titanium Cranioplasty after Decompressive Craniectomy. World Neurosurg. 2015, 83, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Wyleżoł, M. Hybrid Modeling Methods of Cranial Implants. Adv. Sci. Technol. Res. J. 2018, 12, 35–47. [Google Scholar] [CrossRef]
- Al-Ahmari, A.; Nasr, E.A.; Moiduddin, K.; Anwar, S.; Kindi, M.A.; Kamrani, A. A Comparative Study on the Customized Design of Mandibular Reconstruction Plates Using Finite Element Method. Adv. Mech. Eng. 2015, 7, 1687814015593890. [Google Scholar] [CrossRef] [Green Version]
- High-Performance Materials. Available online: https://www.intamsys.com/high-performance-materials/ (accessed on 13 January 2022).
- Marcián, P.; Narra, N.; Borák, L.; Chamrad, J.; Wolff, J. Biomechanical Performance of Cranial Implants with Different Thicknesses and Material Properties: A Finite Element Study. Comput. Biol. Med. 2019, 109, 43–52. [Google Scholar] [CrossRef]
- Ridwan-Pramana, A.; Marcián, P.; Borák, L.; Narra, N.; Forouzanfar, T.; Wolff, J. Finite Element Analysis of 6 Large PMMA Skull Reconstructions: A Multi-Criteria Evaluation Approach. PLoS ONE 2017, 12, e0179325. [Google Scholar] [CrossRef] [Green Version]
- Brandicourt, P.; Delanoé, F.; Roux, F.-E.; Jalbert, F.; Brauge, D.; Lauwers, F. Reconstruction of Cranial Vault Defect with Polyetheretherketone Implants. World Neurosurg. 2017, 105, 783–789. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Noh, H.; Park, K.; Jeon, H.W.; Lim, S. Characterization of Power Demand and Energy Consumption for Fused Filament Fabrication Using CFR-PEEK. Rapid Prototyp. J. 2022, ahead-of-print. [Google Scholar] [CrossRef]
- Jiang, C.-P.; Cheng, Y.-C.; Lin, H.-W.; Chang, Y.-L.; Pasang, T.; Lee, S.-Y. Optimization of FDM 3D Printing Parameters for High Strength PEEK Using the Taguchi Method and Experimental Validation. Rapid Prototyp. J. 2022, ahead-of-print. [Google Scholar] [CrossRef]
- Lalegani Dezaki, M.; Mohd Ariffin, M.K.A.; Hatami, S. An Overview of Fused Deposition Modelling (FDM): Research, Development and Process Optimisation. Rapid Prototyp. J. 2021, 27, 562–582. [Google Scholar] [CrossRef]
- Mahendru, S.; Jain, R.; Aggarwal, A.; Aulakh, H.S.; Jain, A.; Khazanchi, R.K.; Sarin, D. CAD-CAM vs Conventional Technique for Mandibular Reconstruction with Free Fibula Flap: A Comparison of Outcomes. Surg. Oncol. 2020, 34, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Nayman, J.; Pearson, E.S., IX. On the Problem of the Most Efficient Tests of Statistical Hypotheses. Phil. Trans. R. Soc. Lond. A 1933, 231, 289–337. [Google Scholar] [CrossRef] [Green Version]
- Lo Giudice, A.; Ronsivalle, V.; Grippaudo, C.; Lucchese, A.; Muraglie, S.; Lagravère, M.O.; Isola, G. One Step before 3D Printing—Evaluation of Imaging Software Accuracy for 3-Dimensional Analysis of the Mandible: A Comparative Study Using a Surface-to-Surface Matching Technique. Materials 2020, 13, 2798. [Google Scholar] [CrossRef]
- Geomagics Control. X; 3D Systems. Available online: https://www.3dsystems.com/software#inspectionsoftware (accessed on 24 January 2022).
- Hammad Mian, S.; Abdul Mannan, M.; Al-Ahmari, A. The Influence of Surface Topology on the Quality of the Point Cloud Data Acquired with Laser Line Scanning Probe. Sens. Rev. 2014, 34, 255–265. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Vaughan, T.J.; McNamara, L.M. Strain Amplification in Bone Mechanobiology: A Computational Investigation of the in Vivo Mechanics of Osteocytes. J. R Soc. Interface 2012, 9, 2735–2744. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.R.; Fyhrie, D.P.; Whalen, R.T. Trabecular Bone Density and Loading History: Regulation of Connective Tissue Biology by Mechanical Energy. J. Biomech 1987, 20, 785–794. [Google Scholar] [CrossRef]
- Mosley, J.R. Osteoporosis and Bone Functional Adaptation: Mechanobiological Regulation of Bone Architecture in Growing and Adult Bone, a Review. J. Rehabil Res. Dev. 2000, 37, 189–199. [Google Scholar]
- Santos, P.O.; Carmo, G.P.; de Sousa, R.J.A.; Fernandes, F.A.O.; Ptak, M. Mechanical Strength Study of a Cranial Implant Using Computational Tools. Appl. Sci. 2022, 12, 878. [Google Scholar] [CrossRef]

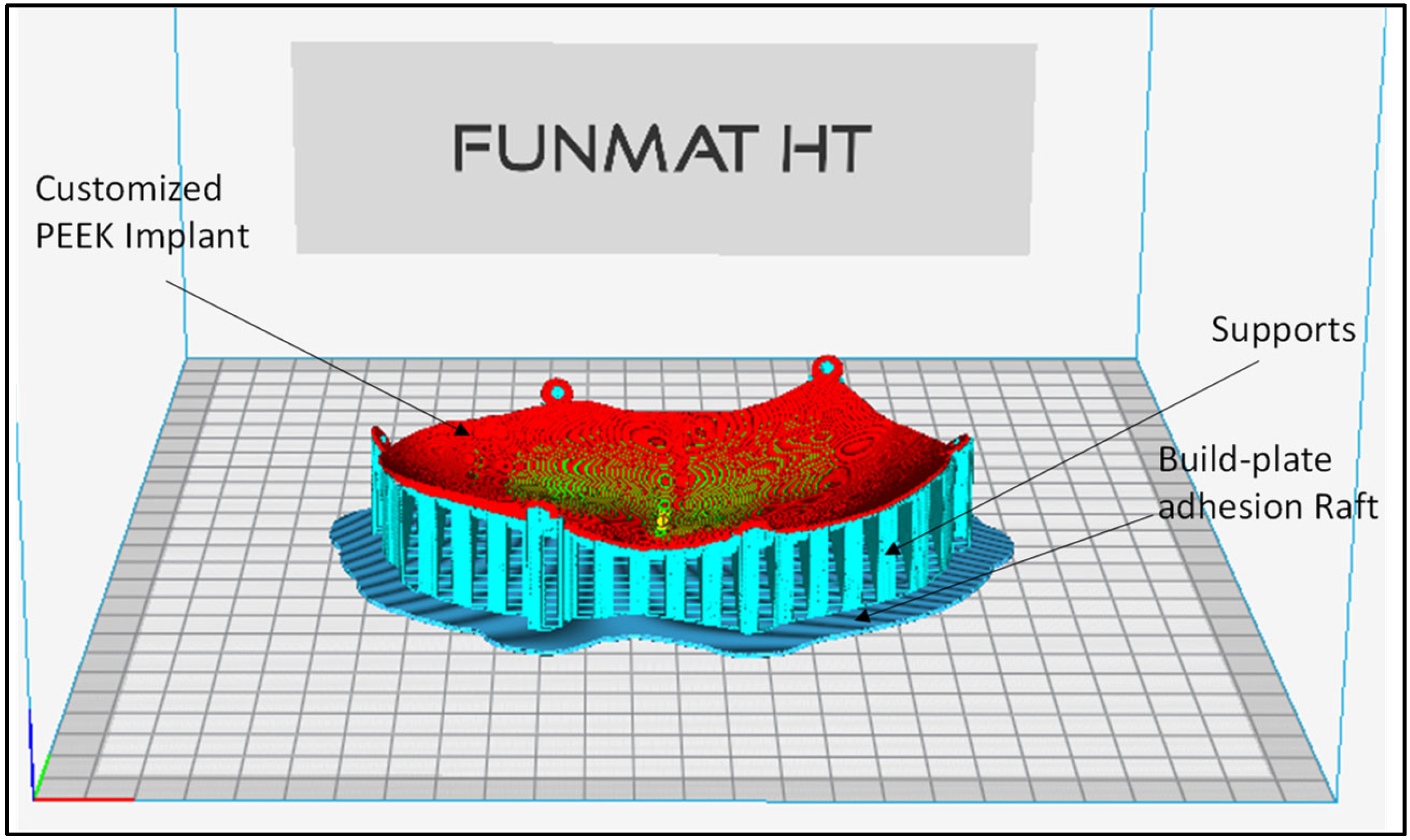
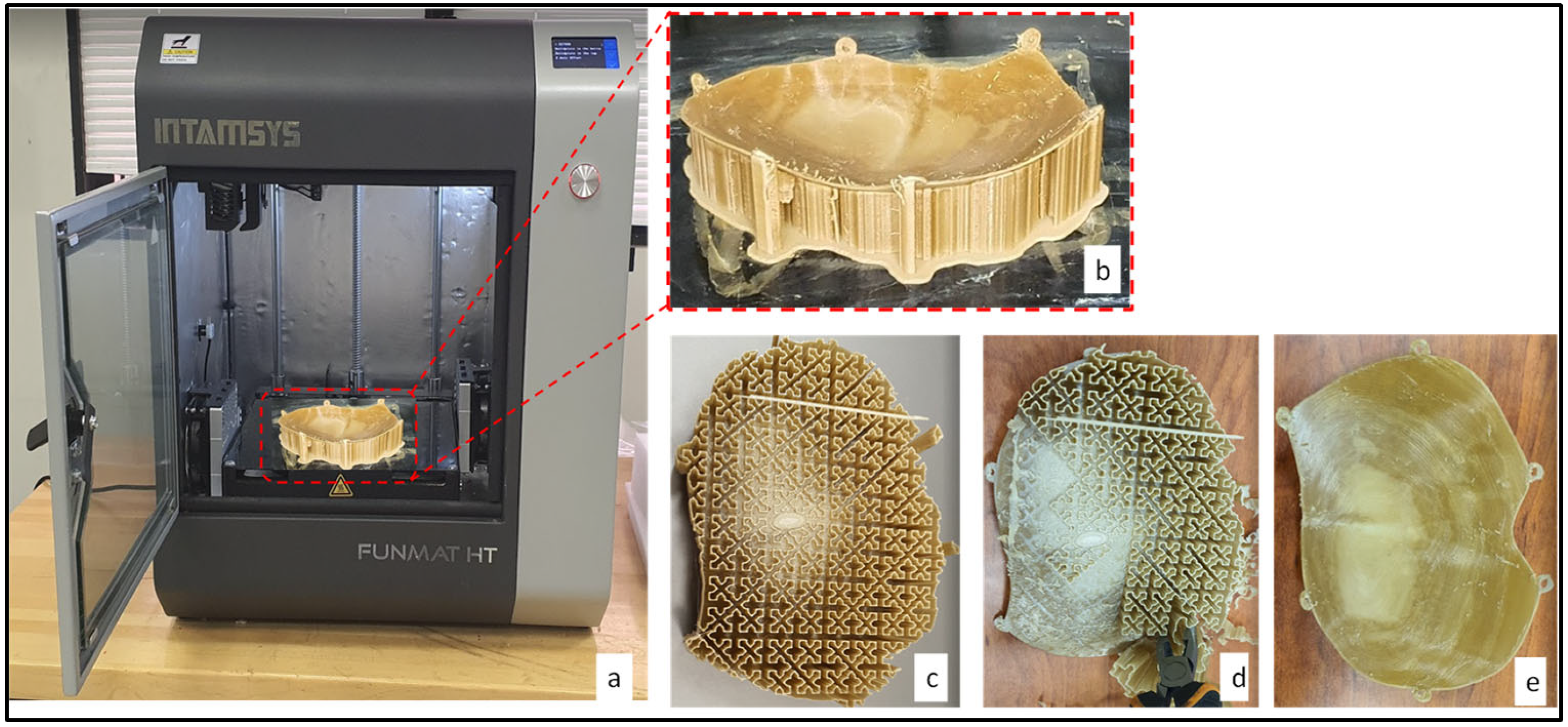

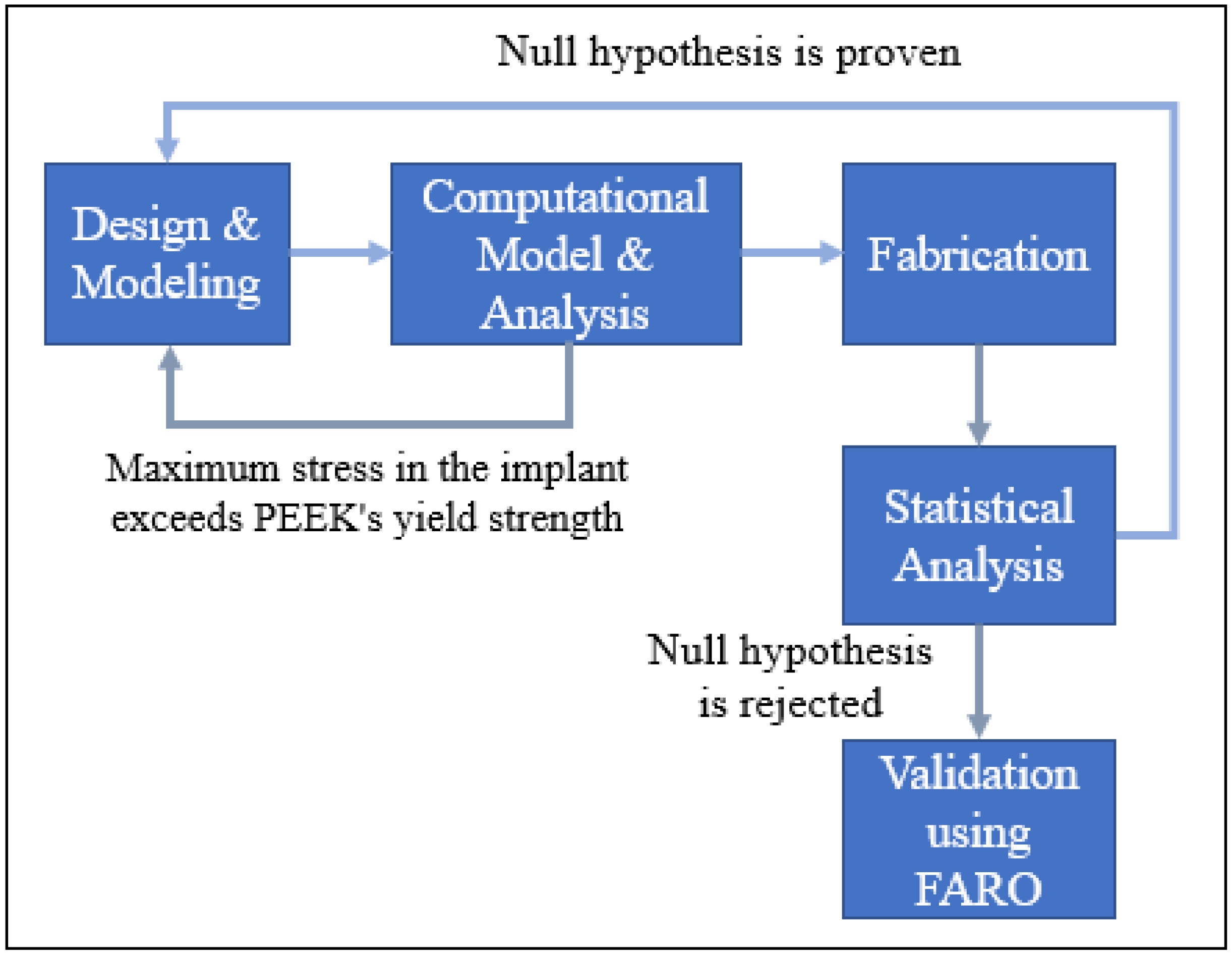
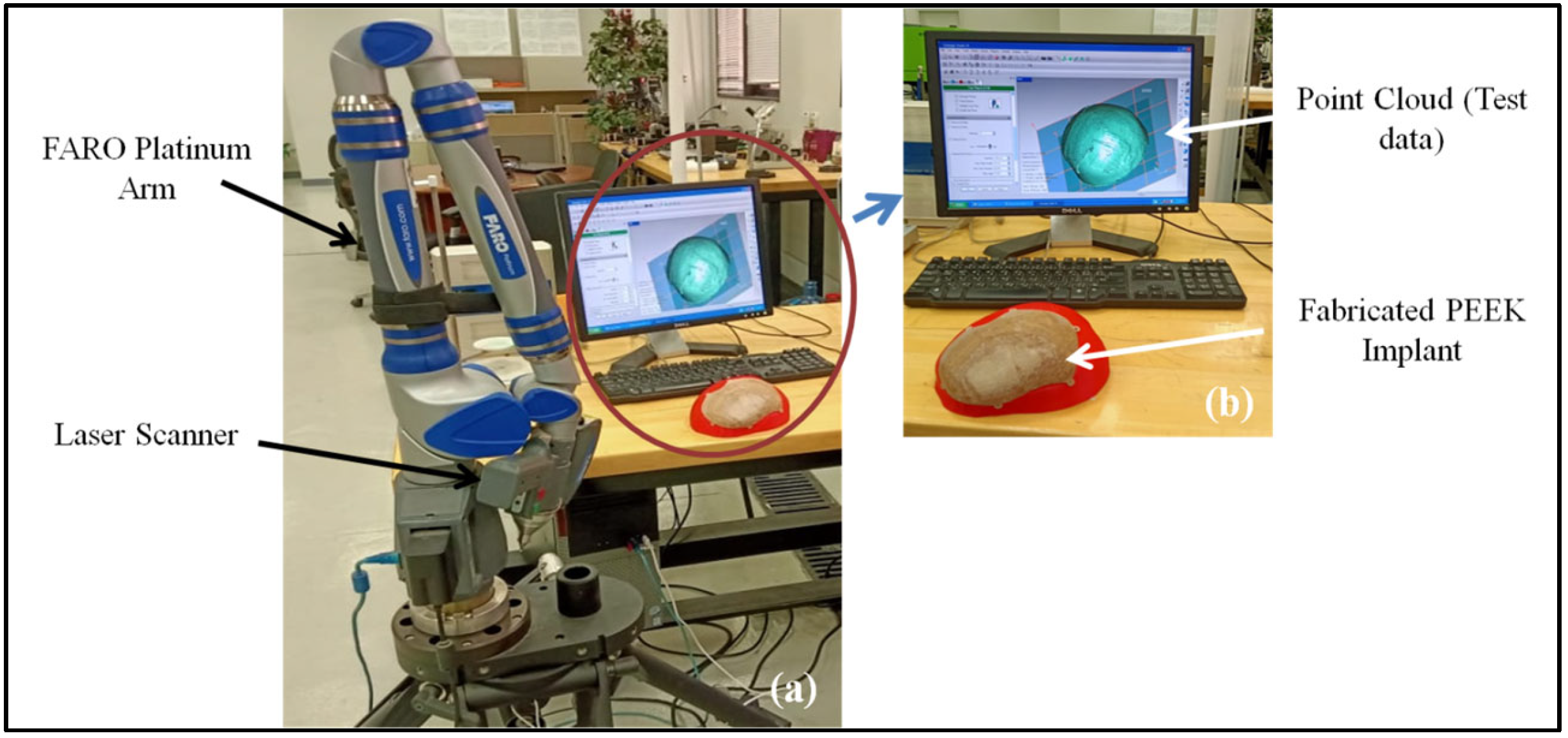


| Material | Elastic Modulus (GPA) | Density (g/cm3) | Thermal Conductivity (W/m.K) | Biocompatible |
|---|---|---|---|---|
| Titanium alloy (Ti6Al4V) | 110 | 4.5 | 7.1 | Yes |
| Cobalt-Chromium | 180–210 | 10 | 9.4 | Yes |
| Zirconia | 210 | 5.68 | 1.7–2.7 | Yes |
| Porcelain | 68.9 | 2.3–2.4 | 1.5 | Yes |
| PMMA | 3–5 | 1.18 | 0.167–0.25 | Yes |
| PEEK | 3–4 | 1.3–1.32 | 0.25–0.93 | Yes |
| CFR-PEEK | 18 | 1.42 | 0.95 | Yes |
| GFR-PEEK | 12 | 1.55 | 0.35 | Yes |
| Cortical Bone | 14 | 1.6–2 | 0.68 | |
| Cancellous bone | 1.34 | 0.05–0.3 | 0.42 | |
| Enamel | 40–83 | 2.6–3 | 0.45–0.93 | |
| Dentin | 15–30 | 1.79–2.12 | 0.11–0.96 |
| Materials | Youngs Modulus (MPa) | Poisson’s Ratio | Yield Strength (MPa) |
|---|---|---|---|
| Cortical bone | 13,700 | 0.3 | 122 |
| PEEK implant | 3738 | 0.4 | 99.9 |
| Titanium screws | 120,000 | 0.3 | 930 |
| Description | Parameters |
|---|---|
| Printing Technology | FFF |
| Extruder | Single |
| Extruder diameter (mm) | 0.4 |
| Layer thickness ((mm) | 0.15 |
| Print speed (mm/s) | 50 |
| Filament diameter (mm) | 1.75 |
| Build adhesion type | Raft |
| Nozzle temperature (℃) | 420 |
| Build temperature (℃) | 130 |
| Chamber temperature (℃) | 90 |
| Nozzle used | High-temperature nozzle set |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mian, S.H.; Moiduddin, K.; Elseufy, S.M.; Alkhalefah, H. Adaptive Mechanism for Designing a Personalized Cranial Implant and Its 3D Printing Using PEEK. Polymers 2022, 14, 1266. https://doi.org/10.3390/polym14061266
Mian SH, Moiduddin K, Elseufy SM, Alkhalefah H. Adaptive Mechanism for Designing a Personalized Cranial Implant and Its 3D Printing Using PEEK. Polymers. 2022; 14(6):1266. https://doi.org/10.3390/polym14061266
Chicago/Turabian StyleMian, Syed Hammad, Khaja Moiduddin, Sherif Mohammed Elseufy, and Hisham Alkhalefah. 2022. "Adaptive Mechanism for Designing a Personalized Cranial Implant and Its 3D Printing Using PEEK" Polymers 14, no. 6: 1266. https://doi.org/10.3390/polym14061266
APA StyleMian, S. H., Moiduddin, K., Elseufy, S. M., & Alkhalefah, H. (2022). Adaptive Mechanism for Designing a Personalized Cranial Implant and Its 3D Printing Using PEEK. Polymers, 14(6), 1266. https://doi.org/10.3390/polym14061266