High-Content Lithium Aluminum Titanium Phosphate-Based Composite Solid Electrolyte with Poly(ionic liquid) Binder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
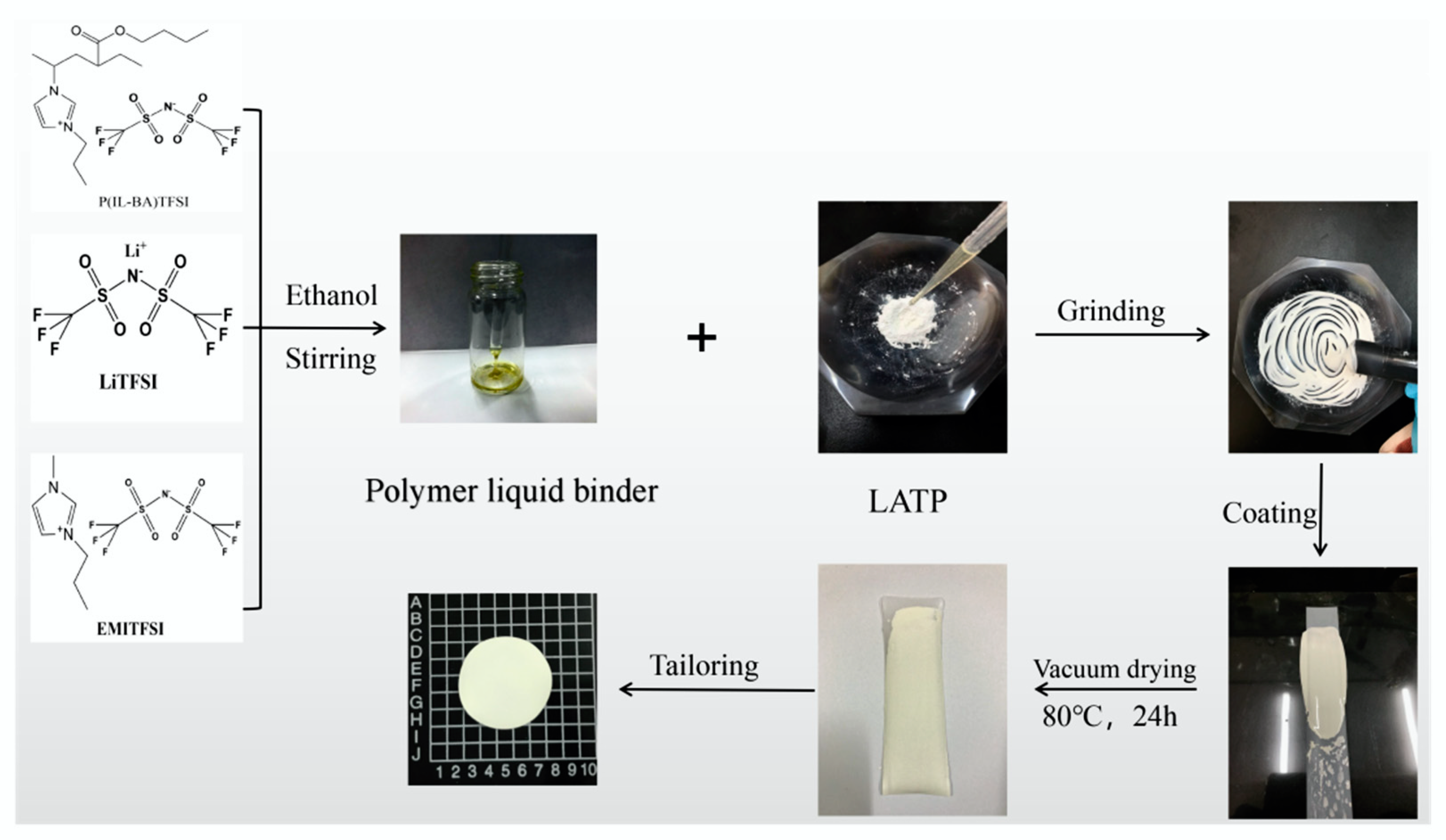
2.2. Synthesis of LATP Composite Electrolytes
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results
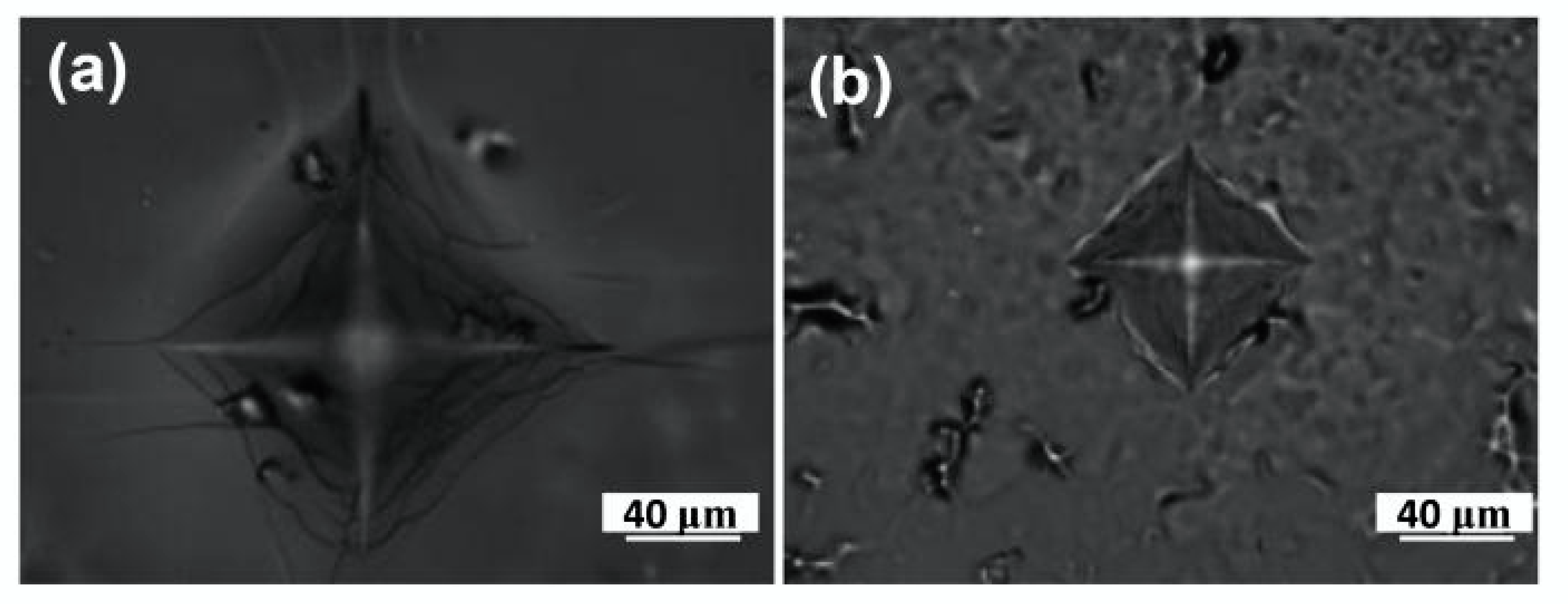
3.1. Structural Characterization of the Composite Electrolyte Materials
3.2. Ion-Conduction Properties of Composite Electrolytes
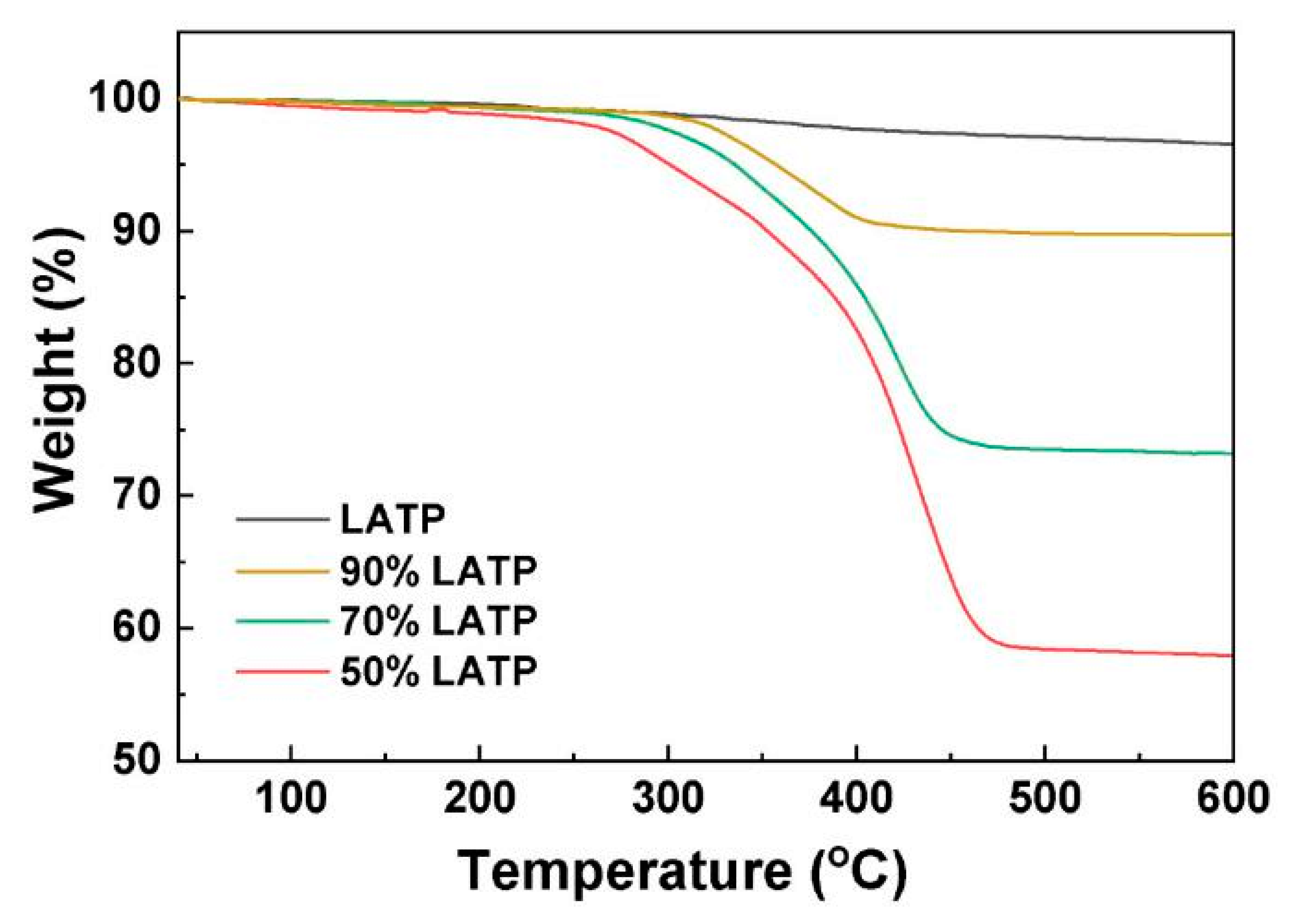
3.3. Hardness and Thermal Stability of Composite Electrolytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Melot, B.C.; Tarascon, J.M. Design and preparation of materials for advanced electrochemical storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, R.; Yang, L.; Zheng, J.; Zhang, K.; Mo, S.; Lin, Z.; Pan, F. Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Mater. 2018, 10, 139–159. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Goodenough, J.B. Electrochemical energy storage in a sustainable modern society. Energy Environ. Sci. 2014, 7, 14–18. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.; Fan, L.; Zhang, J.; Li, X. Recent advances in Li1+xAlxTi2−x(PO4)3 solid-state electrolyte for safe lithium batteries. Energy Storage Mater. 2019, 19, 379–400. [Google Scholar] [CrossRef]
- Guo, H.-L.; Lin, H.-F.; Yang, Y.-C.; Cheng, C.-H.; Tsai, Y.-R.; Wang, F.-M. Modification of LiCoO2 through rough coating with lithium lanthanum zirconium tantalum oxide for high-voltage performance in lithium ion batteries. J. Solid State Electrochem. 2020, 25, 105–115. [Google Scholar] [CrossRef]
- Deng, Y.; Eames, C.; Fleutot, B.; David, R.; Chotard, J.N.; Suard, E.; Masquelier, C.; Islam, M.S. Enhancing the Lithium ion conductivity in LISICON solid electrolytes through a mixed polyanion effect. ACS Appl. Mater. Interfaces 2017, 9, 7050–7058. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhao, R.; Han, S.; Li, S.; Zou, R.; Zhao, Y. Antiperovskite Ionic Conductor Layer for Stabilizing the Interface of NASICON Solid Electrolyte Against Li Metal in All-Solid-State Batteries. Batter. Supercaps 2021, 4, 1491–1498. [Google Scholar] [CrossRef]
- Samsinger, R.F.; Letz, M.; Schuhmacher, J.; Schneider, M.; Roters, A.; Kienemund, D.; Maune, H.; Kwade, A. Fast Ion Conduction of Sintered Glass-Ceramic Lithium Ion Conductors Investigated by Impedance Spectroscopy and Coaxial Reflection Technique. J. Electrochem. Soc. 2020, 167, 140510. [Google Scholar] [CrossRef]
- Aguesse, F.; Roddatis, V.; Roqueta, J.; García, P.; Pergolesi, D.; Santiso, J.; Kilner, J.A. Microstructure and ionic conductivity of LLTO thin films: Influence of different substrates and excess lithium in the target. Solid State Ion. 2015, 272, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Goswami, N.; Indu, M.S.; Murugan, R.; Kant, R. Experimental corroboration of theory for impedance response of solid electrolytes: Doped cubic garnet LLZO. J. Electroanal. Chem. 2021, 897, 115611. [Google Scholar] [CrossRef]
- David, I.N.; Thompson, T.; Wolfenstine, J.; Allen, J.L.; Sakamoto, J. Microstructure and Li-ion conductivity of hot-pressed cubic Li7La3Zr2O12. J. Am. Ceram. Soc. 2015, 98, 1209–1214. [Google Scholar] [CrossRef]
- Arbi, K.; Bucheli, W.; Jiménez, R.; Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li 1+x Al x M 2−x (PO 4) 3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 2015, 35, 1477–1484. [Google Scholar] [CrossRef]
- Wenzheng, C.; Yanan, Y.; Junwen, D.; Yiqiu, L.; Chenghao, C.; Tao, Z. Localization of electrons within interlayer stabilizes NASICON-type solid-state electrolyte. Mater. Today Energy 2021, 22, 100875. [Google Scholar]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Kang, X. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar]
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef] [PubMed]
- Inada, T. Fabrications and properties of composite solid-state electrolytes. Solid State Ion. 2003, 158, 275–280. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.W.; Appleby, A.J. Solvent-Free Composite PEO-Ceramic Fiber/Mat Electrolytes for Lithium Secondary Cells. J. Electrochem. Soc. 2005, 152, A205–A209. [Google Scholar] [CrossRef]
- Inda, Y.; Katoh, T.; Baba, M. Development of all-solid lithium-ion battery using Li-ion conducting glass-ceramics. J. Power Sources 2007, 174, 741–744. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, C.H.; Yu, J.H.; Doh, C.H.; Lee, S.M. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 2015, 274, 458–463. [Google Scholar] [CrossRef]
- Yun-Chae, J.; Sang-Min, L.; Jeong-Hee, C.; Seung Soon, J.; Dong-Won, K. All Solid-State Lithium Batteries Assembled with Hybrid Solid Electrolytes. J. Electrochem. Soc. 2015, 162, A704–A710. [Google Scholar]
- Kim, J.K.; Lim, Y.J.; Kim, H.; Cho, G.B.; Kim, Y. A hybrid solid electrolyte for flexible solid-state sodium batteries. Energy Environ. Sci. 2015, 8, 3589–3596. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Zheng, J.; Cheng, Q.; Hu, Y.Y.; Chan, C.K. Composite polymer electrolytes with Li7La3Zr2O12 garnet-type nanowires as ceramic fillers: Mechanism of conductivity enhancement and role of doping and morphology. ACS Appl. Mater. Interfaces 2017, 9, 21773–21780. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hu, Y.Y. New Insights into the compositional dependence of Li-ion transport in polymer-ceramic composite electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, N.; Sun, J.; Hsu, P.-C.; Li, Y.; Lee, H.-W.; Cui, Y. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers. Nano Lett. 2015, 15, 2740–2745. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Jin, Y.; Zong, X.; Zhang, X.; Liu, C.; Li, D.; Jia, Z.; Li, G.; Zhou, X.; Wei, J.; Xiong, Y. Interface regulation enabling three-dimensional Li1.3Al0.3Ti1.7(PO4)3-reinforced composite solid electrolyte for high-performance lithium batteries. J. Power Sources 2021, 501, 230027. [Google Scholar] [CrossRef]
- Yang, F.J.; Huang, Y.F.; Zhang, M.Q.; Ruan, W.H. Significant improvement of ionic conductivity of high-graphene oxide-loading ice-templated poly (ionic liquid) nanocomposite electrolytes. Polymer 2018, 153, 438–444. [Google Scholar] [CrossRef]
- Osada, I.; de Vries, H.; Scrosati, B.; Passerini, S. Ionic-liquid-based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Sato, Y.; Isawa, Y.; Lo, C.T.; Mori, H. Ionic conductivity and assembled structures of imidazolium salt-based block copolymers with thermoresponsive segments. Polymers 2017, 9, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Liu, C.; Zong, X.; Li, D.; Fu, M.; Tan, S.; Xiong, Y.; Wei, J. Interface engineering of Li1.3Al0.3Ti1.7(PO4)3 ceramic electrolyte via multifunctional interfacial layer for all-solid-state lithium batteries. J. Power Sources 2020, 460, 228125. [Google Scholar] [CrossRef]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Ni, J.E.; Case, E.D.; Sakamoto, J.S.; Rangasamy, E.; Wolfenstine, J.B. Room temperature elastic moduli and Vickers hardness of hot-pressed LLZO cubic garnet. J. Mater. Sci. 2012, 47, 7978–7985. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Miao, C.; Kou, Z.; Xiao, W. Enhanced ionic conductivity and electrochemical stability of Indium doping Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes for all-solid-state lithium-ion batteries. Ionics 2021, 28, 63–72. [Google Scholar] [CrossRef]
- Schroeder, M.; Glatthaar, S.; Binder, J.R. Influence of spray granulation on the properties of wet chemically synthesized Li1.3Ti1.7Al0.3(PO4)3 (LATP) powders. Solid State Ion. 2011, 201, 49–53. [Google Scholar] [CrossRef]
- Rey, I.; Johansson, P.; Lindgren, J.; Lassègues, J.C.; Grondin, J.; Servant, L. Spectroscopic and Theoretical Study of (CF3SO2)2N- (TFSI-) and (CF3SO2)2NH (HTFSI). J. Phys. Chem. A 1998, 102, 3249–3258. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, M.; Ganapathy, S.; Li, C.; Li, Z.; Zhang, X.; He, P.; Zhou, H.; Wagemaker, M. Revealing the impact of space-charge layers on the Li-ion transport in all-solid-state batteries. Joule 2020, 4, 1311–1323. [Google Scholar] [CrossRef]
- Othman, L.; Chew, K.W.; Osman, Z. Impedance spectroscopy studies of poly (methyl methacrylate)-lithium salts polymer electrolyte systems. Ionics 2007, 13, 337–342. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Gwak, E.-J.; Ahn, S.-M.; Kang, N.-R.; Han, H.N.; Jang, J.-I.; Kim, J.-Y. Indentation size effect for spherical nanoindentation on nanoporous gold. Scr. Mater. 2018, 143, 10–14. [Google Scholar] [CrossRef]




| 50% LATP | 60% LATP | 70% LATP | 80% LATP | 90% LATP | LATP | |
|---|---|---|---|---|---|---|
| Rb/Ω | 31.2 | 46.77 | 57.4 | 71.98 | 91.05 | 158 |
| Rgb/Ω | 4922 | 4262 | 4911 | 3335 | 1752 | 600.1 |
| Rint/Ω | 5.54 × 105 | 7.96 × 105 | 2.63 × 105 | 4.45 × 105 | 1.1 × 106 | 4.38 × 104 |
| σb/S·cm−1 | 1.2 × 10−3 | 8.3 × 10−4 | 6.6 × 10−4 | 5.3 × 10−4 | 4.2 × 10−4 | 5.8 × 10−5 |
| σgb/S·cm−1 | 7.78 × 10−6 | 8.98 × 10−6 | 7.80 × 10−6 | 1.15 × 10−5 | 2.18 × 10−5 | 1.53 × 10−5 |
| σint/S cm−1 | 6.91 × 10−8 | 3.64 × 10−8 | 1.45 × 10−7 | 8.6 × 10−8 | 3.48 × 10−8 | 2.09 × 10−8 |
| Sample | Load/Holding Time | Hardness, Hv (gf/mm2) |
|---|---|---|
| 50% LATP | 10 g f (0.098 N)/10 s | 0.9 ± 0.1 |
| 90% LATP | 25 g f (0.245 N)/10 s | 8.6 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Liu, Q.; Xie, W.; Xie, P.; Shang, J.; Shu, X. High-Content Lithium Aluminum Titanium Phosphate-Based Composite Solid Electrolyte with Poly(ionic liquid) Binder. Polymers 2022, 14, 1274. https://doi.org/10.3390/polym14071274
Yang F, Liu Q, Xie W, Xie P, Shang J, Shu X. High-Content Lithium Aluminum Titanium Phosphate-Based Composite Solid Electrolyte with Poly(ionic liquid) Binder. Polymers. 2022; 14(7):1274. https://doi.org/10.3390/polym14071274
Chicago/Turabian StyleYang, Fujie, Qingfeng Liu, Wenfei Xie, Pu Xie, Jingqi Shang, and Xugang Shu. 2022. "High-Content Lithium Aluminum Titanium Phosphate-Based Composite Solid Electrolyte with Poly(ionic liquid) Binder" Polymers 14, no. 7: 1274. https://doi.org/10.3390/polym14071274






