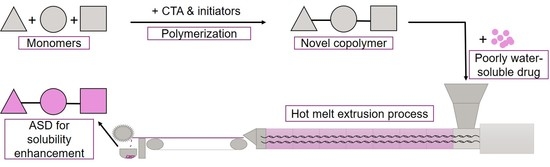
A Novel Aminomethacrylate-Based Copolymer for Solubility Enhancement—From Radical Polymer Synthesis to Manufacture and Characterization of Amorphous Solid Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of ModE Copolymer
2.2.2. Purification and Drying of ModE
2.2.3. Determination of Water Content of ModE
2.2.4. Residual Monomer Analysis and Monomer Conversion Rate
2.2.5. Molecular Weight Analysis by Gel Permeation Chromatography
2.2.6. Thermogravimetric Analysis for Studying ModE Decomposition
2.2.7. Flowability Measurement
2.2.8. Preparation of Amorphous Solid Dispersions via Hot Melt Extrusion
2.2.9. Differential Scanning Calorimetry Analysis
2.2.10. X-ray Powder Diffraction Studies
2.2.11. Fourier-Transform Infrared Spectroscopy Analysis
2.2.12. Determination of Saturation Solubility in Water
2.2.13. Dissolution Studies of ASD
2.2.14. HPLC Setup
HPLC Method for Celecoxib
HPLC Method for Efavirenz
HPLC Method for Fenofibrate
2.2.15. Stability Studies
2.2.16. Data Analysis
3. Results and Discussion
3.1. Synthesis of ModE
3.2. Purification, Residual Monomer Analysis and Water Content of ModE
3.3. Molecular Weight Analysis
3.4. Flowability
3.5. Investigation on Decomposition of ModE via TGA
3.6. ASD Composition and Manufacture
3.7. Thermal Characterization of the Pure Drugs, (Co-)Polymers and ASDs via DSC Analysis
3.8. XRPD Studies of Pure Drugs, (Co-)Polymers and ASDs
3.9. FT-IR Analysis
3.10. Saturation Solubility Studies
3.11. Dissolution Studies
3.12. Stability Studies
3.12.1. Visual Appearance
3.12.2. Thermal Characterization of ASD via DSC Analysis after Three Months of Storage
3.12.3. Dissolution Studies after Three Months of Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamble, R.N.; Mehta, P.P.; Kumar, A. Efavirenz self-nano-emulsifying drug delivery system: In vitro and in vivo evaluation. AAPS PharmSciTech 2016, 17, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.A.; Zhang, G.G.; Alonzo, D.E.; Wu, J.; Zhu, D.; Catron, N.D.; Gao, Y.; Taylor, L.S. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J. Pharm. Sci. 2014, 103, 2736–2748. [Google Scholar] [CrossRef]
- Ullrich, A.; Schiffter, H.A. The influence of polymer excipients on the dissolution and recrystallization behavior of ketoconazole: Application, variation and practical aspects of a pH shift method. Eur. J. Pharm. Biopharm. 2018, 133, 20–30. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmcol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. The effect of HPMCAS functional groups on drug crystallization from the supersaturated state and dissolution improvement. Int. J. Pharm. 2014, 464, 205–213. [Google Scholar] [CrossRef]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Wyttenbach, N.; Janas, C.; Siam, M.; Lauer, M.E.; Jacob, L.; Scheubel, E.; Page, S. Miniaturized screening of polymers for amorphous drug stabilization (SPADS): Rapid assessment of solid dispersion systems. Eur. J. Pharm. Biopharm. 2013, 84, 583–598. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Crystallization of amorphous solid dispersions of resveratrol during preparation and storage-Impact of different polymers. J. Pharm. Sci. 2013, 102, 171–184. [Google Scholar] [CrossRef]
- Song, Y.; Zemlyanov, D.; Chen, X.; Su, Z.; Nie, H.; Lubach, J.W. Acid-base interactions in amorphous solid dispersions of lumefantrine prepared by spray-drying and hot-melt extrusion using X-ray photoelectron spectroscopy. Int. J. Pharm. 2016, 514, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Su, L.; Li, N.; Hu, Y.; Tang, G.; Liu, L. Understanding the mechanism of dissolution enhancement for poorly water-soluble drugs by solid dispersions containing Eudragit. J. Drug Deliv. Sci. Technol. 2018, 48, 328–337. [Google Scholar] [CrossRef]
- Calahan, J.L.; Zanon, R.L.; Alvarez-Nunez, F.; Munson, E.J. Isothermal microcalorimetry to investigate the phase separation for amorphous solid dispersions of AMG 517 with HPMC-AS. Mol. Pharm. 2013, 10, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baek, M.J.; Choi, H.W.; Kim, H.S.; Lee, D.W. Development of poly(methyl methacrylate)-based copolymers with improved heat resistance and reduced moisture absorption. Langmuir 2019, 35, 15880–15886. [Google Scholar] [CrossRef]
- Al-Odayni, A.B.; Saeed, W.S.; Ahmed, A.Y.B.H.; Alrahlah, A.; Al-Kahtani, A.; Aouak, T. New monomer based on eugenol methacrylate, synthesis, polymerization and copolymerization with methyl methacrylate-Characterization and thermal properties. Polymers 2020, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Wang, B.; Chen, J.; Huang, Y.; Fang, T.; Wang, Y.; Liao, B. The effect of acrylamides copolymers on the stability and rheological properties of yellow iron oxide dispersion. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 136–145. [Google Scholar] [CrossRef]
- Harrisson, S. The chain length distribution of an ideal reversible deactivation radical polymerization. Polymers 2018, 10, 887. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Peng, B.; Tan, S.; Tian, X.; Zhang, Z. Grafting PMMA onto P(VDF-TrFE) by C-F activation via a Cu(0) mediated controlled radical polymerization process. Macromol. Rapid Commun. 2020, 41, 1–5. [Google Scholar] [CrossRef]
- Affinisol HPMC HME. Available online: https://www.pharma.dupont.com/content/dam/dupont/amer/us/en/nutrition-health/general/pharmaceuticals/documents/Download_Affinisol%20HPMC%20HME%20Brochure.pdf (accessed on 15 March 2022).
- Hot Melt Extrusion with BASF Pharma Polymers. Available online: https://pharmaceutical.basf.com/global/images/03_120803_hot_melt_extrusion_with_basf_pharma_polymers.pdf (accessed on 15 March 2022).
- Moseson, D.E.; Jordan, M.A.; Shah, D.D.; Corum, I.D.; Alvarenga, B.R., Jr.; Taylor, L.S. Application and limitations of thermogravimetric analysis to delineate the hot melt extrusion chemical stability processing window. Int. J. Pharm. 2020, 590, 119916. [Google Scholar] [CrossRef]
- Hanada, M.; Jermain, S.V.; Williams, R.O., III. Enhanced dissolution of a porous carrier-containing ternary amorphous solid dispersion system prepared by a hot melt method. J. Pharm. Sci. 2018, 107, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Pawar, J.; Suryawanshi, D.; Moravkar, K.; Aware, R.; Shetty, V.; Maniruzzaman, M. Study the influence of formulation process parameters on solubility and dissolution enhancement of efavirenz solid solutions prepared by hot-melt extrusion: A QbD methodology. Drug Deliv. Transl. Res. 2018, 8, 1644–1657. [Google Scholar] [CrossRef] [PubMed]
- Pawar, J.; Tayade, A.; Gangurde, A.; Moravkar, K.; Amin, P. Solubility and dissolution enhancement of efavirenz hot melt extruded amorphous solid dispersions using combination of polymeric blends: A QbD approach. Eur. J. Pharm. Sci. 2016, 88, 37–49. [Google Scholar] [CrossRef]
- Silva, L.A.D.; Almeida, S.L.; Alonso, E.C.; Rocha, P.B.; Martins, F.T.; Freitas, L.A.; Taveira, S.F.; Cunha-Filho, M.S.; Marreto, R.N. Preparation of a solid self-microemulsifying drug delivery system by hot-melt extrusion. Int. J. Pharm. 2018, 541, 1–10. [Google Scholar] [CrossRef]
- Wen, T.; Niu, B.; Wu, Q.; Zhou, Y.; Pan, X.; Quan, G.; Wu, C. Fenofibrate solid dispersion processed by hot-melt extrusion: Elevated bioavailability and its cell transport mechanism. Curr. Drug Deliv. 2019, 16, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Cristofoletti, R.; Nair, A.; Abrahamsson, B.; Groot, D.W.; Kopp, S.; Langguth, P.; Polli, J.E.; Shah, V.P.; Dressman, J.B. Biowaiver monographs for immediate release solid oral dosage forms: Efavirenz. J. Pharm. Sci. 2013, 102, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Schmied, F.P.; Bernhardt, A.; Engel, A.; Klein, S. A customized screening tool approach for the development of a self-nanoemulsifying drug delivery system (SNEDDS). AAPS PharmSciTech 2022, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.M.; Nguyen, J.H.; Becker, C.; Francke, N.M.; Jørgensen, E.B.; Holm, P.; Holm, R.; Mu, H.; Rades, T.; Langguth, P. Influence of polymer molecular weight on in vitro dissolution behavior and in vivo performance of celecoxib: PVP amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2016, 101, 145–151. [Google Scholar] [CrossRef]
- Homayouni, A.; Sadeghi, F.; Nokhodchi, A.; Varshosaz, J.; Garekani, H.A. Preparation and characterization of celecoxib solid dispersions; Comparison of poloxamer-188 and PVP-K30 as carriers. Iran. J. Basic Med. Sci. 2014, 17, 322–331. [Google Scholar]
- Kawakami, K.; Sato, K.; Fukushima, M.; Miyazaki, A.; Yamamura, Y.; Sakuma, S. Phase separation of supersaturated solution created from amorphous solid dispersions: Relevance to oral absorption. Eur. J. Pharm. Biopharm. 2018, 132, 146–156. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Pham, C.V.; Le Thien, G.; Ngoc, B.T.; Le Thi, H.; Huyen, C.P.T.; Thi, T.N. Immediate-released pelletized solid dispersion containing fenofibrate: Formulation, in vitro characterization, and bioequivalence studies in experimental beagle dogs. Int. J. Pharm. 2019, 570, 118661. [Google Scholar] [CrossRef]













| Polymer | Residual Monomer Content | Monomer Conversion Rate | Final Polymer Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DMAPMA * [%] | BMA * [%] | MMA * [%] | DMAPMA [%] | BMA [%] | MMA [%] | DMAPMA [%] | BMA [%] | MMA [%] | |
| E-173 kDa | 6.48 | 0.002 | 0.022 | 87.04 | 99.99 | 99.91 | 46.55 | 26.74 | 26.71 |
| E-254 kDa | 6.79 | 0.002 | 0.037 | 86.42 | 99.99 | 99.85 | 46.38 | 26.83 | 26.79 |
| E-281 kDa | 5.59 | 0.002 | 0.034 | 88.82 | 99.99 | 99.86 | 47.06 | 26.49 | 26.45 |
| E-305 kDa | 5.78 | 0.002 | 0.045 | 88.44 | 99.99 | 99.82 | 46.96 | 26.54 | 26.50 |
| average | 6.16 | 0.002 | 0.035 | 87.68 | 99.99 | 99.86 | 46.74 | 26.65 | 26.61 |
| Polymer | DMAPMA * [%] | BMA * [%] | MMA * [%] | Water Content after 5 Days [%] ± S.D. | Water Content after 10 Days [%] ± S.D. |
|---|---|---|---|---|---|
| E-173 kDa | 0.016 ± 0.001 | <0.002 ± 0 | <0.002 ± 0 | 1.11 ± 0.05 | 0.60 ± 0.07 |
| E-254 kDa | 0.025 ± 0.002 | <0.002 ± 0 | <0.002 ± 0 | 1.31 ± 0.08 | 0.90 ± 0.03 |
| E-281 kDa | 0.028 ± 0.002 | <0.002 ± 0 | <0.002 ± 0 | 1.10 ± 0.05 | 0.90 ± 0.04 |
| E-305 kDa | 0.042 ± 0.003 | <0.002 ± 0 | <0.002 ± 0 | 1.11 ± 0.07 | 0.80 ± 0.06 |
| Concentration of n-Dodecylmercaptan [%] | Number-Average Molecular Weight (Mn) [kDa] | Weight-Average Molecular Weight (Mw) [kDa] | Polydispersity Index (PDI) |
|---|---|---|---|
| 1.5 | 41.5 ± 1.8 | 173 ± 0.5 | 4.17 ± 0.09 |
| 0.9 | 64.1 ± 0.4 | 254 ± 0.5 | 3.97 ± 0.02 |
| 0.5 | 71.7 ± 1.3 | 281 ± 1.0 | 3.92 ± 0.05 |
| 0.3 | 81.4 ± 0.1 | 305 ± 0.5 | 3.75 ± 0.01 |
| E-173 kDa | E-254 kDa | E-281 kDa | E-305 kDa | EPO * | |
|---|---|---|---|---|---|
| polymer [g] | 99.90 ± 0.45 | 99.17 ± 0.54 | 99.35 ± 0.55 | 99.87 ± 0.33 | 99.65 ± 0.41 |
| flow time [s] | 30.51 ± 0.60 | 25.37 ± 0.71 | 24.09 ± 0.40 | 23.11 ± 1.46 | - |
| flow rate [g/s] | 3.28 ± 0.06 | 3.91 ± 0.13 | 4.13 ± 0.09 | 4.34 ± 0.29 | - |
| slope angle [°] | 34.58 ± 0.43 | 31.33 ± 0.52 | 31.87 ± 0.54 | 32.20 ± 1.00 | - |
| Polymer | Drug Load [%] | Extrusion Temperature [°C] | Torque [N·cm] | Screw Speed [rpm] |
|---|---|---|---|---|
| Soluplus® | 5.1/6.3/4.2 | 150/150/150 | 130/105/95 | 200/200/200 |
| Kollidon® VA 64 | 5.1/6.3/4.2 | 160/165/170 | 70/90/65 | 200/200/200 |
| Kollidon® 17 PF | 5.1/6.3/4.2 | 180/180/180 | 55/85/65 | 200/200/200 |
| AQOAT® AS-MMP | 5.1/6.3/4.2 | 170/175/175 | 140/200/140 | 200/200/200 |
| E-173 kDa | 5.1/6.3/4.2 | 150/160/160 | 200/185/180 | 200/200/200 |
| E-254 kDa | 5.1/6.3/4.2 | 165/160/165 | 190/180/180 | 200/200/200 |
| E-281 kDa | 5.1/6.3/4.2 | 160/160/160 | 200/200/200 | 200/200/200 |
| E-305 kDa | 5.1/6.3/4.2 | 165/165/165 | 230/200/220 | 200/200/200 |
| Affinisol® HPMC 100 LV | 5.1/6.3/4.2 | 165/165/170 | 150/150/120 | 100/100/100 |
| EUDRAGIT® E PO | 5.1/6.3/4.2 | 150/150/150 | 50/45/45 | 200/200/200 |
| Polymer | Tg (Polymer) [°C] | Tg (Celecoxib ASD) [°C] | Tg (Efavirenz ASD) [°C] | Tg (Fenofibrate ASD) [°C] |
|---|---|---|---|---|
| Soluplus® | 70 ± 0 | 67 ± 1 | 69 ± 0 | 62 ± 1 |
| Kollidon® VA 64 | 107 ± 1 | 100 ± 2 | 100 ± 1 | 92 ± 2 |
| Kollidon® 17 PF | 136 ± 3 | 126 ± 3 | 96 ± 1 | 90 ± 3 |
| AQOAT® AS-MMP | 113 ± 1 | 106 ± 1 | 100 ± 2 | 101 ± 1 |
| E-173 kDa | 77 ± 1 | 77 ± 1 | 76 ± 1 | 74 ± 1 |
| E-254 kDa | 85 ± 2 | 79 ± 0 | 78 ± 1 | 77 ± 1 |
| E-281 kDa | 89 ± 0 | 84 ± 1 | 83 ± 2 | 76 ± 2 |
| E-305 kDa | 91 ± 1 | 84 ± 1 | 82 ± 1 | 80 ± 2 |
| Affinisol® HPMC 100 LV | 103 ± 2 | 90 ± 0 | 87 ± 0 | 84 ± 1 |
| EUDRAGIT® E PO | 42 ± 1 | 41 ± 1 | 40 ± 1 | 34 ± 2 |
| Sample | Saturation Solubility of Celecoxib [µg/mL] | Saturation Solubility of Efavirenz [µg/mL] | Saturation Solubility of Fenofibrate [µg/mL] |
|---|---|---|---|
| Drug substance | 0.6 ± 0.1 | 0.7 ± 0 | 0.1 ± 0 |
| Soluplus® ASD | 34.4 ± 0.3 | 8.8 ± 0 | 1.0 ± 0 |
| Kollidon® VA 64 ASD | 2.5 ± 0.1 | 15.5 ± 0.1 | 8.5 ± 0 |
| Kollidon® 17 PF ASD | 0.6 ± 0 | 0.5 ± 0 | 2.3 ± 0 |
| AQOAT® AS-MMP ASD | 0.7 ± 0 | 0.4 ± 0 | 0.1 ± 0 |
| E-173 kDa ASD | 3.5 ± 0.3 | 2.1 ± 0.2 | 0.9 ± 0.3 |
| E-254 kDa ASD | 3.0 ± 0.1 | 1.9 ± 0.1 | 1.2 ± 0.4 |
| E-281 kDa ASD | 2.7 ± 0.2 | 1.8 ± 0.2 | 0.8 ± 0.2 |
| E-305 kDa ASD | 2.1 ± 0.2 | 1.1 ± 0.1 | 0.6 ± 0.1 |
| Affinisol® HPMC 100 LV ASD | 3.6 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0 |
| EUDRAGIT® E PO ASD | 2.6 ± 0.3 | 1.8 ± 0.1 | 0.4 ± 0 |
| Polymer | Tg (Polymer) [°C] | Tg (Celecoxib ASD) [°C] | Tg (Efavirenz ASD) [°C] | Tg (Fenofibrate ASD) [°C] |
|---|---|---|---|---|
| Soluplus® | 70 ± 1 | 66 ± 2 | 65 ± 1 | 62 ± 3 |
| Kollidon® VA 64 | 106 ± 0 | 99 ± 3 | 86 ± 2 | 92 ± 0 |
| Kollidon® 17 PF | 135 ± 2 | 88 ± 1 | 103 ± 0 | 78 ± 2 |
| AQOAT® AS-MMP | 111 ± 1 | 103 ± 2 | 84 ± 2 | 86 ± 2 |
| E-173 kDa | 78 ± 1 | 78 ± 3 | 74 ± 1 | 70 ± 1 |
| E-254 kDa | 84 ± 1 | 79 ± 2 | 77 ± 0 | 77 ± 0 |
| E-281 kDa | 89 ± 1 | 85 ± 1 | 82 ± 1 | 78 ± 3 |
| E-305 kDa | 91 ± 0 | 85 ± 2 | 80 ± 1 | 70 ± 1 |
| Affinisol® HPMC 100 LV | 103 ± 3 | 91 ± 1 | 98 ± 2 | 94 ± 2 |
| EUDRAGIT® E PO | 41 ± 1 | 40 ± 2 | 38 ± 2 | 34 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmied, F.-P.; Bernhardt, A.; Moers, C.; Meier, C.; Endres, T.; Klein, S. A Novel Aminomethacrylate-Based Copolymer for Solubility Enhancement—From Radical Polymer Synthesis to Manufacture and Characterization of Amorphous Solid Dispersions. Polymers 2022, 14, 1281. https://doi.org/10.3390/polym14071281
Schmied F-P, Bernhardt A, Moers C, Meier C, Endres T, Klein S. A Novel Aminomethacrylate-Based Copolymer for Solubility Enhancement—From Radical Polymer Synthesis to Manufacture and Characterization of Amorphous Solid Dispersions. Polymers. 2022; 14(7):1281. https://doi.org/10.3390/polym14071281
Chicago/Turabian StyleSchmied, Fabian-Pascal, Alexander Bernhardt, Christian Moers, Christian Meier, Thomas Endres, and Sandra Klein. 2022. "A Novel Aminomethacrylate-Based Copolymer for Solubility Enhancement—From Radical Polymer Synthesis to Manufacture and Characterization of Amorphous Solid Dispersions" Polymers 14, no. 7: 1281. https://doi.org/10.3390/polym14071281