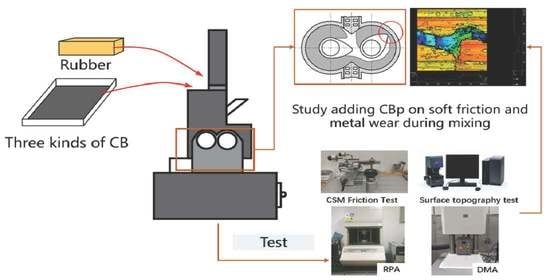
Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Methods
- 1.
- Friction and wear changes between common CBp and the mixer chamber wall in different mixing stages: To study the friction and wear between C-CBp and the mixer chamber wall materials at different stages in the mixing process, compare the test results with ordinary carbon black. The reason why the performance of cracked carbon black rubber is lower than that of ordinary carbon black rubber is analyzed from the surface morphology of rubber and metal materials before and after friction. The sample formula is TSR20: 50 phr, BR9000: 50 phr, CBp: 58 phr.
- 2.
- Influence of replacing I-CB with CBp in different formulations on the friction properties between the compound and mixer chamber metal in the mixing process: C-CBp and M-CBp were replaced with I-CB in the original formula, which was dominated by carbon black or silica, to explore the influence of friction and wear with the mixer chamber wall during mixing. Based on the tribological point of view, the effect of C-CBp and M-CBp replacing I-CB on the friction coefficient between the compound and mixer chamber wall, as well as properties of the compound, were analyzed. By comparing the changes in various properties of rubber to reveal the relationship between the friction coefficient changing and the comprehensive mechanical properties of CB compound. At the same time, the surface wear of the mixing chamber wall was studied after the equivalent amount of C-CBp and M-CBp replaced the I-CB in the original formula in the mixing process. The formulation is shown in Table 1.
2.3. Experimental Process
- (1)
- Comprehensive mechanical property test
- (2)
- Friction and wear test
3. Results and Discussion
3.1. Friction and Wear Changes between Common CBp and Mixer Chamber Wall in Different Mixing Stages
3.2. Influence of Replacing I-CB with CBp in Different Formulations on the Friction Properties between the Compound and Mixer Chamber Metal in the Mixing Process
3.2.1. Comparison of SEM Morphology of Three Kinds of Carbon Black
3.2.2. Changes of Friction and Wear during Mixing and the Relationship between Friction Coefficient and Physical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czajczynska, D.; Krzyzynska, R.; Jouhara, H.; Spencer, N. Use of pyrolytic gasfrom waste tire as a fuel: A review. Energy 2017, 134, 1121–1131. [Google Scholar]
- Lampman, R.; Hanson, S.; Novak, R. Seasonal abundanceand distribution of mosquitoes ata rural waste tire stein illinois. J. Am. Mosq. Control. Assoc. 1997, 13, 193–200. [Google Scholar] [PubMed]
- Jing, W.; Yigang, H.; Yue, W.; Jiankong, W.; Zhipeng, L. Application of waste tire pyrolysis carbon black in the inner liner of all steel radial truck tire. Tire Ind. 2019, 39, 30–33. [Google Scholar]
- Lee, J.M.; Lee, J.S.; Kim, J.R.; Sang, D.K. Pyrolysis of waste tyres with partial oxidation in a fluidized-bed reactor. Energy 1995, 20, 969–976. [Google Scholar]
- Xie, M.; Zhang, R. Pyrolysis treatment of waste tire and its product application. Compr. Util. Tire Resour. China 2015, 7, 44–48. [Google Scholar]
- Wang, F.; Hong, R.Y.; Feng, W.G.; Badami, D.; Zeng, K. Electrical and mechanical propertiesof ABS/EPDM composites filled with carbon black. Mater. Lett. 2014, 125, 48–50. [Google Scholar]
- Amer, M.R.; Alaskar, Y.; Qasem, H. Integration of low-dimensionalmaterials for energy-harvesting applications: Current progress, scope, challenges, and opportunities. Nanotechnol. Rev. 2016, 5, 549–566. [Google Scholar]
- Kameya, Y.; Hayashi, T.; Motosuke, M. Stability of platinum nanoparticles supported on surface-treated carbon black. Appl. Catal. B-Environ. 2016, 189, 219–225. [Google Scholar]
- Jaffe, A.; Valdes, A.S.; Karunadasa, H.I. Quinone-functionalizedcarbon black cathodes for lithium batteries with high power densities. Chem. Mater. 2015, 27, 3568–3571. [Google Scholar]
- Li, S.; Wan, C.; Wu, X.; Wang, S. Core-shell structured carbon nanoparticles derived from light pyrolysis of waste tires. Polym. Degrad. Stab. 2016, 129, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Hoshikawa, Y.; An, B.; Kashihara, S.; Ishii, T.; Ando, M.; Fujisawa, S.; Hayakawa, K.; Hamatani, S.; Yamada, H.; Kyotani, T. Analysis of the interaction between rubber polymer and carbon black surfaces by efficient removal of physisorbed polymer from carbon-rubber composites. Carbon 2016, 99, 148–156. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Choi, H.S.; Park, H.C. Influence of process conditions on product yield of waste tyre pyrolysis—A review. Korean J. Chem. Eng. 2016, 33, 2268–2286. [Google Scholar]
- Wang, C.; Tang, Y.; Chen, G. Application of pyrolysis carbon black eco in inner tube compound of motorcycle tire. Rubber Technol. Mark. 2011, 9, 31–33. [Google Scholar]
- Choi, G.-G.; Jung, S.-H.; Oh, S.-J. Total utilization of waste tire rubber through pyrolysis to obtain oils and CO2 activation of pyrolysis char. Euel Processing Technol. 2014, 123, 57–64. [Google Scholar]
- Yiren, P.; Lin, Z.; Huaqiao, L.; Meng, Z.; Wenwen, H.; Chuansheng, W.; Huiguang, B. Friction and wear between polymer and metal in the mixing process. Materials 2019, 12, 4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, V.; Gupta, S.; Saxena, S. Surface characterization of carbon black obtained from waste tyres. Int. J. Sci. Technol. 2015, 5, 1–7. [Google Scholar]
- Akyıldız, V.; Ozkan, A.; Cokaygil, Z.; Banar, M.; Baydar, S. Improvement of solid product quality in pyrolysis of Tyre Derived Fuels (TDF). Chem. Eng. Technol. 2010, 21, 775–780. [Google Scholar]
- Bik, J.; Głuszewski, W.; Rzymski, W.; Zagórski, Z. EB radiation crosslinking of elastomers. Radiat. Phys. Chem. 2003, 67, 421–423. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, S.; Shen, J.; Jiang, J.; Hu, S.; Zhang, L.; Liu, L. Improved dynamic properties of natural rubber filled with irradiation-modified carbon black. Radiat. Phys. Chem. 2015, 111, 91–97. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S. Heat-treatment of carbon blacks obtained by pvrolvsis ofused tires. Effect on the surface chemistry porosity and electrical conductivity. J. Anal. Appl. Pyrolysis 2003, 67, 55–76. [Google Scholar]


















| Raw Material | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| BR9000 | 25.5 | 25.5 | 25.5 | 25.5 | 25.5 | 25.5 |
| RC2557S | 81.81 | 81.81 | 81.81 | 81.81 | 81.81 | 81.81 |
| TSR20 | 15 | 15 | 15 | 15 | 15 | 15 |
| I-CB | 70 | / | / | 25 | / | / |
| C-CBp | / | 70 | / | / | 25 | / |
| M-CBp | / | / | 70 | / | / | 25 |
| Silica115MP | / | / | / | 45 | 45 | 45 |
| Si69mix | / | / | / | 6 | 6 | 6 |
| DPG | 1 | 1 | 1 | 0.8 | 0.8 | 0.8 |
| S | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| CZ | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 |
| Others | Protection system: 3.5 phr; activation system: 4 phr | |||||
| List | I-CB | C-CBp | M-CBp | I-CB/Silica | C-CBp/Silica | M-CBp/Silica |
|---|---|---|---|---|---|---|
| ML/dNm | 2.42 | 2.42 | 2.38 | 3.33 | 3.74 | 3.56 |
| MH/dNm | 13.77 | 11.53 | 13.57 | 14.25 | 15.8 | 15.15 |
| MH-ML/dNm | 11.35 | 9.11 | 11.19 | 10.92 | 12.06 | 11.59 |
| TS2/min | 5.43 | 8.88 | 5.65 | 5.87 | 5.48 | 5.56 |
| T10/min | 4.27 | 7.51 | 4.56 | 1.67 | 1.86 | 1.74 |
| T50/min | 7.04 | 10.47 | 8.63 | 14.29 | 15.2 | 14.89 |
| T90/min | 12.87 | 15.7 | 14.34 | 28.07 | 33.38 | 29.45 |
| 100% Tensile Modulus/MPa | 3.84 | 3.65 | 3.58 | 3.51 | 3.68 | 3.71 |
| 300% Tensile Modulus/MPa | 8.11 | 4.64 | 7.13 | 5.26 | 5.35 | 5.32 |
| Tensile Strength/MPa | 15.89 | 6.79 | 20.82 | 17.38 | 13.82 | 18.56 |
| Abrasion/% | 0.127 | 0.325 | 0.130 | 0.116 | 0.2377 | 0.195 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Pan, Y.; Wang, Z.; Han, S.; Han, W.; Bian, H. Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing. Polymers 2022, 14, 1319. https://doi.org/10.3390/polym14071319
Pan Y, Pan Y, Wang Z, Han S, Han W, Bian H. Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing. Polymers. 2022; 14(7):1319. https://doi.org/10.3390/polym14071319
Chicago/Turabian StylePan, Yiren, Yi Pan, Zhilin Wang, Shuang Han, Wenwen Han, and Huiguang Bian. 2022. "Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing" Polymers 14, no. 7: 1319. https://doi.org/10.3390/polym14071319
APA StylePan, Y., Pan, Y., Wang, Z., Han, S., Han, W., & Bian, H. (2022). Effect of Adding Pyrolysis Carbon Black (CBp) on Soft Friction and Metal Wear during Mixing. Polymers, 14(7), 1319. https://doi.org/10.3390/polym14071319