Improved Thermal and Electromagnetic Shielding of PEEK Composites by Hydroxylating PEK-C Grafted MWCNTs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.3. Composites Synthesis
2.3.1. Synthesis of Hydroxyphenolphthalein Polyether Ketones
2.3.2. Synthesis of Hydroxyphenolphthalein Polyetherketone Grafted Carbon Nanotubes
2.3.3. Synthesis of Hydroxyphenolphthalein-Based Polyetheretherketone Grafted Carbon Nanotube/Polyetheretheretherketone Composites
3. Results and Discussion
3.1. Characteristics of Specimens
3.2. Composite Performance
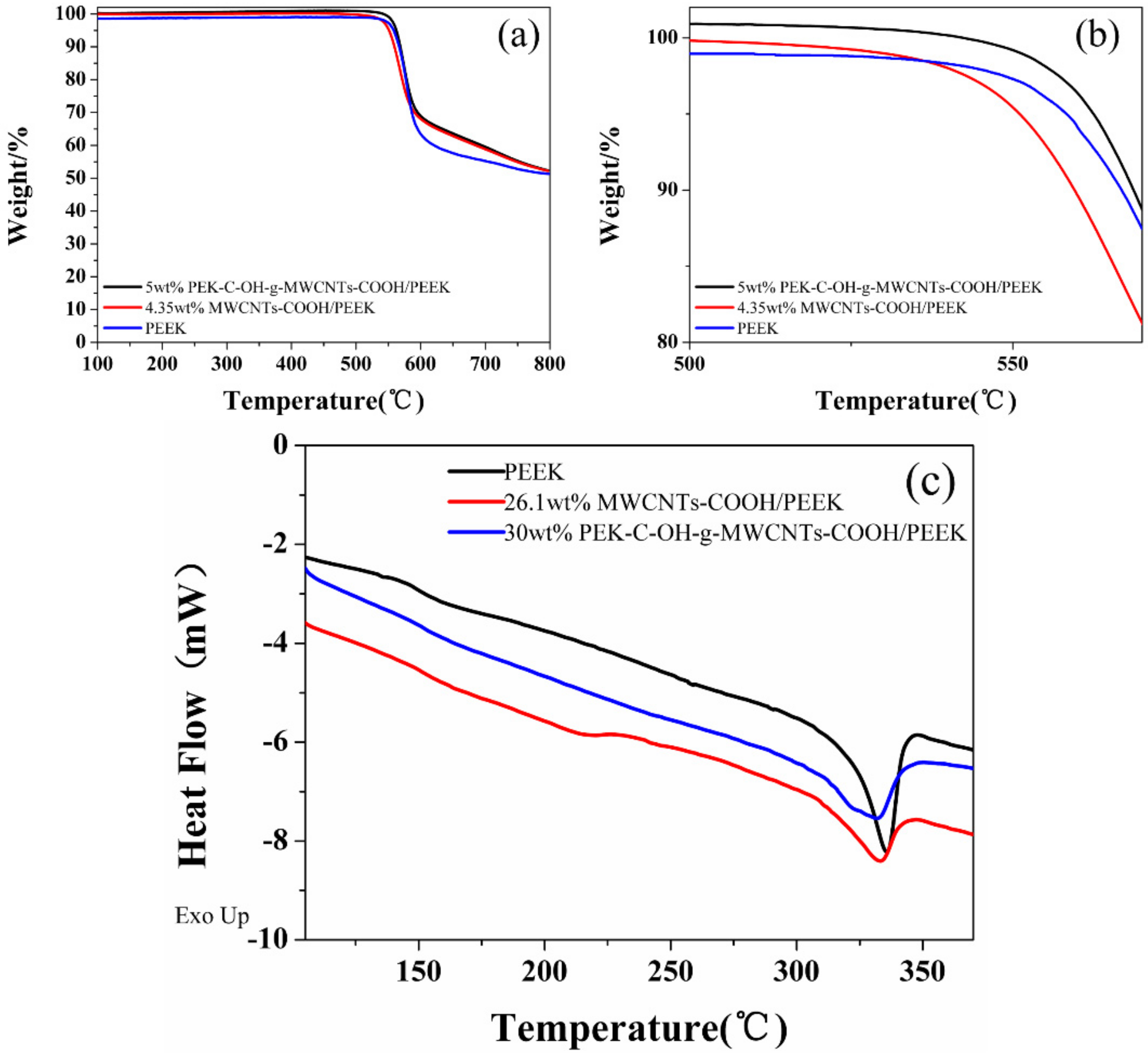
3.2.1. Thermal Performance Analysis of MWCNTs/PEEK Composites
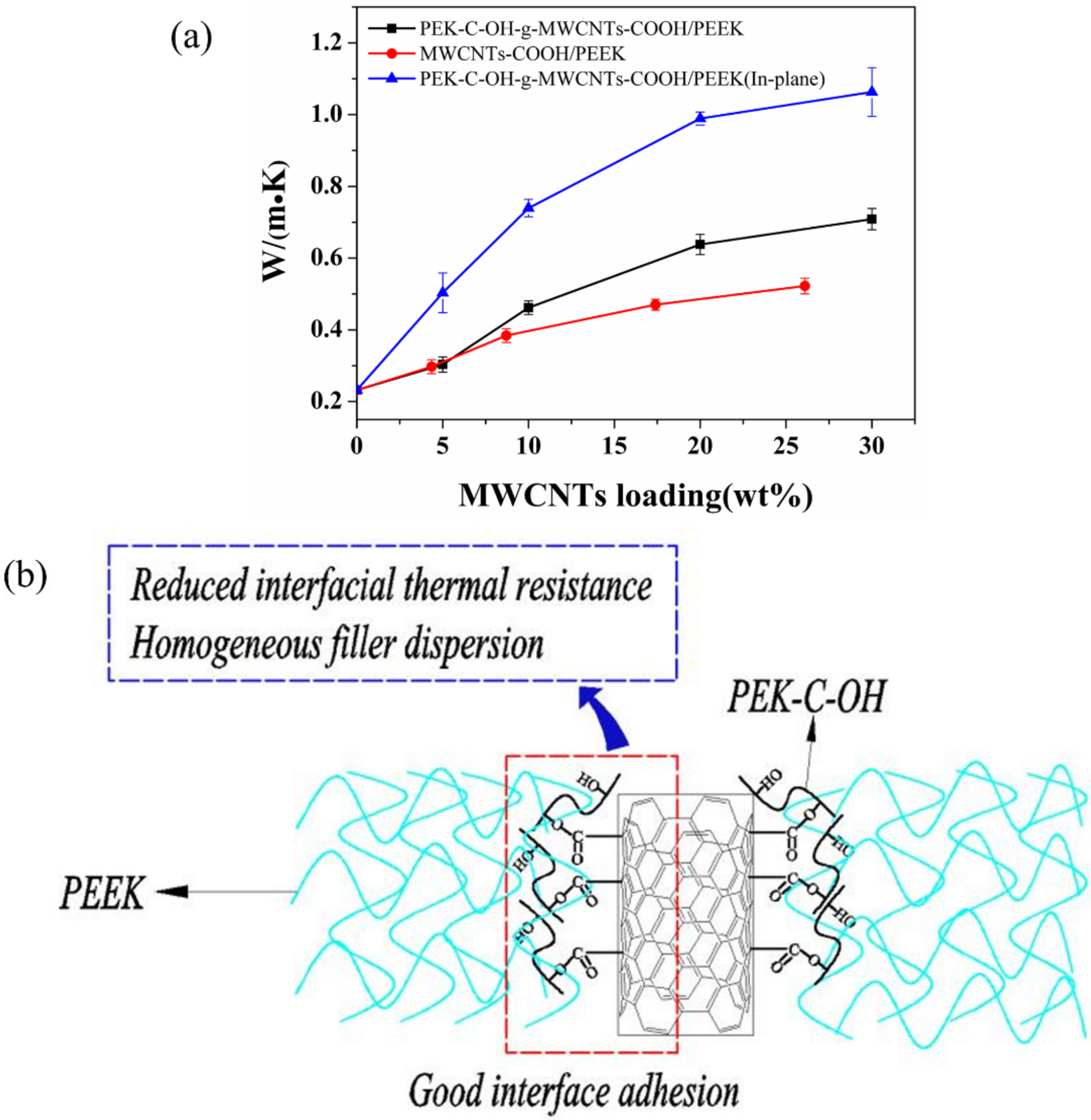
3.2.2. Thermal Conductivity Analysis of MWCNTs/PEEK Composites
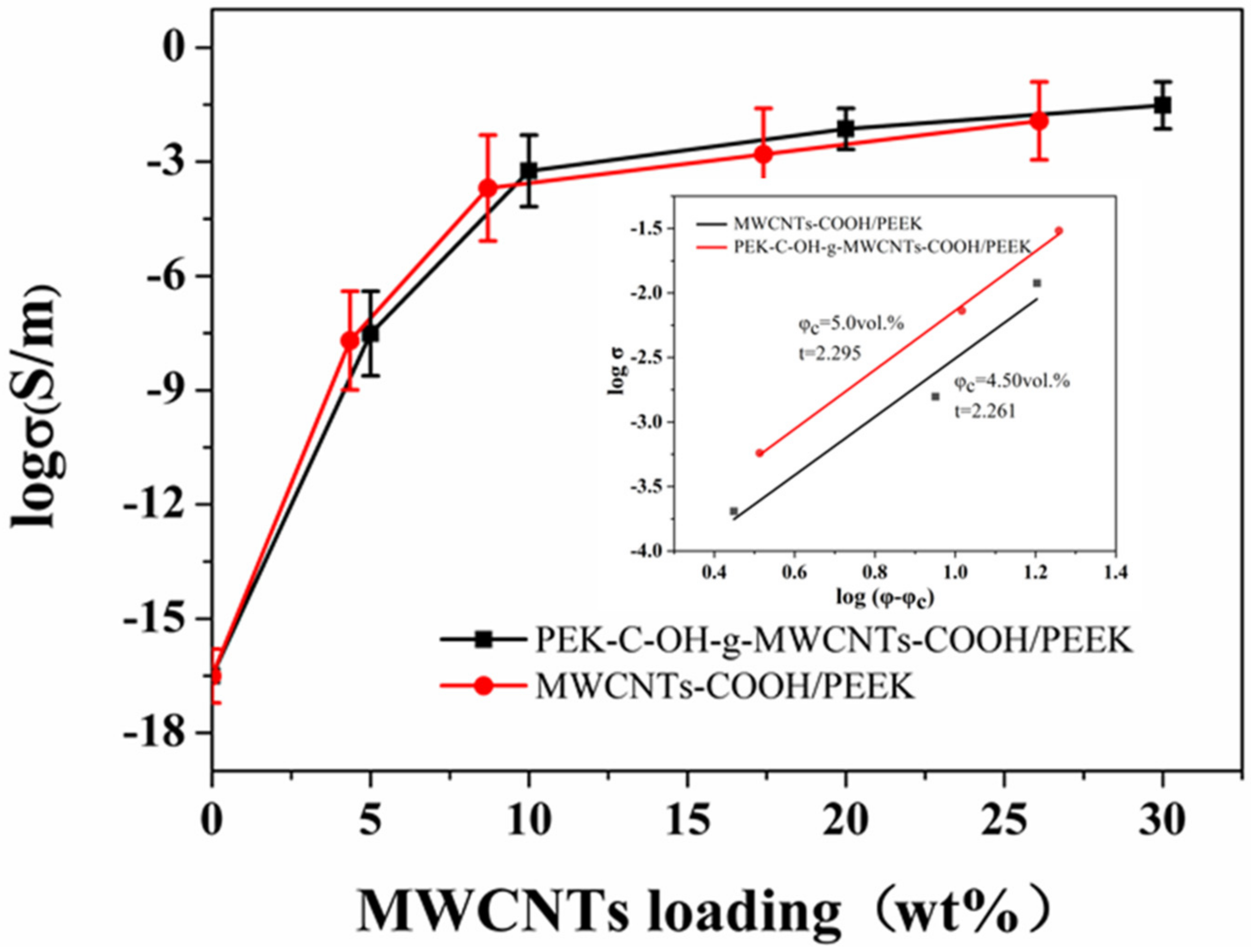
3.2.3. Electrical Conductivity and Electromagnetic Shielding Effectiveness Analysis of MWCNTs/PEEK Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, H.C.; Lee, C.Y.; Tai, N.H. Extremely high thermal conductivity of nanodiamond-polydopamine/thin-layer graphene composite films. Compos. Sci. Technol. 2018, 167, 313–322. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral oligosilsesquioxane-modified boron nitride nanotube based epoxy nanocomposites: An ideal dielectric material with high thermal conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Q.; Lv, Y.; Liu, X.; Wang, X.; Huang, P.Y.; Cahill, D.G.; Lv, B. High thermal conductivity in cubic boron arsenide crystals. Science 2018, 361, 579–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Ren, Z. High Thermal Conductivity in Boron Arsenide: From Prediction to Reality. Angew. Chem. 2019, 131, 5882–5889. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Gao, Q.; Tang, M.; Ma, Z.; Qin, J.; Wang, M.; Kim, J.K. Multifunctional microcellular PVDF/Ni-chains composite foams with enhanced electromagnetic interference shielding and superior thermal insulation performance. Chem. Eng. J. 2020, 379, 122304. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, H.; Liang, C.; Song, P.; Han, Y.; Han, Y.; Gu, J.; Kong, J.; Pan, D.; Guo, Z. Electromagnetic interference shielding MWCNT-Fe3O4@Ag/epoxy nanocomposites with satisfactory thermal conductivity and high thermal stability. Carbon 2019, 141, 506–514. [Google Scholar] [CrossRef]
- Sefadi, J.S.; Luyt, A.S.; Pionteck, J.; Piana, F.; Gohs, U. Effect of surfactant and electron treatment on the electrical and thermal conductivity as well as thermal and mechanical properties of ethylene vinyl acetate/expanded graphite composites. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Kang, H.; Wei, Q.; Guo, B.; Zhang, L.; Jia, D. Incorporation of graphene into polyester/carbon nanofibers composites for better multi-stimuli responsive shape memory performances. Carbon 2013, 64, 487–498. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal transport measurements of individual multiwalled nanotubes. Phys. Rev. Lett. 2001, 87, 215502-1–215502-4. [Google Scholar] [CrossRef] [Green Version]
- Mehra, N.; Mu, L.; Ji, T.; Yang, X.; Kong, J.; Gu, J.; Zhu, J. Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 2018, 12, 92–130. [Google Scholar] [CrossRef]
- Dong, P.; Long, C.; Peng, Y.; Peng, X.; Guo, F. Effect of coatings on thermal conductivity and tribological properties of aluminum foam/polyoxymethylene interpenetrating composites. J. Mater. Sci. 2019, 54, 13135–13146. [Google Scholar] [CrossRef]
- Lin, T.; Bajpai, V.; Ji, T.; Dai, L. Chemistry of carbon nanotubes. Aust. J. Chem. 2003, 56, 635–651. [Google Scholar] [CrossRef]
- Luo, T.; Lloyd, J.R. Enhancement of thermal energy transport across graphene/graphite and polymer interfaces: A molecular dynamics study. Adv. Funct. Mater. 2012, 22, 2495–2502. [Google Scholar] [CrossRef]
- Ding, P.; Zhuang, N.; Cui, X.; Shi, L.; Song, N.; Tang, S. Enhanced thermal conductive property of polyamide composites by low mass fraction of covalently grafted graphene nanoribbons. J. Mater. Chem. C 2015, 3, 10990–10997. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Huang, C.; Su, F.; Wu, B.; Sun, Z.; Xu, F.; Sun, L.; Xia, Y.; Peng, H.; et al. Construction of double cross-linking PEG/h-BN@GO polymeric energy-storage composites with high structural stability and excellent thermal performances. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128193. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Castro, M.; Morris, J.A.; Ríos, R.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Noiset, O.; Schneider, Y.J.; Marchand-Brynaert, J. Surface modification of poly(aryl ether ether ketone) (PEEK) film by covalent coupling of amines and amino acids through a spacer arm. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 3779–3790. [Google Scholar] [CrossRef]
- Feng, S.; Shang, Y.; Liu, G.; Dong, W.; Xie, X.; Xu, J.; Mathur, V.K. Novel modification method to prepare crosslinked sulfonated poly(ether ether ketone)/silica hybrid membranes for fuel cells. J. Power Sources 2010, 195, 6450–6458. [Google Scholar] [CrossRef]
- Xu, R.; He, L.; Li, L.; Hou, M.; Wang, Y.; Zhang, B.; Liang, C.; Wang, T. Ultraselective carbon molecular sieve membrane for hydrogen purification. J. Energy Chem. 2020, 50, 16–24. [Google Scholar] [CrossRef]
- Shaheen, H.A.; Marwani, H.M.; Soliman, E.M. Selective solid phase extraction and determination of trace Pd(II) using multi-walled carbon nanotubes modified with 8-aminoquinoline. J. Mol. Liq. 2017, 232, 139–146. [Google Scholar] [CrossRef]
- Wisdom, K.S.; Bhat, I.A.; Chanu, T.I.; Kumar, P.; Pathakota, G.B.; Nayak, S.K.; Walke, P.; Sharma, R. Chitosan grafting onto single-walled carbon nanotubes increased their stability and reduced the toxicity in vivo (catfish) model. Int. J. Biol. Macromol. 2020, 155, 697–707. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Sinani, V.A.; Gheith, M.K.; Yaroslavov, A.A.; Rakhnyanskaya, A.A.; Sun, K.; Mamedov, A.A.; Wicksted, J.P.; Kotov, N.A. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. J. Am. Chem. Soc. 2005, 127, 3463–3472. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Q.; Rochette, J.F.; Sacher, E. Controlled chemical functionalization of multiwalled carbon nanotubes by kiloelectronvolt argon ion treatment and air exposure. Langmuir 2005, 21, 8539–8545. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liao, X.; Sheng, X.; Hao, Z.; He, L.; Liu, P.; Quan, H.; Zhang, Y. Effect of structure regulation of hyper-branched polyester modified carbon nanotubes on toughening performance of epoxy/carbon nanotube nanocomposites. RSC Adv. 2019, 9, 12864–12876. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Peng, Q.; Zhao, X.; Sun, H.; Wang, S.; Zhu, Y.; Liu, Z.; Wang, C.; He, X. Grafting carbon nanotubes densely on carbon fibers by poly(propylene imine) for interfacial enhancement of carbon fiber composites. Carbon 2020, 158, 704–710. [Google Scholar] [CrossRef]
- Papagelis, K.; Kalyva, M.; Tasis, D.; Parthenios, J.; Siokou, A.; Galiotis, C. Covalently functionalized carbon nanotubes as macroinitiators for radical polymerization. Phys. Status Solidi Basic Res. 2007, 244, 4046–4050. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Yang, K.; Gu, M.; Guo, Y.; Pan, X.; Mu, G. Effects of carbon nanotube functionalization on the mechanical and thermal properties of epoxy composites. Carbon 2009, 47, 1723–1737. [Google Scholar] [CrossRef]
- Dewapriya, M.A.N.; Miller, R.E. Molecular dynamics study of the penetration resistance of multilayer polymer/ceramic nanocomposites under supersonic projectile impacts. Extrem. Mech. Lett. 2021, 44, 101238. [Google Scholar] [CrossRef]
- Román-Manso, B.; Moyano, J.J.; Pérez-Coll, D.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Polymer-derived ceramic/graphene oxide architected composite with high electrical conductivity and enhanced thermal resistance. J. Eur. Ceram. Soc. 2018, 38, 2265–2271. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Antonowicz, M.; Paszenda, Z. Surface modification methods of ceramic filler in ceramic-carbon fibre composites for bioengineering applications—A systematic review. Rev. Adv. Mater. Sci. 2020, 59, 586–609. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A ‘defect’ peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Blokhin, A.; Stolyarov, R.; Burmistrov, I.; Gorshkov, N.; Kolesnikov, E.; Yagubov, V.; Tkachev, A.; Zaytsev, I.; Tarov, D.; Galunin, E.; et al. Increasing electrical conductivity of PMMA-MWCNT composites by gas phase iodination. Compos. Sci. Technol. 2021, 214, 108972. [Google Scholar] [CrossRef]
- Imai, M.; Akiyama, K.; Tanaka, T.; Sano, E. Highly strong and conductive carbon nanotube/cellulose composite paper. Compos. Sci. Technol. 2010, 70, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. A comparative study of EMI shielding properties of carbon nanofiber and multi-walled carbon nanotube filled polymer composites. J. Nanosci. Nanotechnol. 2005, 5, 927–931. [Google Scholar] [CrossRef]
- Jelmy, E.J.; Lakshmanan, M.; Kothurkar, N.K. Microwave absorbing behavior of glass fiber reinforced MWCNT-PANi/epoxy composite laminates. Mater. Today Proc. 2018, 26, 36–43. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Yan, Q.; Zheng, W.G.; He, Z.; Yu, Z.Z. Tough graphene-polymer microcellular foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2011, 3, 918–924. [Google Scholar] [CrossRef]
- Liu, H.; Liang, C.; Chen, J.; Huang, Y.; Cheng, F.; Wen, F.; Xu, B.; Wang, B. Novel 3D network porous graphene nanoplatelets /Fe3O4/epoxy nanocomposites with enhanced electromagnetic interference shielding efficiency. Compos. Sci. Technol. 2019, 169, 103–109. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Han, L.; Lin, J.; Jin, P. High-performance hierarchical graphene/metal-mesh film for optically transparent electromagnetic interference shielding. Carbon 2017, 115, 34–42. [Google Scholar] [CrossRef]


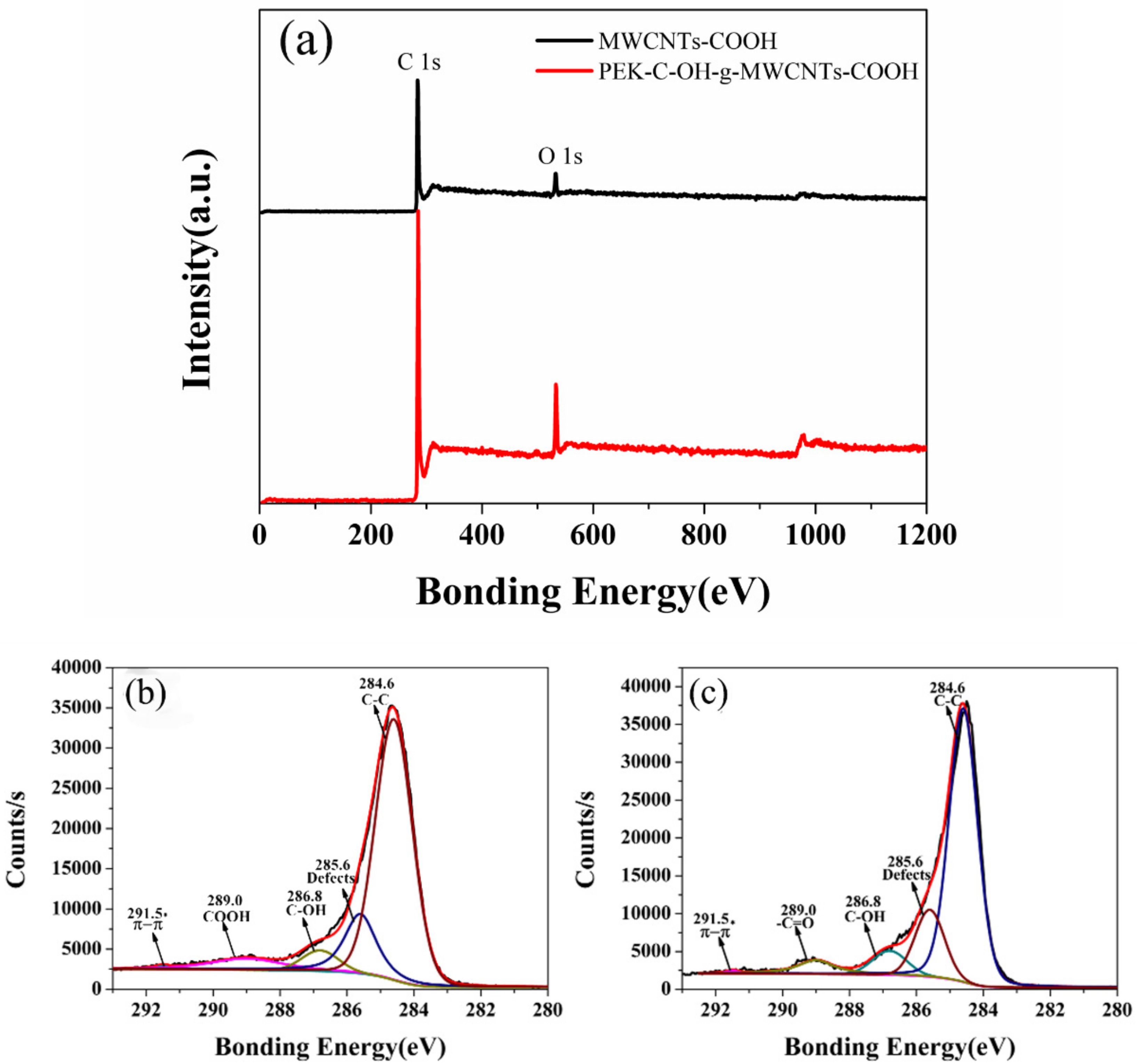





| Samples | D/cm−1 | G/cm−1 | ID | IG | ID/IG |
|---|---|---|---|---|---|
| MWCNTs-COOH | 1335.0 | 1572.2 | 24,808.3 | 23,174.2 | 1.07 |
| PEK-C-OH-g-MWCNTs-COOH | 1335.0 | 1567.5 | 61,982.3 | 55,392.0 | 1.12 |
| Samples | C1s(%) | O1s(%) | nO/nC |
|---|---|---|---|
| MWCNTs-COOH | 91.96 | 8.04 | 0.087 |
| PEK-C-OH-g-MWCNTs-COOH | 88.59 | 11.41 | 0.12 |
| MWCNTs-COOH | PEK-C-OH-g-MWCNTs-COOH | |||
|---|---|---|---|---|
| E(eV) | A(%) | E(eV) | A(%) | Assignation |
| 291.5 | 0.44 | 291.5 | 0.435 | π-π |
| 289.0 | 7.35 | 289 | 6.15 | -COOH(-C=O) |
| 286.8 | 5.27 | 286.8 | 6.88 | C-OH |
| 284.6 | 69.82 | 284.6 | 69.75 | C-C |
| 285.6 | 17.12 | 285.6 | 16.585 | Defects |
| Samples | Tg/°C | Tm/°C |
|---|---|---|
| PEEK | 148.63 | 335.66 |
| 26.1 wt%MWCNTs-COOH/PEEK | 150.77 | 332.72 |
| 30 wt%PEK-C-OH-g-MWCNTs-COOH/PEEK | 152.27 | 331.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, F.; Li, S.; Chen, R.; He, Y.; Li, L.; Cheng, L.; Ma, J.; Mu, J. Improved Thermal and Electromagnetic Shielding of PEEK Composites by Hydroxylating PEK-C Grafted MWCNTs. Polymers 2022, 14, 1328. https://doi.org/10.3390/polym14071328
Wen F, Li S, Chen R, He Y, Li L, Cheng L, Ma J, Mu J. Improved Thermal and Electromagnetic Shielding of PEEK Composites by Hydroxylating PEK-C Grafted MWCNTs. Polymers. 2022; 14(7):1328. https://doi.org/10.3390/polym14071328
Chicago/Turabian StyleWen, Fengyu, Shu Li, Rui Chen, Yashu He, Lei Li, Lin Cheng, Jierun Ma, and Jianxin Mu. 2022. "Improved Thermal and Electromagnetic Shielding of PEEK Composites by Hydroxylating PEK-C Grafted MWCNTs" Polymers 14, no. 7: 1328. https://doi.org/10.3390/polym14071328
APA StyleWen, F., Li, S., Chen, R., He, Y., Li, L., Cheng, L., Ma, J., & Mu, J. (2022). Improved Thermal and Electromagnetic Shielding of PEEK Composites by Hydroxylating PEK-C Grafted MWCNTs. Polymers, 14(7), 1328. https://doi.org/10.3390/polym14071328





