Optimized Anticorrosion of Polypyrrole Coating by Inverted-Electrode Strategy: Experimental and Molecular Dynamics Investigations
Abstract
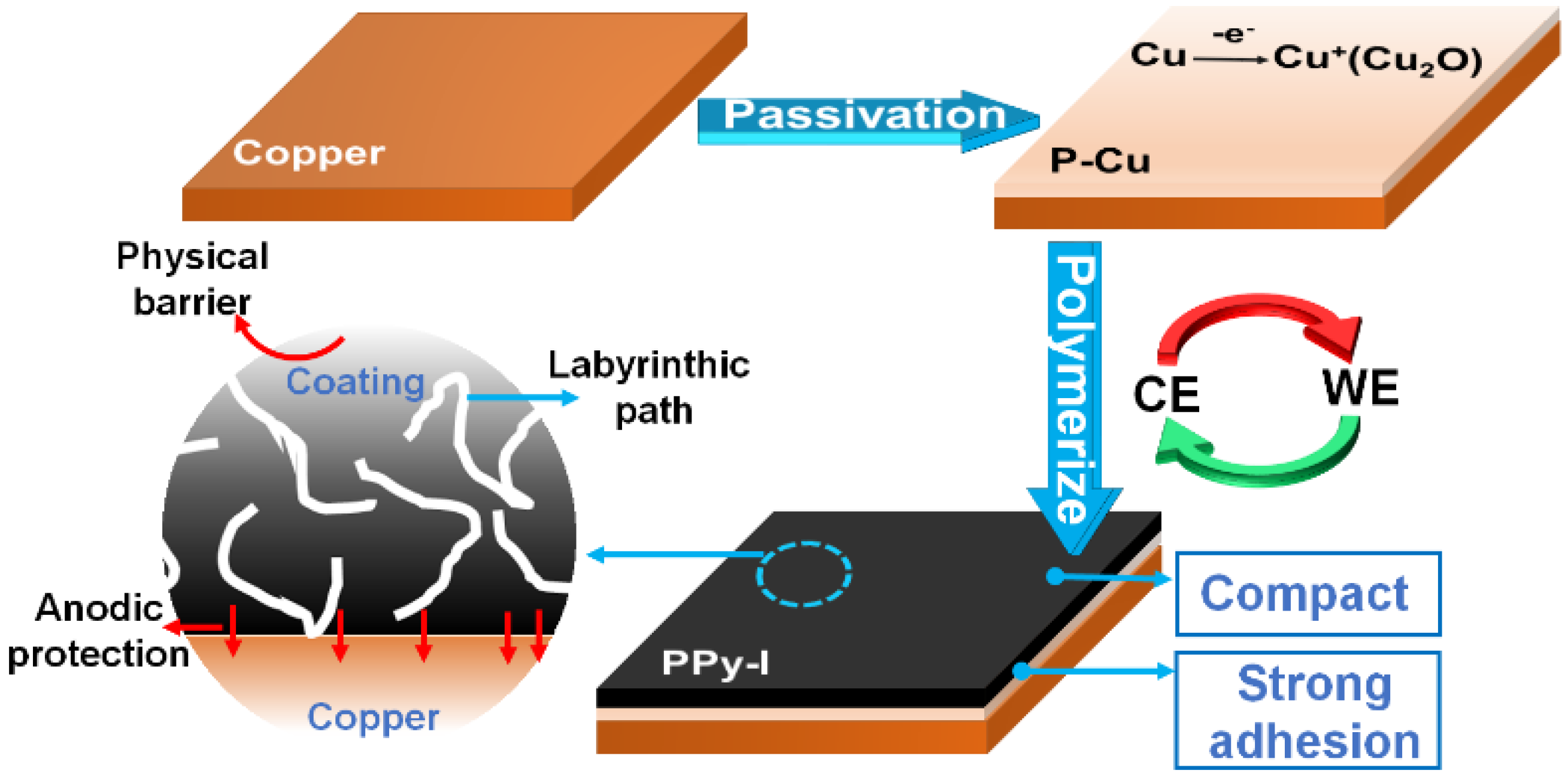
:1. Introduction
2. Materials and Methods
2.1. Materials and Solutions
2.2. Electropolymerization Procedure
2.3. Characterization of Electropolymerized Coatings
2.4. Electrochemical Evaluations
2.5. Immersion Tests
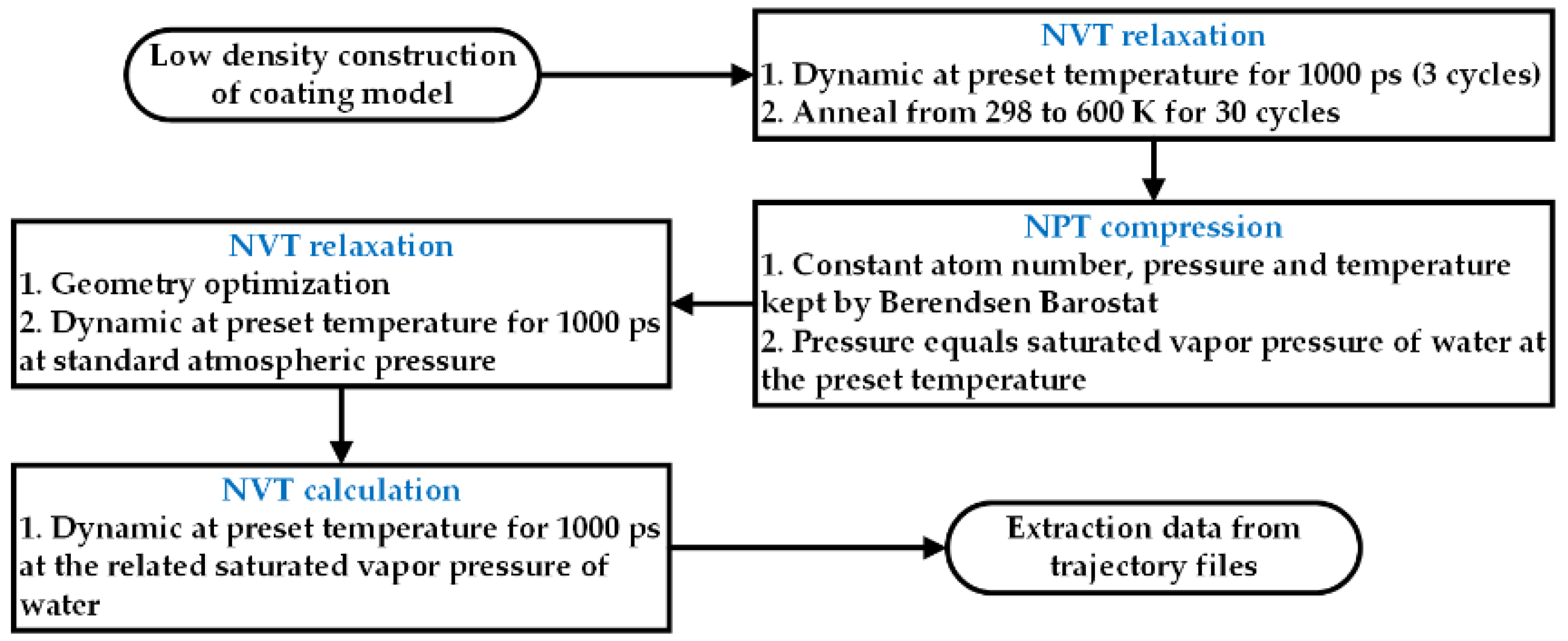
2.6. Theoretical Modeling
3. Results and Discussion
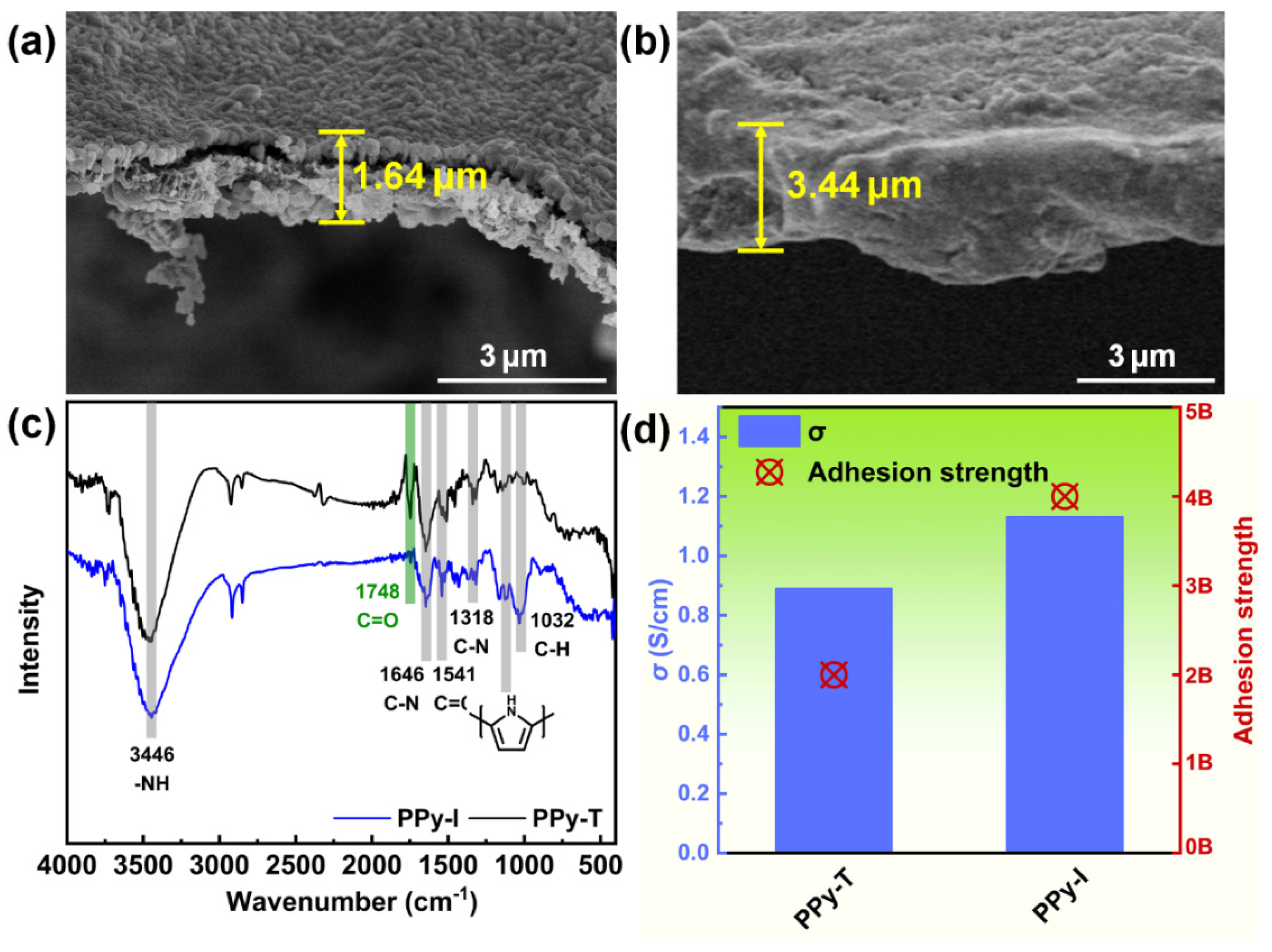
3.1. Preparation and Characterization of PPy Coatings
3.2. Electrochemical Analyses
3.2.1. Electrochemical Thermodynamics and Kinetics
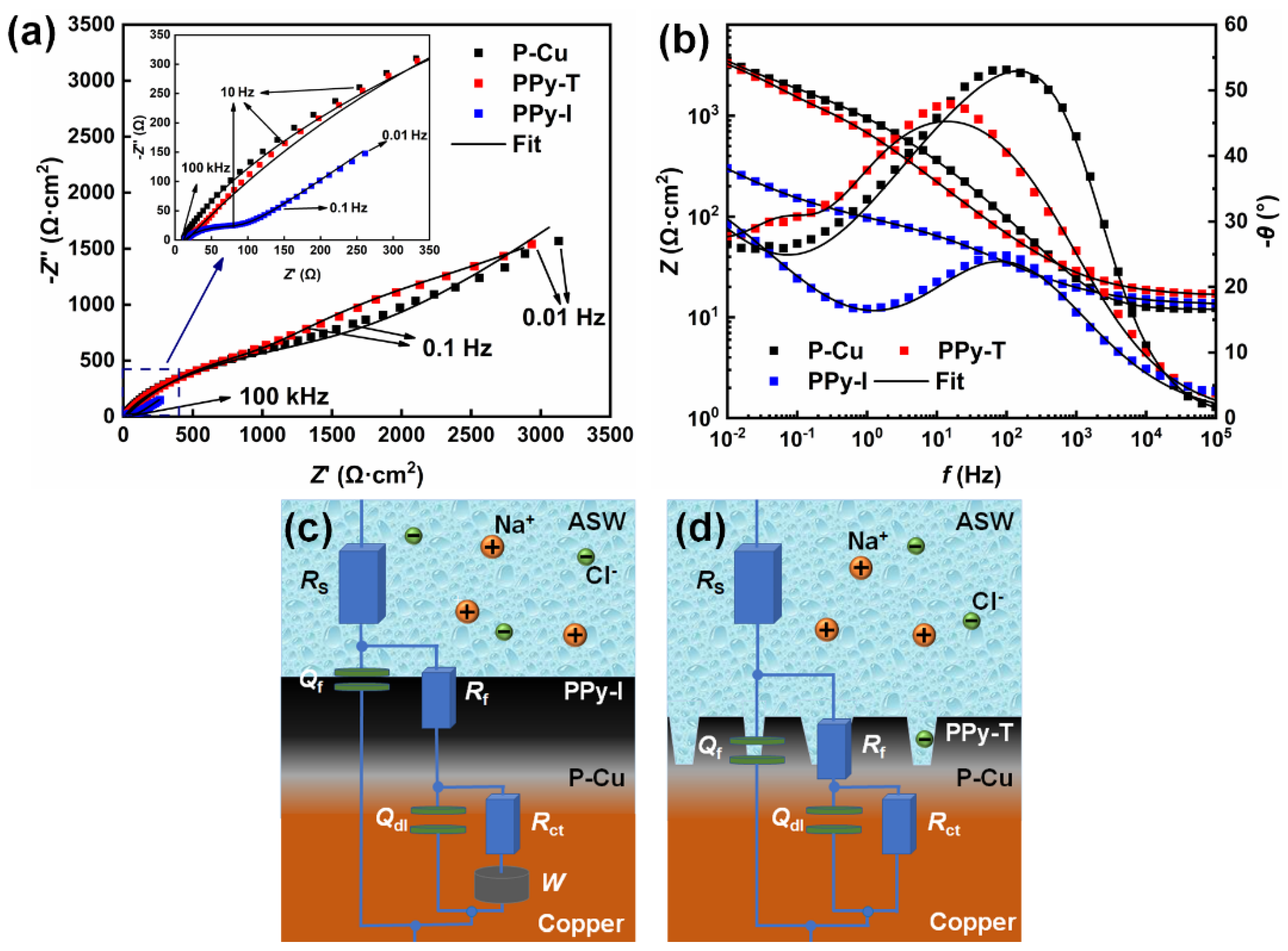
3.2.2. EIS
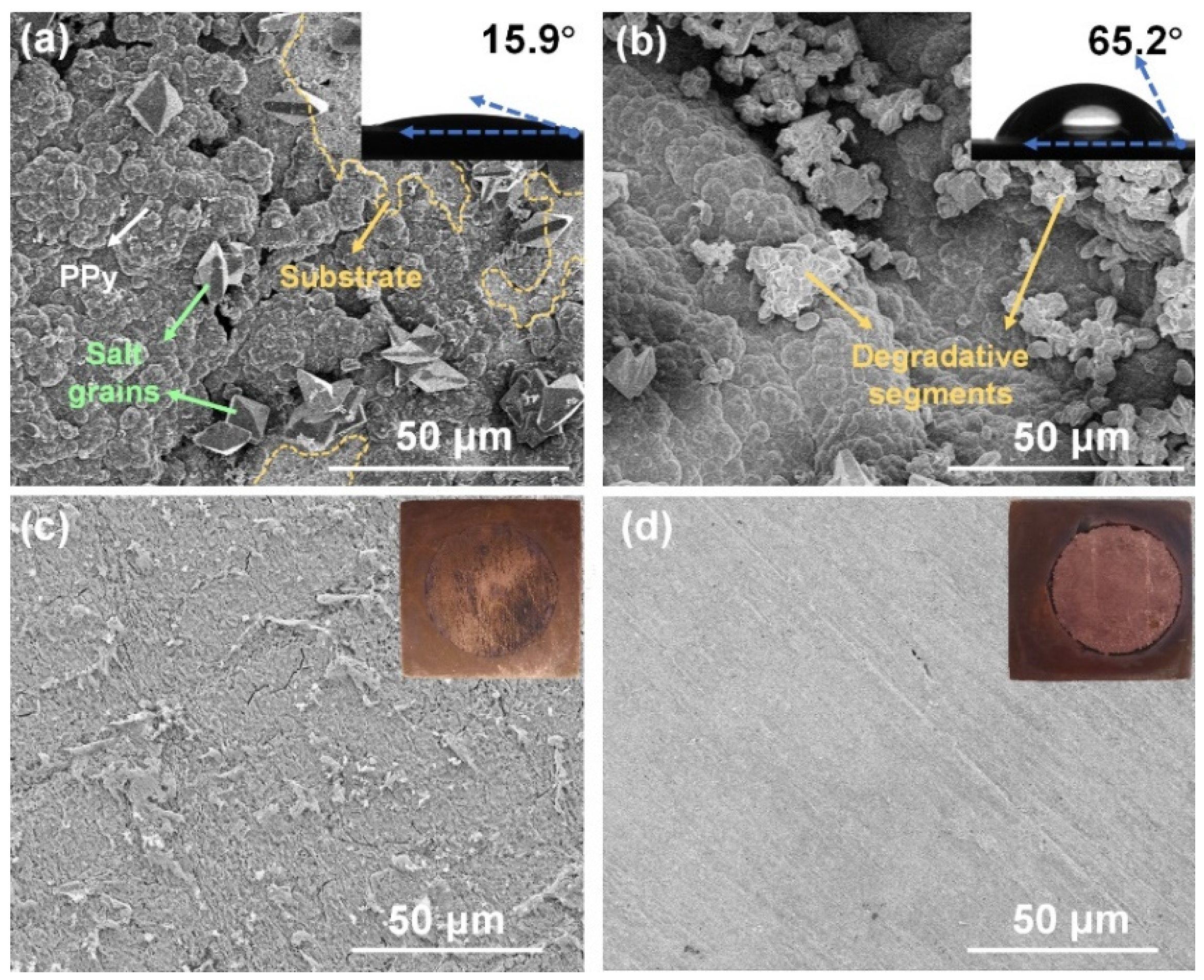
3.3. Surface Examination
3.4. Theoretical Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Peng, L.L.; Ding, Y.; Zhao, Y.; Yu, G.H. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayathilaka, W.A.D.M.; Chinnappan, A.; Tey, J.N.; Wei, J.; Ramakrishna, S. Alternative Current Electroluminescence and Flexible Light Emitting Devices. J. Mater. Chem. C 2019, 7, 5553–5572. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.J.; Yu, G.H. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadeh, M.K.; Yeganeh, M.; Shoushtari, M.T.; Esmaeilkhanian, A. Corrosion Performance of Polypyrrole-Coated Metals: A Review of Perspectives and Recent Advances. Synth. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.J.; Yu, G.H. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Çakmakcı, İ.; Duran, B.; Duran, M.; Bereket, G. Experimental and Theoretical Studies on Protective Properties of Poly(Pyrrole-co-N-Methyl Pyrrole) Coatings on Copper in Chloride Media. Corros. Sci. 2013, 69, 252–261. [Google Scholar] [CrossRef]
- Bahramian, A.; Eyraud, M.; Maria, S.; Vacandio, F.; Djenizian, T.; Knauth, P. Enhancing the Corrosion Resistance of Cu/Ni-P/Au Electrical Contacts by Electropolymerized Poly(Methyl Methacrylate). Corros. Sci. 2019, 149, 75–86. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Jadhav, N.G.; Gelling, V.J.; Sazou, D. Conducting Polymers for Corrosion Protection: A Review. J. Coat. Technol. Res. 2014, 11, 473–494. [Google Scholar] [CrossRef]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Feng, Y.; Zhang, X.; Guo, R.; Yan, H. Flatness and Corrosion Protection of Polypyrrole Film Enhanced by Two-Dimensional g-C3N4 Nanosheets. Mater. Today Commun. 2021, 29, 102934. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Salvo-Comino, C.; Garcia-Hernandez, C.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. Nanocomposites of Conductive Polymers and Nanoparticles Deposited on Porous Material as a Strategy to Improve Its Corrosion Resistance. Surf. Coat. Technol. 2020, 403, 126395. [Google Scholar] [CrossRef]
- Jin, Y.J.; Chen, Z.H.; Yang, W.Z.; Yin, X.S.; Chen, Y.; Liu, Y. Electrosynthesis of Molybdate-Doped P(ANI-co-PY) Copolymer Coating in Ionic Liquid for Corrosion Protection of 304 Stainless Steel. J. Taiwan Inst. Chem. Eng. 2020, 117, 171–181. [Google Scholar] [CrossRef]
- Akula, S.; Kalaiselvi, P.; Sahu, A.K.; Chellammal, S. Electrodeposition of Conductive PAMT/PPY Bilayer Composite Coatings on 316L Stainless Steel Plate for PEMFC Application. Int. J. Hydrogen Energy 2021, 46, 17909–17921. [Google Scholar] [CrossRef]
- Polishchuk, P. Interpretation of Quantitative Structure–Activity Relationship Models: Past, Present, and Future. J. Chem. Inf. Model. 2017, 57, 2618–2639. [Google Scholar] [CrossRef] [PubMed]
- Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. The Role of Chrome and Zinc Free-Based Neodymium Oxide Nanofilm on Adhesion and Corrosion Protection Properties of Polyester/Melamine Coating on Mild Steel: Experimental and Molecular Dynamics Simulation Study. J. Clean. Prod. 2019, 210, 872–886. [Google Scholar] [CrossRef]
- Moradi, M.; Rezaei, M. Construction of Highly Anti-Corrosion and Super-Hydrophobic Polypropylene/Graphene Oxide Nanocomposite Coatings on carbon Steel: Experimental, Electrochemical and Molecular Dynamics Studies. Constr. Build. Mater. 2022, 317, 126136. [Google Scholar] [CrossRef]
- Liu, H.; Fan, B.M.; Fan, G.F.; Zhao, X.Q.; Liu, Z.N.; Hao, H.; Yang, B. Long-Term Protective Mechanism of Poly(N-Methylaniline)/Phosphate One-Step Electropolymerized Coatings for Copper in 3.5% NaCl Solution. J. Alloy. Compd. 2021, 872, 159752. [Google Scholar] [CrossRef]
- Liu, H.; Fan, B.M.; Fan, G.F.; Ma, Y.C.; Hao, H.; Zhang, W. Anti-Corrosive Mechanism of Poly (N-Ethylaniline)/Sodium Silicate Electrochemical Composites for Copper: Correlated Experimental and in-silico Studies. J. Mater. Sci. Technol. 2021, 72, 202–216. [Google Scholar] [CrossRef]
- Wan, S.; Miao, C.H.; Wang, R.M.; Zhang, Z.F.; Dong, Z.H. Enhanced Corrosion Resistance of Copper by Synergetic Effects of Silica and BTA Codoped in Polypyrrole Film. Prog. Org. Coat. 2019, 129, 187–198. [Google Scholar] [CrossRef]
- Nautiyal, A.; Qiao, M.Y.; Cook, J.E.; Zhang, X.Y.; Huang, T.S. High Performance Polypyrrole Coating for Corrosion Protection and Biocidal Applications. Appl. Surf. Sci. 2018, 427, 922–930. [Google Scholar] [CrossRef]
- Kumar, A.M.; Adesina, A.Y.; Hussein, M.A.; Umoren, S.A.; Ramakrishna, S.; Saravanan, S. Preparation and Characterization of Pectin/Polypyrrole Based Multifunctional Coatings on TiNbZr Alloy for Orthopaedic Applicationse. Carbohyd. Polym. 2020, 242, 116285. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Liu, H.; Fan, G.F.; Hao, H.; Yang, B. Enhanced Corrosion Inhibition of Aniline Derivatives Electropolymerized Coatings on Copper: Preparation, Characterization and Mechanism Modeling. Appl. Surf. Sci. 2020, 514, 146086. [Google Scholar] [CrossRef]
- Liu, H.; Fan, B.M.; Liu, Z.N.; Zhao, X.Q.; Yang, B.; Zheng, X.W.; Hao, H. Electronic Effects on Protective Mechanism of Electropolymerized Coatings Based on N-Substituted Aniline Derivatives for Mild Steel in Saline Solution. J. Ind. Eng. Chem. 2021, 106, 297–310. [Google Scholar] [CrossRef]
- Wang, W.K.; Xu, H.T.; Zhao, W.W.; Zhao, J.D.; Jiang, M.Y.; Liu, S.J.; Huang, W.; Zhao, Q. Porphyrin-Assisted Synthesis of Hierarchical Flower-like Polypyrrole Arrays Based Flexible Electrode with High Areal Capacitance. Chem. Eng. J. 2022, 428, 131089. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yang, W.Z.; Yin, X.S.; Chen, Y.; Liu, Y.; Xu, B. Corrosion Protection of 304 Stainless Steel from A Smart Conducting Polypyrrole Coating Doped with pH-Sensitive Molybdate-Loaded TiO2 Nanocontainers. Prog. Org. Coat. 2020, 146, 105750. [Google Scholar] [CrossRef]
- Meng, S.G.; Liu, Z.N.; Zhao, X.Q.; Fan, B.M.; Liu, H.; Guo, M.; Hao, H. Efficient Corrosion Inhibition by Sugarcane Purple Rind Extract for Carbon Steel in HCl Solution: Mechanism Analyses by Experimental and in silico Insights. RSC Adv. 2021, 11, 31693–31711. [Google Scholar] [CrossRef]
- Fan, B.M.; Hao, H.; Guo, A.R.; Yang, R.P. Fabrication and Evaluation of An Attapulgite Membrane as the Filter for Recycling Blowdown Water from Industrial Boilers. J. Water Reuse Desal. 2016, 6, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Menkuer, M.; Ozkazanc, H. Electrodeposition of Polypyrrole on Copper Surfaces in OXA-DBSA Mix Electrolyte and Their Corrosion Behaviour. Prog. Org. Coat. 2019, 130, 149–157. [Google Scholar] [CrossRef]
- Li, D.J.; Zhao, X.Q.; Liu, Z.N.; Liu, H.; Fan, B.M.; Yang, B.; Zheng, X.W.; Li, W.Z.; Zou, H.J. Synergetic Anticorrosion Mechanism of Main Constituents in Chinese Yam Peel for Copper in Artificial Seawater. ACS Omega 2021, 6, 29965–29981. [Google Scholar] [CrossRef]
- Fan, G.F.; Liu, H.; Fan, B.M.; Ma, Y.C.; Hao, H.; Yang, B. Trazodone as an Efficient Corrosion Inhibitor for Carbon Steel in Acidic and Neutral Chloride-Containing Media: Facile Synthesis, Experimental and Theoretical Evaluations. J. Mol. Liq. 2020, 311, 113302. [Google Scholar] [CrossRef]
- Shahryari, Z.; Gheisari, K.; Yeganeh, M.; Ramezanzadeh, B. Corrosion Mitigation Ability of Differently Synthesized Polypyrrole (PPy-FeCl3 & PPy-APS) Conductive Polymers Modified with Na2MoO4 on Mild Steel in 3.5% NaCl Solution: Comparative Study and Optimization. Corros. Sci. 2021, 193, 109894. [Google Scholar] [CrossRef]
- Cao, H.J.; Fang, M.X.; Jia, W.H.; Liu, X.D.; Xu, Q.J. Remarkable Improvement of Corrosion Resistance of Silane Composite Coating with Ti3C2Tx MXene on Copper. Compos. Part B Eng. 2022, 228, 109427. [Google Scholar] [CrossRef]
- Wang, M.X.; Yun, H.; Tan, K.Q.; Guo, A.Q.; Ling, J.W.; Jiang, F.F.; Shen, X.X.; Xu, Q.J. One-Step Electrochemical Synthesis of Poly(Vinyl Pyrrolidone) Modified Polyaniline Coating on Stainless Steel for High Corrosion Protection Performance. Prog. Org. Coat. 2020, 149, 105908. [Google Scholar] [CrossRef]
- Madhusudhana, A.M.; Shetty Mohana, K.N.; Hegde, M.B.; Nayak, S.R.; Rajitha, K.; Swamy, N.K. Development of Al2O3.ZnO/GO-phenolic Formaldehyde Amine Derivative Nanocomposite: A New Hybrid Anticorrosion Coating Material for Mild Steel. Colloids Surf. A 2020, 601, 125036. [Google Scholar] [CrossRef]
- Fan, B.M.; Hao, H.; Yang, B.; Li, Y. Insights into the Inhibition Mechanism of A Novel Supramolecular Complex Towards the Corrosion of Mild Steel in the Condensate Water: Experimental and Theoretical Studies. Res. Chem. Intermediat. 2018, 44, 5711–5736. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Zhou, T.T.; Hao, H.; Yang, B.; Sun, H. Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd El-Hafeez, G.M.; El-Rabeie, M.M.; Gaber, A.F.; Farag, Z.R. Electropolymerized Durable Coatings Deposited onto Pt-Electrode as Corrosion Inhibitor for Mild Steel. J. Adhes. Sci. Technol. 2021, 1–19. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Hu, S.J.; Ghaderi, S.; Ghanbari, A.; Ahmadipour, M.; Pung, S.Y.; Li, S.B.; Feilizadeh, M.; Arjmand, M. Amino-Functionalized MXene Nanosheets Doped with Ce(III) as Potent Nanocontainers toward Self-Healing Epoxy Nanocomposite Coating for Corrosion Protection of Mild Steel. ACS Appl. Mater. Inter. 2021, 13, 42074–42093. [Google Scholar] [CrossRef]
- Obot, I.B.; Onyeachu, I.B. Electrochemical Frequency Modulation (EFM) Technique: Theory and Recent Practical Applications in Corrosion Research. J. Mol. Liq. 2018, 249, 83–96. [Google Scholar] [CrossRef]
- Lalvani, S.; Kerr, L.; Lalvani, S.; Olaguera-Delogu, D. Electrochemical Frequency Modulation: New Approach and Revision of Previous Model. J. Electrochem. Soc. 2021, 168, 121508. [Google Scholar] [CrossRef]
- Bosch, R.W.; Hubrecht, J.; Bogaerts, W.F.; Syrett, B.C. Electrochemical Frequency Modulation: A New Electrochemical Technique for Online Corrosion Monitoring. Corrosion 2001, 57, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Shalabi, K.; El-Gammal, O.A.; Abdallah, Y.M. Adsorption and Inhibition Effect of Tetraaza-Tetradentate Macrocycle Ligand and Its Ni (II), Cu (II) Complexes on the Corrosion of Cu10Ni Alloy in 3.5% NaCl Solutions. Colloids Surf. A 2021, 609, 125653. [Google Scholar] [CrossRef]
- Fan, B.M.; Liu, Z.N.; Zhao, X.Q.; Liu, H.; Fan, G.F.; Hao, H. Fabrication, Characterization and Efficient Surface Protection Mechanism of Poly(trans-Cinnamaldehyde) Electropolymerized Coatings for EH36 Steel in Simulated Seawater. Colloids Surf. A 2021, 629, 127434. [Google Scholar] [CrossRef]
- Li, M.X.; Ji, X.Q.; Cui, L.; Liu, J.Q. In Situ Preparation Of Graphene/Polypyrrole Nanocomposite via Electrochemical Co-Deposition Methodology for Anti-Corrosion Application. J. Mater. Sci. 2017, 52, 12251–12265. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yang, W.Z.; Xu, B.; Chen, Y.; Qian, M.Q.; Su, X.; Li, Z.H.; Yin, X.S.; Liu, Y. Corrosion Protection of Carbon Steels by Electrochemically Synthesized V-TiO2/Polypyrrole Composite Coatings in 0.1 M HCl Solution. J. Alloy. Compd. 2019, 771, 857–868. [Google Scholar] [CrossRef]
- Khorrammaslack, N.; Farhadi, K.; Seyedahmadian, S.M.; Habibi, B. Electrochemical Preparation of poly 3-Amino-5-Hydroxypyrazole on Copper and Its Corrosion Protection Efficiency. J. Coat. Technol. Res. 2020, 17, 1269–1276. [Google Scholar] [CrossRef]
- Koleva, D.A.; Boshkov, N.; Bachvarov, V.; Zhan, H.; de Wit, J.H.W.; van Breugel, K. Application of PEO113–b-PS218 Nano-Aggregates for Improved Protective Characteristics of Composite Zinc Coatings in Chloride-Containing Environment. Surf. Coat. Technol. 2010, 204, 3760–3772. [Google Scholar] [CrossRef]
- Shen, S.Y.; Ke, T.; Rajavel, K.; Yang, K.; Lin, D.H. Dispersibility and Photochemical Stability of Delaminated MXene Flakes in Water. Small 2020, 16, 2002433. [Google Scholar] [CrossRef]
- Fan, B.M.; Ma, Y.C.; Wang, M.M.; Hao, H.; Yang, B.; Lv, J.Y.; Sun, H. Revealing the Assembly Mechanism of An Octadecylamine Based Supramolecular Complex on Mild Steel in Condensate Water: Correlative Experimental and Theoretical Studies. J. Mol. Liq. 2019, 292, 111446. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Hao, H.; Lu, J.Y.; Feng, Y.H.; Yang, B. Experimental and Theoretical Studies of Action Mechanism of an Octadecylamine-Based Molecular Assembly on Mild Steel. Chem. J. Chin. Univ. 2019, 40, 96–107. [Google Scholar] [CrossRef]
- Ma, Y.C.; Fan, B.M.; Wang, M.M.; Yang, B.; Hao, H.; Sun, H.; Zhang, H.J. Two-Step Preparation of Trazodone and Its Corrosion Inhibition Mechanism for Carbon Steel. Chem. J. Chin. Univ. 2019, 40, 1706–1716. [Google Scholar] [CrossRef]
- Wang, M.M.; Fan, B.M.; Wen, B.Y.; Jiang, C. Experimental and Theoretical Studies on the Removal Mechanism of Formaldehyde from Water by Mesoporous Calcium Silicate. Sci. China Technol. Sci. 2020, 63, 2098–2112. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhou, T.T.; Zhu, W.Q.; Fan, B.M.; Liu, H.; Fan, G.F.; Hao, H.; Sun, H.; Yang, B. Understanding the Anticorrosive Mechanism of A Cross-Linked Supramolecular Polymer for Mild Steel in the condensate Water: Comprehensive Experimental, Molecular Docking, and Molecular Dynamics Investigations. J. Mol. Model. 2020, 26, 81. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.M.; Wei, G.; Hao, H.; Guo, A.R.; Li, J. Preparation of A Ceramic Membrane from Prevalent Natural Clay for the Purification of Phosphate Wastewater. Desalin. Water Treat. 2016, 57, 17308–17321. [Google Scholar] [CrossRef]
- Xiong, X.H.; Ding, D.; Wang, Z.X.; Huang, B.; Guo, H.J.; Li, X.H. Surface Modification of LiNi0.8Co0.1Mn0.1O2 with Conducting Polypyrrole. J. Solid State Electr. 2014, 18, 2619–2624. [Google Scholar] [CrossRef]




| Item | NaCl | MgCl2 | Na2SO4 | NaHCO3 | H3BO3 | CaCl2 | KCl | SrCl2 | KBr | NaF |
|---|---|---|---|---|---|---|---|---|---|---|
| C (g/mL) | 24.50 | 5.20 | 4.09 | 2.01 × 10−1 | 2.70 × 10−2 | 1.16 | 6.95 × 10−1 | 2.50 × 10−2 | 1.01 × 10−1 | 3.00 × 10−3 |
| Samples | icorr (μA/cm2) | Ecorr (mV) | βa (mV/dec) | −βc (mV/dec) |
|---|---|---|---|---|
| P-Cu | 1.79 | −186.64 | 87.06 | 116.47 |
| PPy-T | 2.58 | −180.67 | 100.52 | 112.41 |
| PPy-I | 43.81 | −171.07 | 173.53 | 96.88 |
| Samples | Rs (Ω·cm2) | Qf | Rf (Ω·cm2) | Qdl | Rct (Ω·cm2) | W × 104 (Ω·cm2·s1/2) | χ2 (×10−4) | ||
|---|---|---|---|---|---|---|---|---|---|
| Cf × 104 (μF/cm2) | nf | Cdl × 104 (μF/cm2) | ndl | ||||||
| P-Cu | 11.95 | 113.06 | 0.64 | 35.86 | 2.92 | 0.46 | 2413.06 | 14.63 | 6.07 |
| PPy-T | 13.18 | 125.53 | 0.58 | 68.73 | 17.15 | 0.71 | 1875.35 | / | 6.82 |
| PPy-I | 13.23 | 151.90 | 0.54 | 71.40 | 145.20 | 0.74 | 122.95 | 15.57 | 2.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Liu, X.; Fan, B.; Zheng, X. Optimized Anticorrosion of Polypyrrole Coating by Inverted-Electrode Strategy: Experimental and Molecular Dynamics Investigations. Polymers 2022, 14, 1356. https://doi.org/10.3390/polym14071356
Zhao X, Liu X, Fan B, Zheng X. Optimized Anticorrosion of Polypyrrole Coating by Inverted-Electrode Strategy: Experimental and Molecular Dynamics Investigations. Polymers. 2022; 14(7):1356. https://doi.org/10.3390/polym14071356
Chicago/Turabian StyleZhao, Xiaoqi, Xiaoyan Liu, Baomin Fan, and Xingwen Zheng. 2022. "Optimized Anticorrosion of Polypyrrole Coating by Inverted-Electrode Strategy: Experimental and Molecular Dynamics Investigations" Polymers 14, no. 7: 1356. https://doi.org/10.3390/polym14071356






