3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optimization of Filament Extrusion and 3D Printing
2.2. Physicochemical Characterization of Extruded Filaments and 3D Printed Samples
3. Results and Discussion
3.1. Filament Characterization
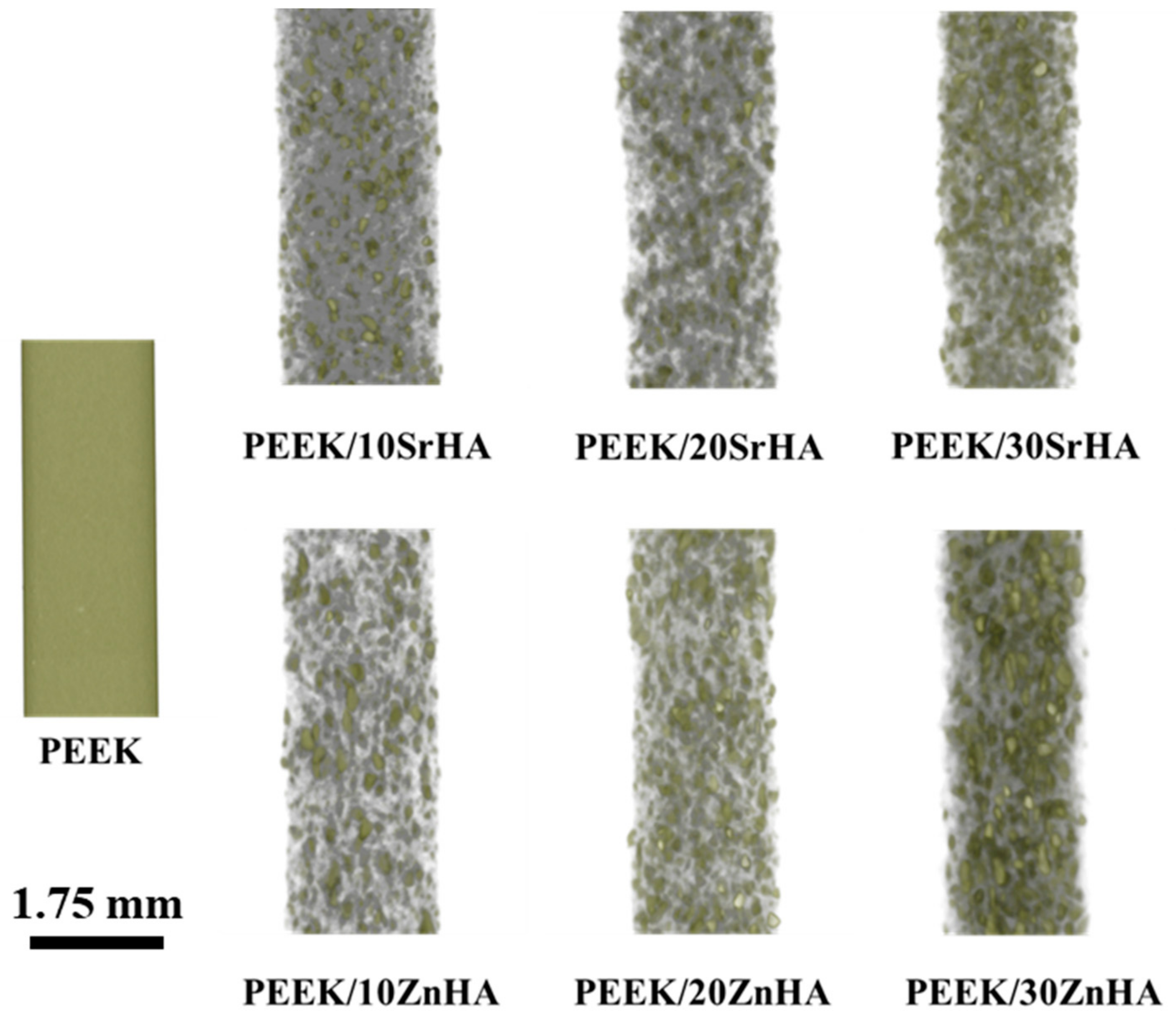
3.1.1. Micro-CT Analysis of Filaments
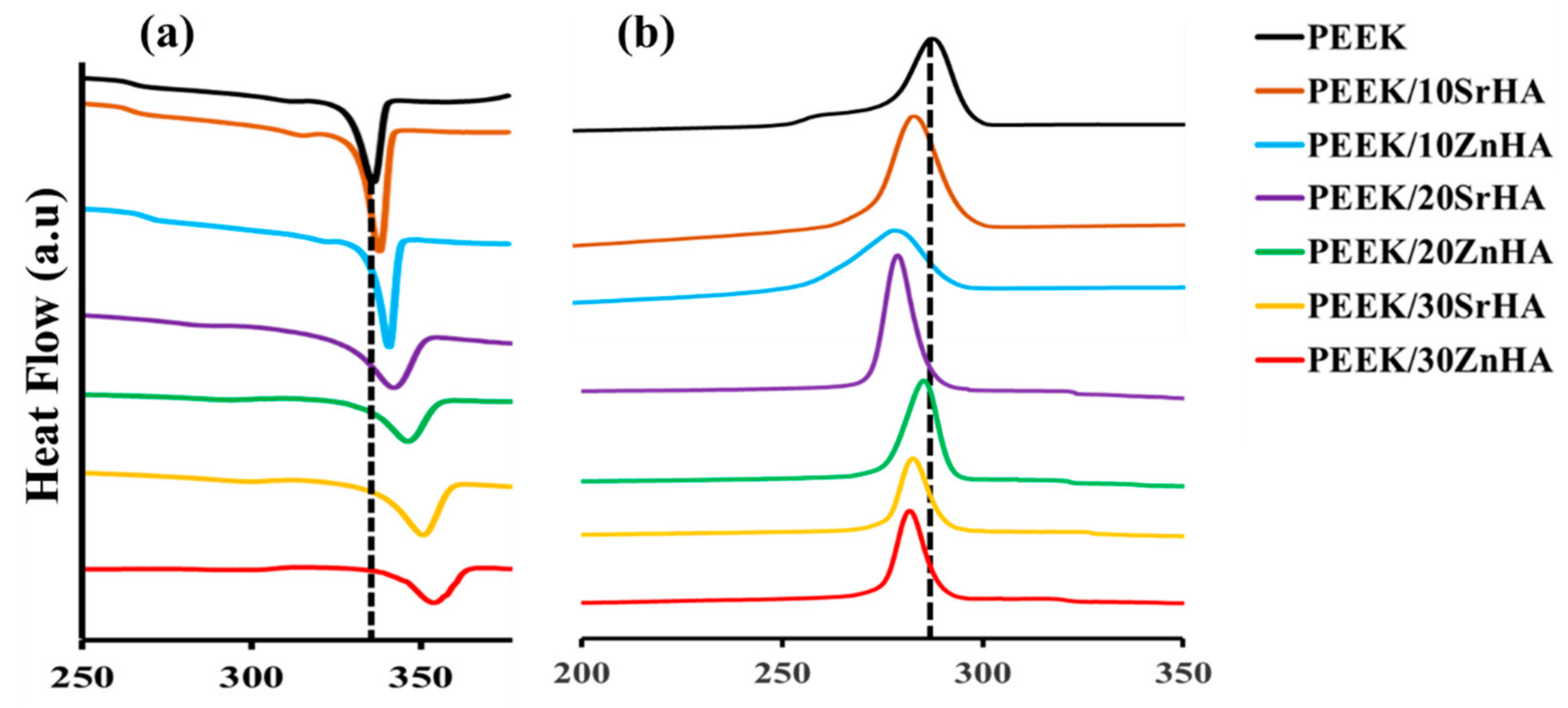
3.1.2. Thermal Analysis of Filaments
3.2. 3D-Printed Sample Characterization
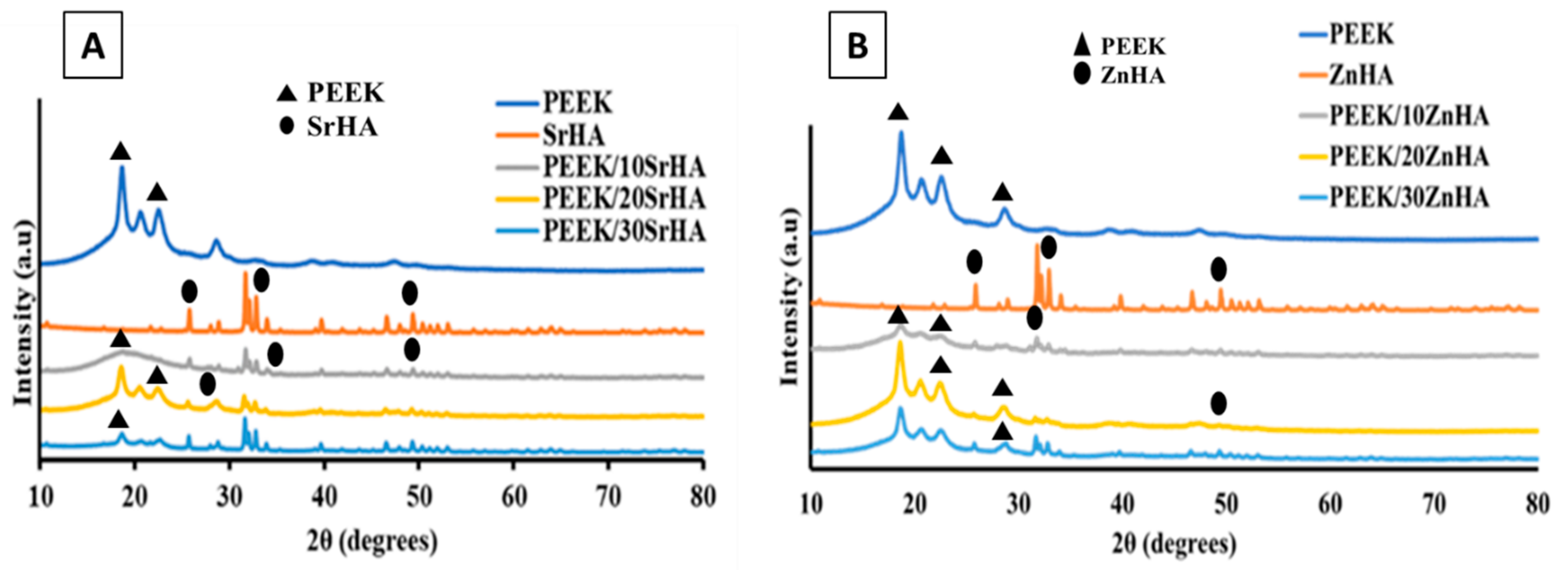
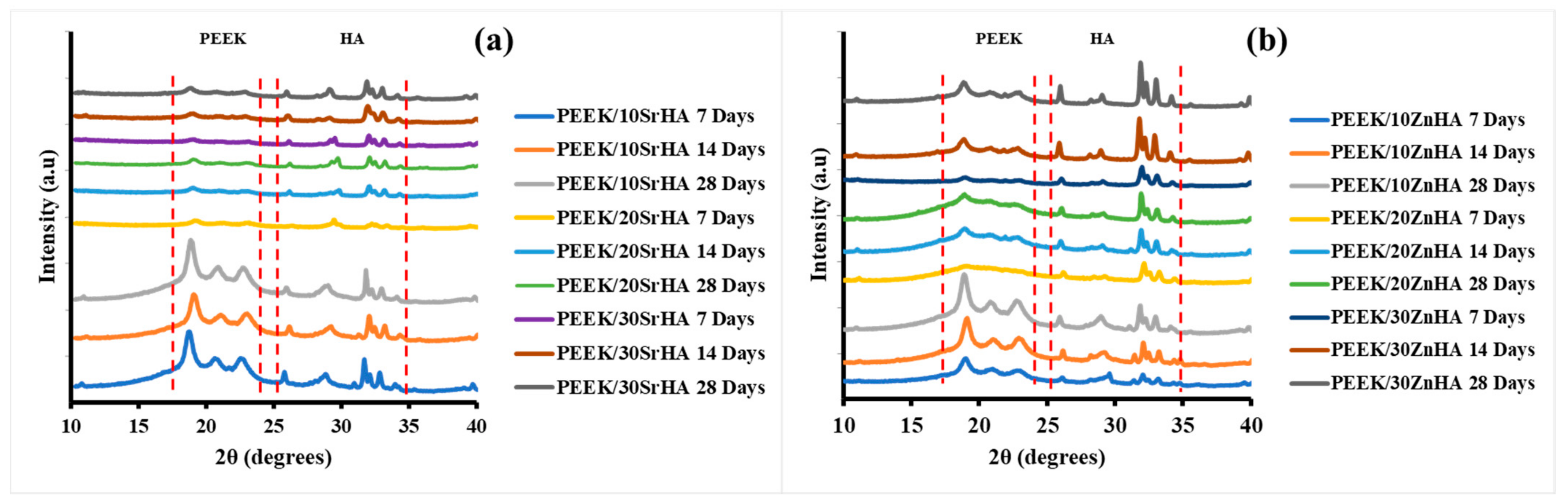
3.2.1. X-ray Diffraction (XRD)
3.2.2. Scanning Electron Microscopy (SEM)
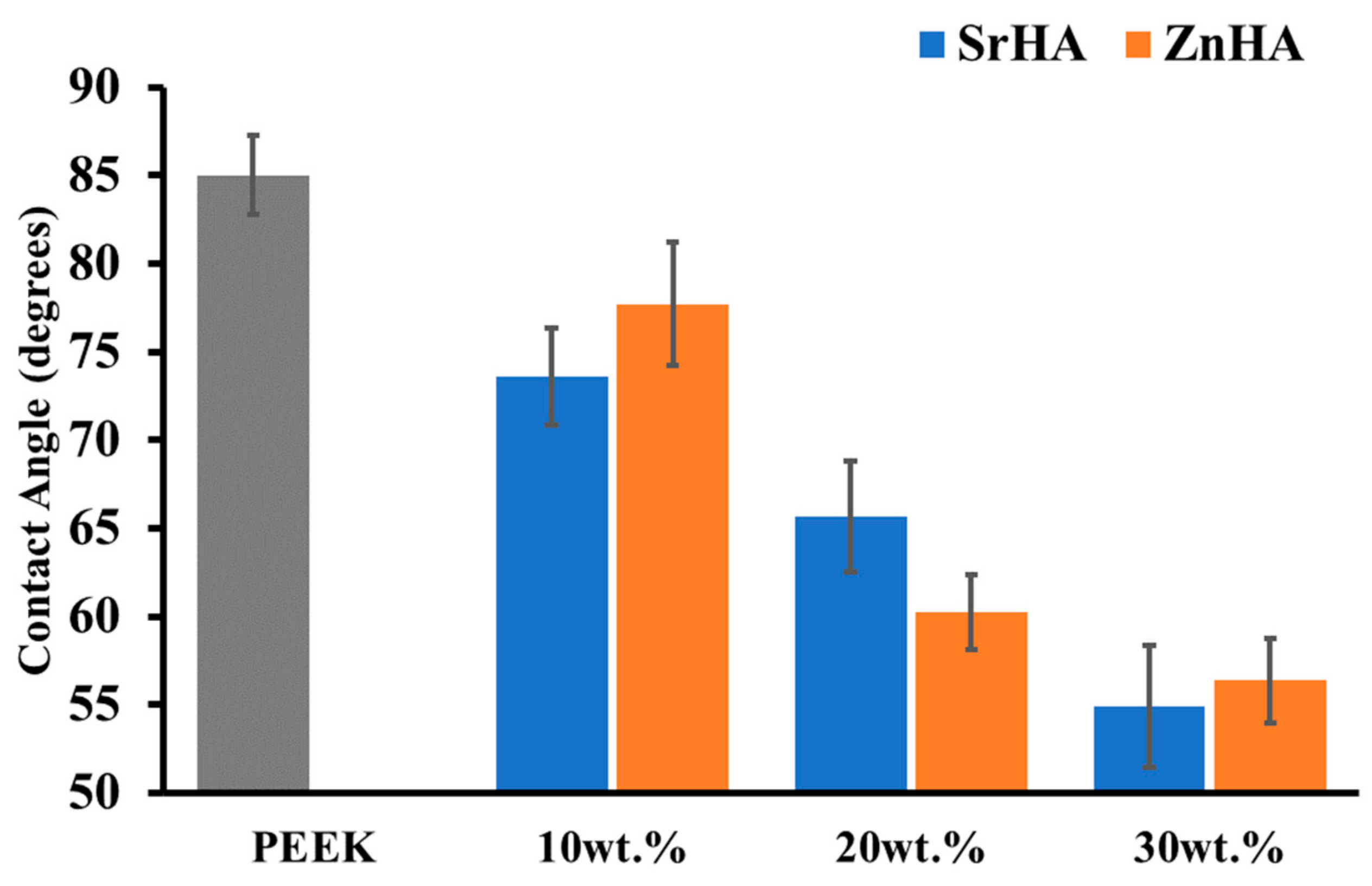
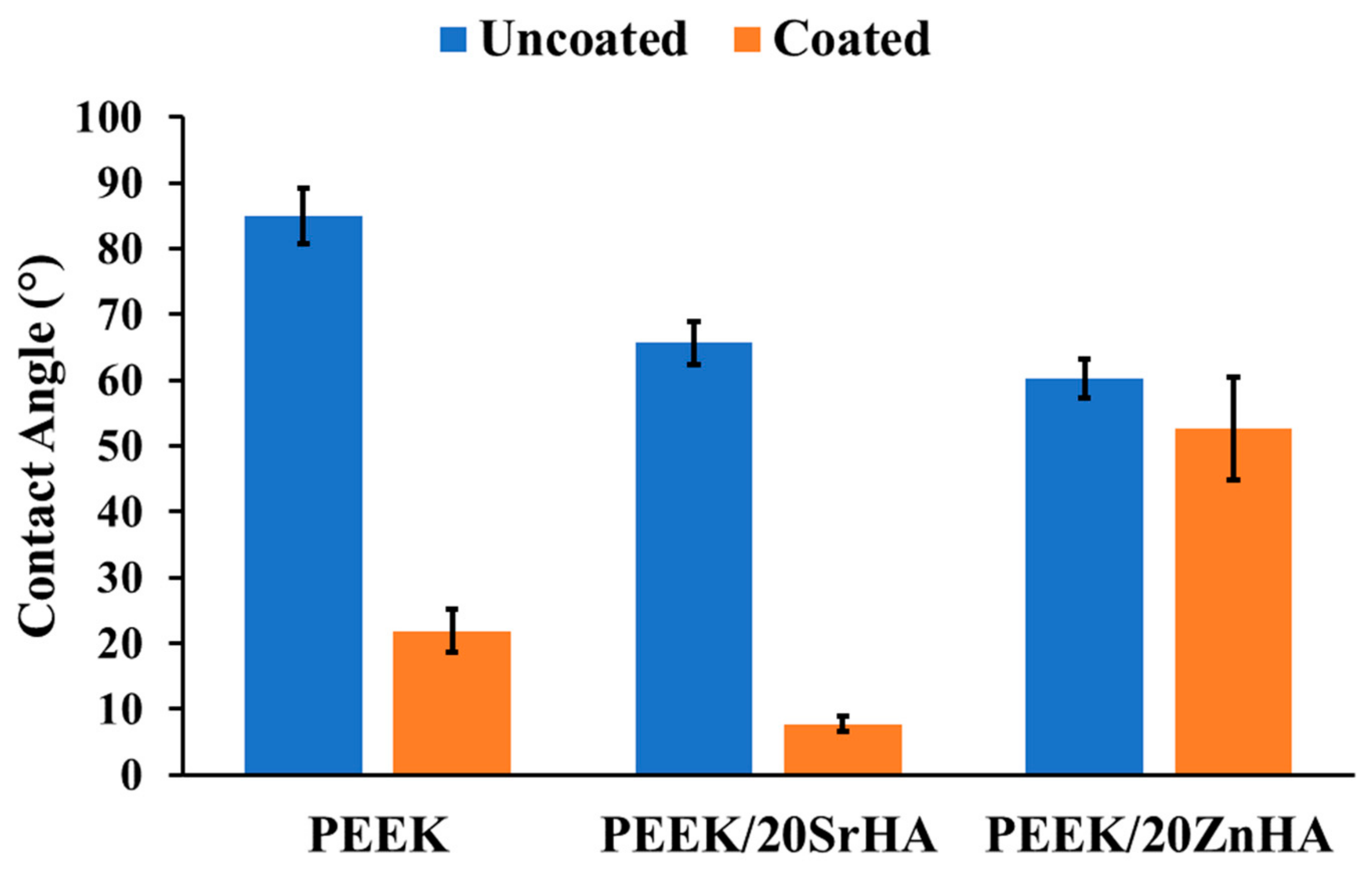
3.2.3. Water Contact Angle Measurement
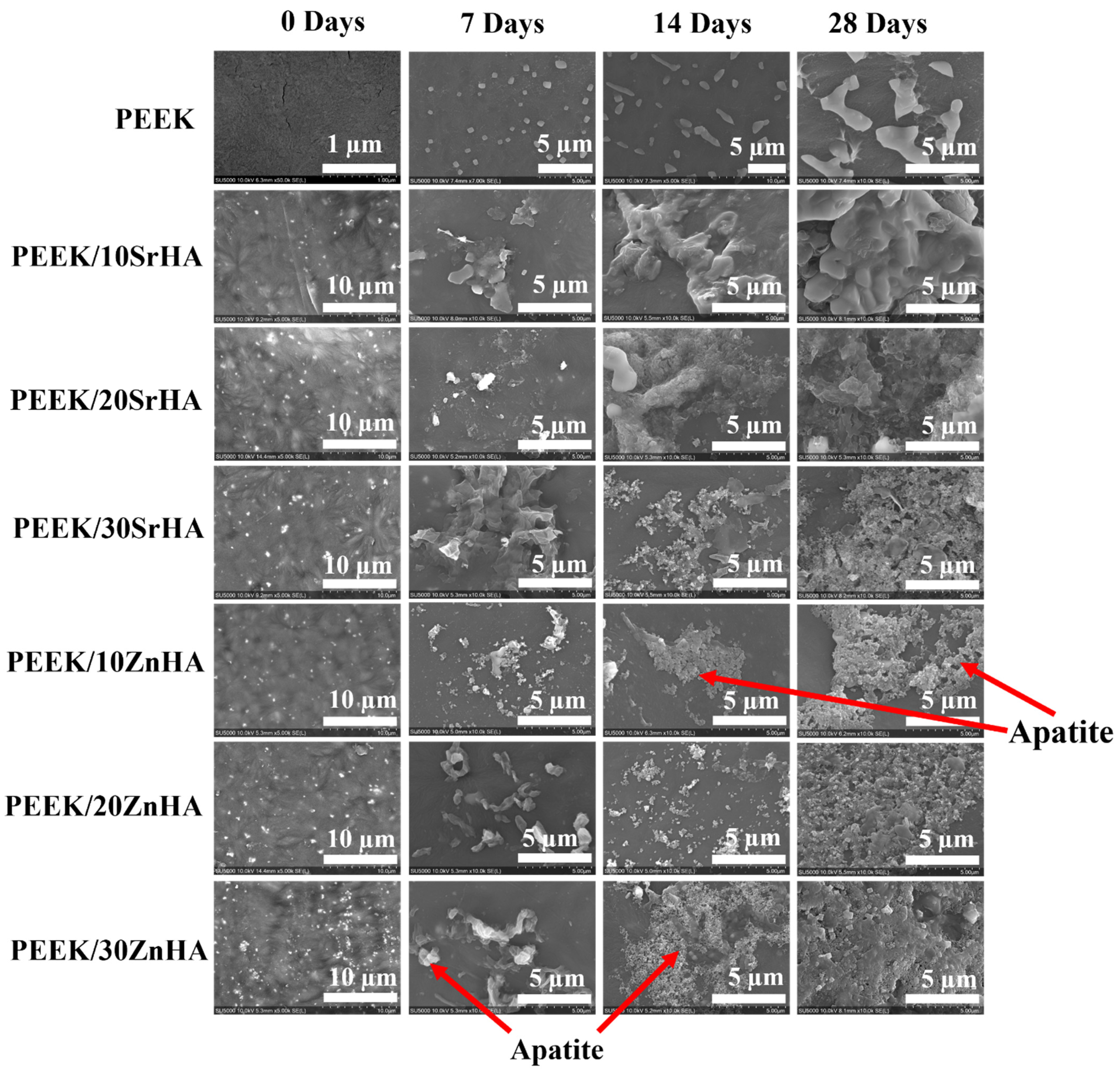
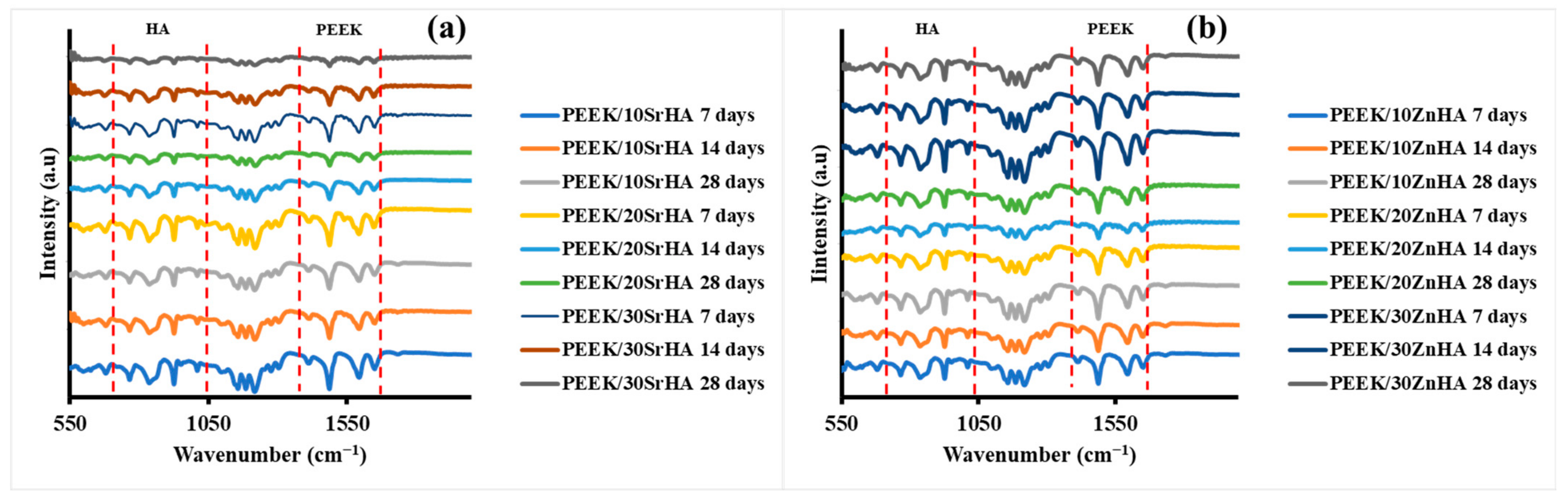
3.2.4. Bioactivity Testing
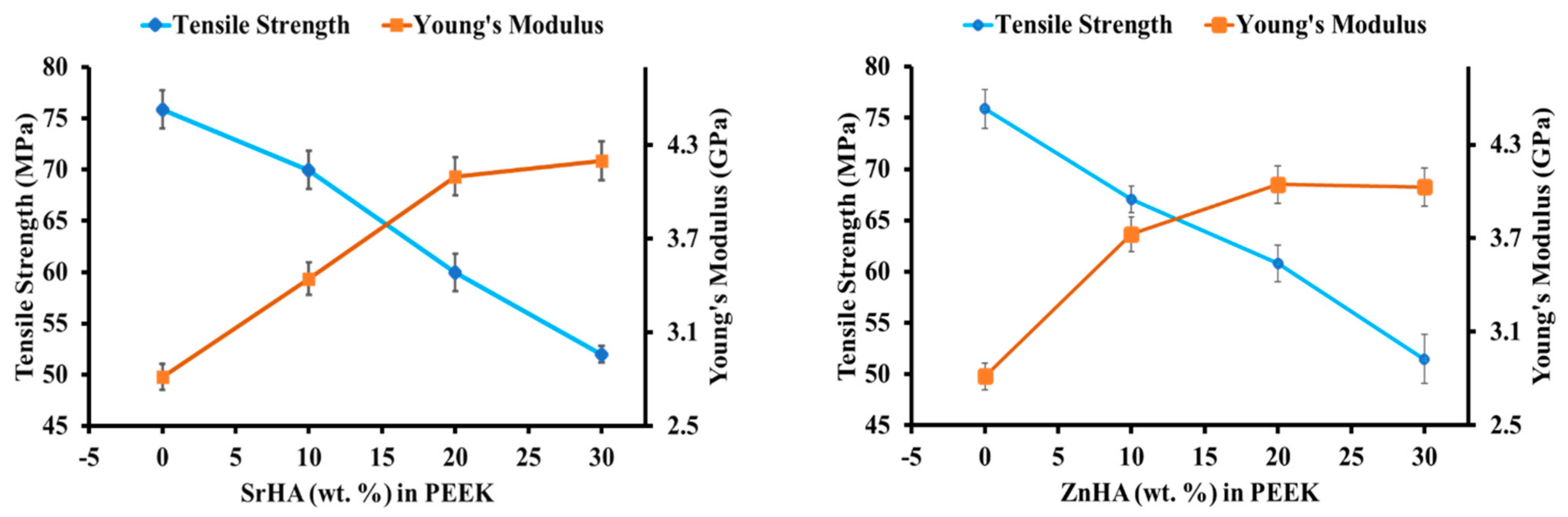
3.2.5. Mechanical Performance before SBF Immersion
3.2.6. Mechanical Performance after SBF Immersion
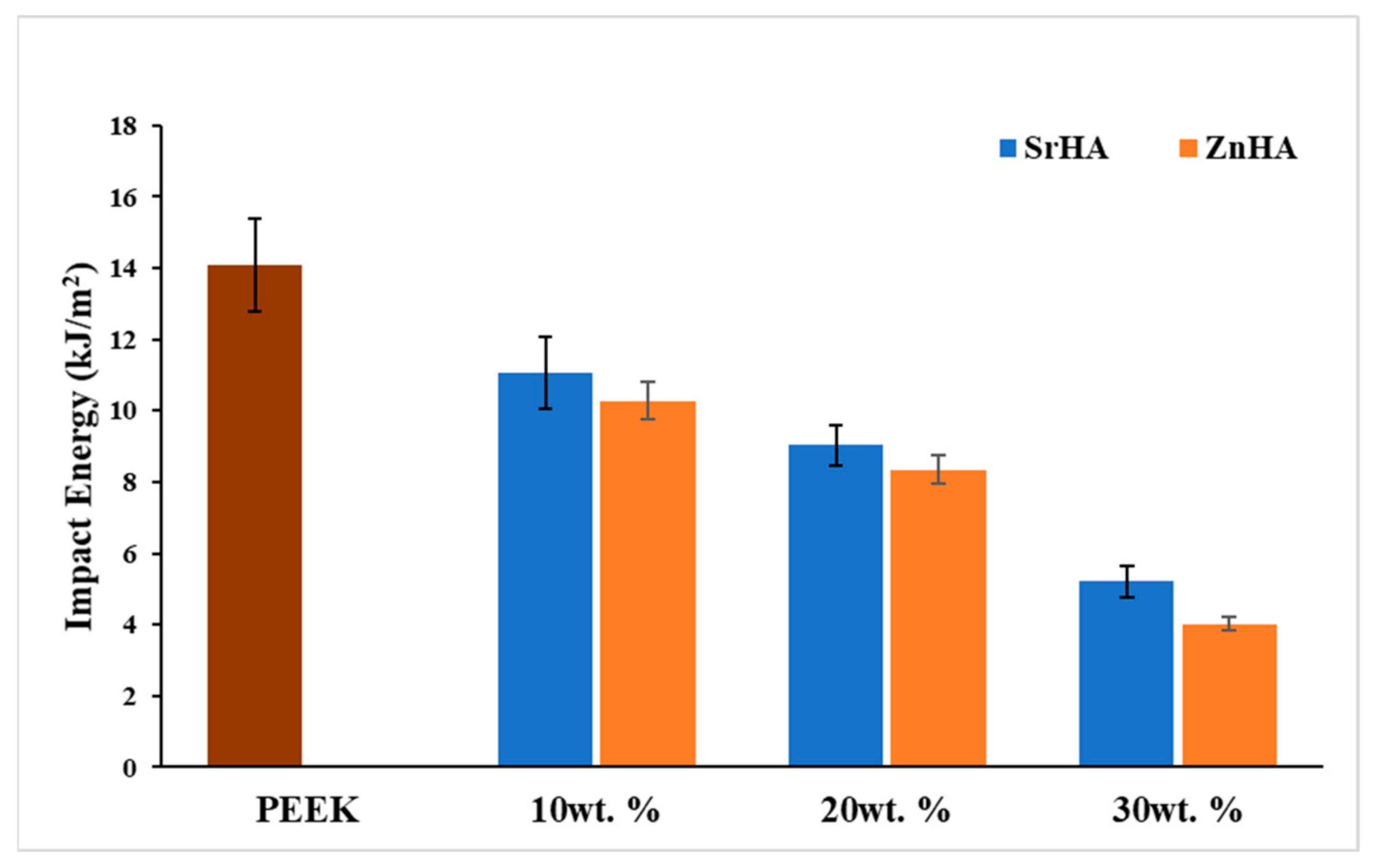
3.2.7. Impact Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, Z.-X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Zou, B.; Huang, C.; Gao, H. Study on microstructure, mechanical properties and machinability of efficiently additive manufactured AISI 316L stainless steel by high-power direct laser deposition. J. Mater. Process. Technol. 2017, 240, 12–22. [Google Scholar] [CrossRef]
- Davies, R.; Yi, N.; McCutchion, P.; Ghita, O. Mechanical property variance amongst vertical fused filament fabricated specimens via four different printing methods. Polym. Int. 2021, 70, 1073–1079. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M.C. Additive manufacturing: Technology, applications and research needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Heller, M.; Bauer, H.-K.; Goetze, E.; Gielisch, M.; Roth, K.E.; Drees, P.; Maier, G.S.; Dorweiler, B.; Ghazy, A.; Neufurth, M.; et al. Applications of patient-specific 3D printing in medicine. Int. J. Comput. Dent. 2016, 19, 323–339. [Google Scholar]
- Ren, Z.-H.; Wu, H.-J.; Tan, H.-Y.; Wang, K.; Zhang, S. Transfer of anterolateral thigh flaps in elderly oral cancer patients: Complications in oral and maxillofacial reconstruction. J. Oral Maxillofac. Surg. 2015, 73, 534–540. [Google Scholar] [CrossRef]
- Rohner, D.; Guijarro-Martínez, R.; Bucher, P.; Hammer, B. Importance of patient-specific intraoperative guides in complex maxillofacial reconstruction. J. Cranio-Maxillofac. Surg. 2013, 41, 382–390. [Google Scholar] [CrossRef]
- Bauer, T.W.; Muschler, G.F. Bone graft materials: An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Hallman, M.; Thor, A. Bone substitutes and growth factors as an alternative/complement to autogenous bone for grafting in implant dentistry. Periodontology 2000 2008, 47, 172–192. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.; Valiev, R.; Meyers, M. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Bougherara, H.; Bureau, M.N.; Yahia, L.H. Bone remodeling in a new biomimetic polymer-composite hip stem. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 164–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Lu, T.; Meng, F.; Zhu, H.; Liu, X. Enhanced osteoblast responses to poly ether ether ketone surface modified by water plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2014, 117, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-D.; Jang, T.-S.; Lee, J.E.; Park, S.J.; Son, Y.; Park, S.-H. Enhanced bioactivity of titanium-coated polyetheretherketone implants created by a high-temperature 3D printing process. Biofabrication 2019, 11, 045014. [Google Scholar] [CrossRef] [PubMed]
- Berretta, S.; Evans, K.E.; Ghita, O. Processability of PEEK, a new polymer for high temperature laser sintering (HT-LS). Eur. Polym. J. 2015, 68, 243–266. [Google Scholar] [CrossRef] [Green Version]
- Berretta, S.; Evans, K.; Ghita, O. Additive manufacture of PEEK cranial implants: Manufacturing considerations versus accuracy and mechanical performance. Mater. Des. 2018, 139, 141–152. [Google Scholar] [CrossRef]
- Han, X.; Yang, D.; Yang, C.; Spintzyk, S.; Scheideler, L.; Li, P.; Li, D.; Geis-Gerstorfer, J.; Rupp, F. Carbon fiber reinforced PEEK composites based on 3D-printing technology for orthopedic and dental applications. J. Clin. Med. 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Noiset, O.; Schneider, Y.-J.; Marchand-Brynaert, J. Fibronectin adsorption or/and covalent grafting on chemically modified PEEK film surfaces. J. Biomater. Sci. Polym. Ed. 1999, 10, 657–677. [Google Scholar] [CrossRef]
- Ma, R.; Guo, D. Evaluating the bioactivity of a hydroxyapatite-incorporated polyetheretherketone biocomposite. J. Orthop. Surg. Res. 2019, 14, 32. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Omigbodun, F.T.; Bowoto, O.K.; Olawumi, M.A.; Muhammad, M.A. 3D printing of PEEK–cHAp scaffold for medical bone implant. Bio-Des. Manuf. 2021, 4, 44–59. [Google Scholar] [CrossRef]
- Yu, S.; Hariram, K.P.; Kumar, R.; Cheang, P.; Aik, K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials 2005, 26, 2343–2352. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Liu, X.; Wei, J.; Ji, J.; Yang, S.; Hu, Z.; Wei, S. Response of human osteoblast to n-HA/PEEK—quantitative proteomic study of bio-effects of nano-hydroxyapatite composite. Sci. Rep. 2016, 6, 22832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Yu, X.; Zhu, H. Fabrication of Mg Coating on PEEK and Antibacterial Evaluation for Bone Application. Coatings 2021, 11, 1010. [Google Scholar] [CrossRef]
- Shannon, A.; Manolakis, I. A Facile Route to Bio-Inspired Supramolecular Oligo (Ethylene Glycol) Catecholates. Macromol. Chem. Phys. 2019, 220, 1800412. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, X.; Zhao, J.; Zhao, Z.; Wang, Q.; Zhang, C. Biologically Modified Polyether Ether Ketone as Dental Implant Material. Front. Bioeng. Biotechnol. 2020, 8, 620537. [Google Scholar] [CrossRef]
- Fathi, M.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Sun, H.; Lu, Y.; Zhao, F.; Liu, C.; Wang, Q.; Zheng, C.; Lu, R.; Song, K. Strontium/Chitosan/Hydroxyapatite/Norcantharidin Composite That Inhibits Osteosarcoma and Promotes Osteogenesis In Vitro. BioMed Res. Int. 2020, 2020, 9825073. [Google Scholar] [CrossRef]
- Porter, A.; Patel, N.; Skepper, J.; Best, S.; Bonfield, W. Comparison of in vivo dissolution processes in hydroxyapatite and silicon-substituted hydroxyapatite bioceramics. Biomaterials 2003, 24, 4609–4620. [Google Scholar] [CrossRef]
- Stipniece, L.; Salma-Ancane, K.; Borodajenko, N.; Sokolova, M.; Jakovlevs, D.; Berzina-Cimdina, L. Characterization of Mg-substituted hydroxyapatite synthesized by wet chemical method. Ceram. Int. 2014, 40, 3261–3267. [Google Scholar] [CrossRef]
- Thian, E.; Konishi, T.; Kawanobe, Y.; Lim, P.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef]
- Feng, P.; Wu, P.; Gao, C.; Yang, Y.; Guo, W.; Yang, W.; Shuai, C. A multimaterial scaffold with tunable properties: Toward bone tissue repair. Adv. Sci. 2018, 5, 1700817. [Google Scholar] [CrossRef] [PubMed]
- Xian, P.; Chen, Y.; Gao, S.; Qian, J.; Zhang, W.; Udduttula, A.; Huang, N.; Wan, G. Polydopamine (PDA) mediated nanogranular-structured titanium dioxide (TiO2) coating on polyetheretherketone (PEEK) for oral and maxillofacial implants application. Surf. Coat. Technol. 2020, 401, 126282. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Smitha, S.; Shajesh, P.; Mukundan, P.; Nair, T.; Warrier, K. Synthesis of biocompatible hydrophobic silica–gelatin nano-hybrid by sol–gel process. Colloids Surf. B Biointerfaces 2007, 55, 38–43. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly (ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Wang, L.; He, S.; Wu, X.; Liang, S.; Mu, Z.; Wei, J.; Deng, F.; Deng, Y.; Wei, S. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef]
- Rodzeń, K.; McIvor, M.J.; Sharma, P.K.; Acheson, J.G.; McIlhagger, A.; Mokhtari, M.; McFerran, A.; Ward, J.; Meenan, B.J.; Boyd, A.R. The Surface Characterisation of Fused Filament Fabricated (FFF) 3D Printed PEEK/Hydroxyapatite Composites. Polymers 2021, 13, 3117. [Google Scholar] [CrossRef]
- Rodzeń, K.; Sharma, P.K.; McIlhagger, A.; Mokhtari, M.; Dave, F.; Tormey, D.; Sherlock, R.; Meenan, B.J.; Boyd, A. The Direct 3D Printing of Functional PEEK/Hydroxyapatite Composites via a Fused Filament Fabrication Approach. Polymers 2021, 13, 545. [Google Scholar] [CrossRef]
- Zheng, J.; Kang, J.; Sun, C.; Yang, C.; Wang, L.; Li, D. Effects of printing path and material components on mechanical properties of 3D-printed polyether-ether-ketone/hydroxyapatite composites. J. Mech. Behav. Biomed. Mater. 2021, 118, 104475. [Google Scholar] [CrossRef]
- Manzoor, F.; Golbang, A.; Jindal, S.; Dixon, D.; McIlhagger, A.; Harkin-Jones, E.; Crawford, D.; Mancuso, E. 3D printed PEEK/HA composites for bone tissue engineering applications: Effect of material formulation on mechanical performance and bioactive potential. J. Mech. Behav. Biomed. Mater. 2021, 121, 104601. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Wong, C.; Liu, W.; Pan, H.; Fong, M.; Lam, W.; Cheung, W.; Tang, W.; Chiu, K.; Luk, K.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Baştan, F.E. Fabrication and characterization of an electrostatically bonded PEEK-Hydroxyapatite composites for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Deng, X.; Zeng, Z.; Peng, B.; Yan, S.; Ke, W. Mechanical properties optimization of poly-ether-ether-ketone via fused deposition modeling. Materials 2018, 11, 216. [Google Scholar] [CrossRef] [Green Version]
- Berretta, S.; Davies, R.; Shyng, Y.; Wang, Y.; Ghita, O. Fused Deposition Modelling of high temperature polymers: Exploring CNT PEEK composites. Polym. Test. 2017, 63, 251–262. [Google Scholar] [CrossRef]
- Mutyala, R.S. Effect of FDM Process Parameters on the Mechanical Properties of CFR-PEEK. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2019. [Google Scholar]
- Ding, S.; Zou, B.; Wang, P.; Ding, H. Effects of nozzle temperature and building orientation on mechanical properties and microstructure of PEEK and PEI printed by 3D-FDM. Polym. Test. 2019, 78, 105948. [Google Scholar] [CrossRef]
- Lowry, N.; Brolly, M.; Han, Y.; McKillop, S.; Meenan, B.; Boyd, A. Synthesis and characterisation of nanophase hydroxyapatite co-substituted with strontium and zinc. Ceram. Int. 2018, 44, 7761–7770. [Google Scholar] [CrossRef]
- Hench, L.L. An introduction to Bioceramics; World Scientific, Toh Tuck Link: Singapore, 1993; Volume 1. [Google Scholar]
- Sikder, P.; Ferreira, J.A.; Fakhrabadi, E.A.; Kantorski, K.Z.; Liberatore, M.W.; Bottino, M.C.; Bhaduri, S.B. Bioactive amorphous magnesium phosphate-polyetheretherketone composite filaments for 3D printing. Dent. Mater. 2020, 36, 865–883. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Koo, J.H.; Wardle, B.L.; Kumar, S. Additively manufactured polyetheretherketone (PEEK) with carbon nanostructure reinforcement for biomedical structural applications. Adv. Eng. Mater. 2020, 22, 2000483. [Google Scholar] [CrossRef]
- Hao, L.; Hu, Y.; Zhang, Y.; Wei, W.; Hou, X.; Guo, Y.; Hu, X.; Jiang, D. Enhancing the mechanical performance of poly (ether ether ketone)/zinc oxide nanocomposites to provide promising biomaterials for trauma and orthopedic implants. RSC Adv. 2018, 8, 27304–27317. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.K.; Chatterjee, S.; Spieckermann, F.; Ghosh, P.; Samanta, S. Reversing and non-reversing effects of PEEK-HA composites on tuning cooling rate during crystallization. J. Polym. Res. 2019, 26, 279. [Google Scholar] [CrossRef]
- Comelli, C.A.; Davies, R.; van der Pol, H.; Ghita, O. PEEK filament characteristics before and after extrusion within fused filament fabrication process. J. Mater. Sci. 2022, 57, 766–788. [Google Scholar] [CrossRef]
- Temprom, L.; Seet, S.L.; Tippayawat, P.; Suwanna, P. Bioactivity, cytotoxicity and antibacterial evaluation of undoped, Zn-doped, Sr-doped, and Zn/Sr-codoped hydroxyapatites synthesized by a sol-gel method. Chiang Mai J. Sci. 2016, 43, 630–639. [Google Scholar]
- Liu, J.; Yao, R.; Guo, J.; Gao, T.; He, J.; Meng, G.; Wu, F. The regulating effect of trace elements Si, Zn and Sr on mineralization of gelatin-hydroxyapatite electrospun fiber. Colloids Surf. B Biointerfaces 2021, 204, 111822. [Google Scholar] [CrossRef]
- Pantasri, T.; Seet, S.; Suwanna, P. Preparation of strontium-and/or zinc-doped hydroxyapatite nanoparticles and their polycaprolactone composite fibrous scaffolds. J. Phys. Conf. Ser. 2017, 901, 012029. [Google Scholar] [CrossRef] [Green Version]
- Rego, B.T.; Neto, W.A.R.; de Paula, A.C.C.; Góes, A.M.; Bretas, R.E.S. Mechanical properties and stem cell adhesion of injection-molded poly (ether ether ketone) and hydroxyapatite nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41748. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.-R.; Jin, K.; Hong, K.S.; Noh, J.H.; Lee, H.-K. In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater. 2013, 9, 6177–6187. [Google Scholar] [CrossRef]
- Elawadly, T.; Radi, I.A.; El Khadem, A.; Osman, R.B. Can PEEK be an implant material? Evaluation of surface topography and wettability of filled versus unfilled PEEK with different surface roughness. J. Oral Implantol. 2017, 43, 456–461. [Google Scholar] [CrossRef]
- Huang, R.; Shao, P.; Burns, C.; Feng, X. Sulfonation of poly (ether ether ketone)(PEEK): Kinetic study and characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- De Gennes, P.-G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Kaygili, O.; Keser, S.; Kom, M.; Bulut, N.; Dorozhkin, S.V. The effect of simulating body fluid on the structural properties of hydroxyapatite synthesized in the presence of citric acid. Prog. Biomater. 2016, 5, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Ghomi, H.; Fathi, M.; Edris, H. Preparation of nanostructure hydroxyapatite scaffold for tissue engineering applications. J. Sol-Gel Sci. Technol. 2011, 58, 642–650. [Google Scholar] [CrossRef]
- Shuai, C.; Shuai, C.; Wu, P.; Yuan, F.; Feng, P.; Yang, Y.; Guo, W.; Fan, X.; Su, T.; Peng, S.; et al. Characterization and bioactivity evaluation of (polyetheretherketone/polyglycolicacid)-hydroyapatite scaffolds for tissue regeneration. Materials 2016, 9, 934. [Google Scholar] [CrossRef]
- Golbang, A.; Harkin-Jones, E.; Wegrzyn, M.; Campbell, G.; Archer, E.; McIlhagger, A. Production and characterization of PEEK/IF-WS2 nanocomposites for additive manufacturing: Simultaneous improvement in processing characteristics and material properties. Addit. Manuf. 2020, 31, 100920. [Google Scholar] [CrossRef]
- Jeyachandran, P.; Bontha, S.; Bodhak, S.; Balla, V.K.; Kundu, B.; Doddamani, M. Mechanical behaviour of additively manufactured bioactive glass/high density polyethylene composites. J. Mech. Behav. Biomed. Mater. 2020, 108, 103830. [Google Scholar] [CrossRef]
- Delille, R.; Lesueur, D.; Potier, P.; Drazetic, P.; Markiewicz, E. Experimental study of the bone behaviour of the human skull bone for the development of a physical head model. Int. J. Crashworthiness 2007, 12, 101–108. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Santos, J.D.; Monteiro, F.J.; da Silva, M.H.P. Dissolution studies of hydroxyapatite and glass-reinforced hydroxyapatite ceramics. Mater. Charact. 2003, 50, 197–202. [Google Scholar] [CrossRef]
- Kim, Y.G.; Seo, D.S.; Lee, J.K. Comparison of dissolution resistance in artificial hydroxyapatite and biologically derived hydroxyapatite ceramics. J. Phys. Chem. Solids 2008, 69, 1556–1559. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Gamys, C.G.; Gosselin, M.; Zhao, F. In-Vitro Dissolution and Mineralization of Silicon-Doped Hydroxyapatite Produced by a Thermal Technique. Available online: https://www.frontiersin.org/10.3389/conf.fbioe.2016.01.00837/event_abstract (accessed on 1 February 2022).
- Motherway, J.A.; Verschueren, P.; Van der Perre, G.; Vander Sloten, J.; Gilchrist, M.D. The mechanical properties of cranial bone: The effect of loading rate and cranial sampling position. J. Biomech. 2009, 42, 2129–2135. [Google Scholar] [CrossRef] [PubMed]



| Parameters/ Material | PEEK | PEEK/10SrHA & PEEK/10ZnHA | PEEK/20SrHA & PEEK/20ZnHA | PEEK/30SrHA & PEEK/30ZnHA |
|---|---|---|---|---|
| Temperature (°C) Z1, Z2, Z3, Z4 | 355, 365, 375, 390 | 360, 370, 380, 400 | 365, 380, 400, 410 | 380, 400, 410, 420 |
| Feeding rate (g/min) | 3.3 | 2.8 | 2.3 | 2.1 |
| Extruder Screw speed (RPM) | 5.5 | 5.5 | 5.5 | 5.5 |
| Cooling fan speed (%) | 100 | 90 | 80 | 80 |
| Nozzle diameter (mm) | 4 | 4 | 4 | 4 |
| Puller wheel speed (RPM) | 1100 | 1000 | 950 | 900 |
| PEEK | PEEK/10SrHA & PEEK/10ZnHA | PEEK/20SrHA & PEEK/20ZnHA | PEEK/30SrHA & PEEK/30ZnHA | |
|---|---|---|---|---|
| Nozzle temperature (°C) | 390 | 410 | 420 | 430 |
| Bed temperature (°C) | 150 | 160 | 180 | 200 |
| Chamber temperature (°C) | 75 | 80 | 80 | 80 |
| Layer thickness (mm) | 0.2 | 0.2 | 0.2 | 0.2 |
| Printing speed (mm/s) | 30 | 30 | 30 | 30 |
| Infill density (%) | 100 | 100 | 100 | 100 |
| Infill pattern | −45, +45 | −45, +45 | −45, +45 | −45, +45 |
| Nozzle diameter (mm) | 0.5 | 0.5 | 0.5 | 0.5 |
| Tg, Mid-Point (°C) | Tm (°C) | Tc (°C) | Xc (%) | |
|---|---|---|---|---|
| PEEK | 143.3 | 343.1 | 285.8 | 27.7 |
| PEEK/10SrHA | 144.1 | 348.3 | 283.3 | 28.1 |
| PEEK/10ZnHA | 143.2 | 347.5 | 279.4 | 28.7 |
| PEEK/20SrHA | 146.7 | 351.5 | 277.5 | 30.5 |
| PEEK/20ZnHA | 144.4 | 350.1 | 282.2 | 31.5 |
| PEEK/30SrHA | 148.1 | 356.7 | 276.8 | 32.3 |
| PEEK/30ZnHA | 145.3 | 355.0 | 277.2 | 34.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzoor, F.; Golbang, A.; Dixon, D.; Mancuso, E.; Azhar, U.; Manolakis, I.; Crawford, D.; McIlhagger, A.; Harkin-Jones, E. 3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants. Polymers 2022, 14, 1376. https://doi.org/10.3390/polym14071376
Manzoor F, Golbang A, Dixon D, Mancuso E, Azhar U, Manolakis I, Crawford D, McIlhagger A, Harkin-Jones E. 3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants. Polymers. 2022; 14(7):1376. https://doi.org/10.3390/polym14071376
Chicago/Turabian StyleManzoor, Faisal, Atefeh Golbang, Dorian Dixon, Elena Mancuso, Usaid Azhar, Ioannis Manolakis, Daniel Crawford, Alistair McIlhagger, and Eileen Harkin-Jones. 2022. "3D Printed Strontium and Zinc Doped Hydroxyapatite Loaded PEEK for Craniomaxillofacial Implants" Polymers 14, no. 7: 1376. https://doi.org/10.3390/polym14071376







