Structural Characterization of Degraded Lycium barbarum L. Leaves’ Polysaccharide Using Ascorbic Acid and Hydrogen Peroxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
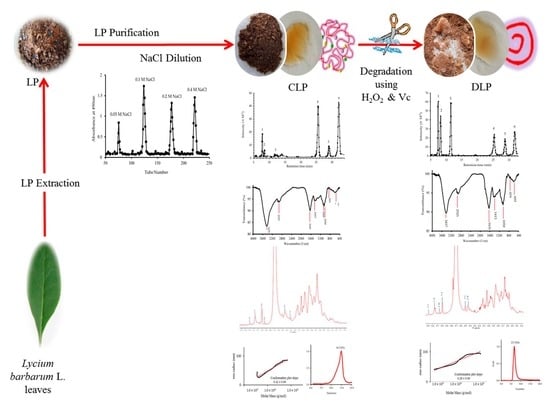
2.2. LP Extraction
2.3. Protien Removal
2.4. LP Purification
2.5. Degradation of LP
2.6. Uronic Acid Content
2.7. Mineral Elements Content
2.8. Monosaccharide Analysis
2.9. Fourier Transform Infrared Spectrometer
2.10. Nuclear Magnetic Resonance Spectra
2.11. Molecular Weight (Mw) and LP Conformation
2.12. Scanning Electron Microscopy (SEM) Analysis
2.13. Statistical Analysis
3. Results
3.1. LP Characteristics
3.2. LP Proximate Composition
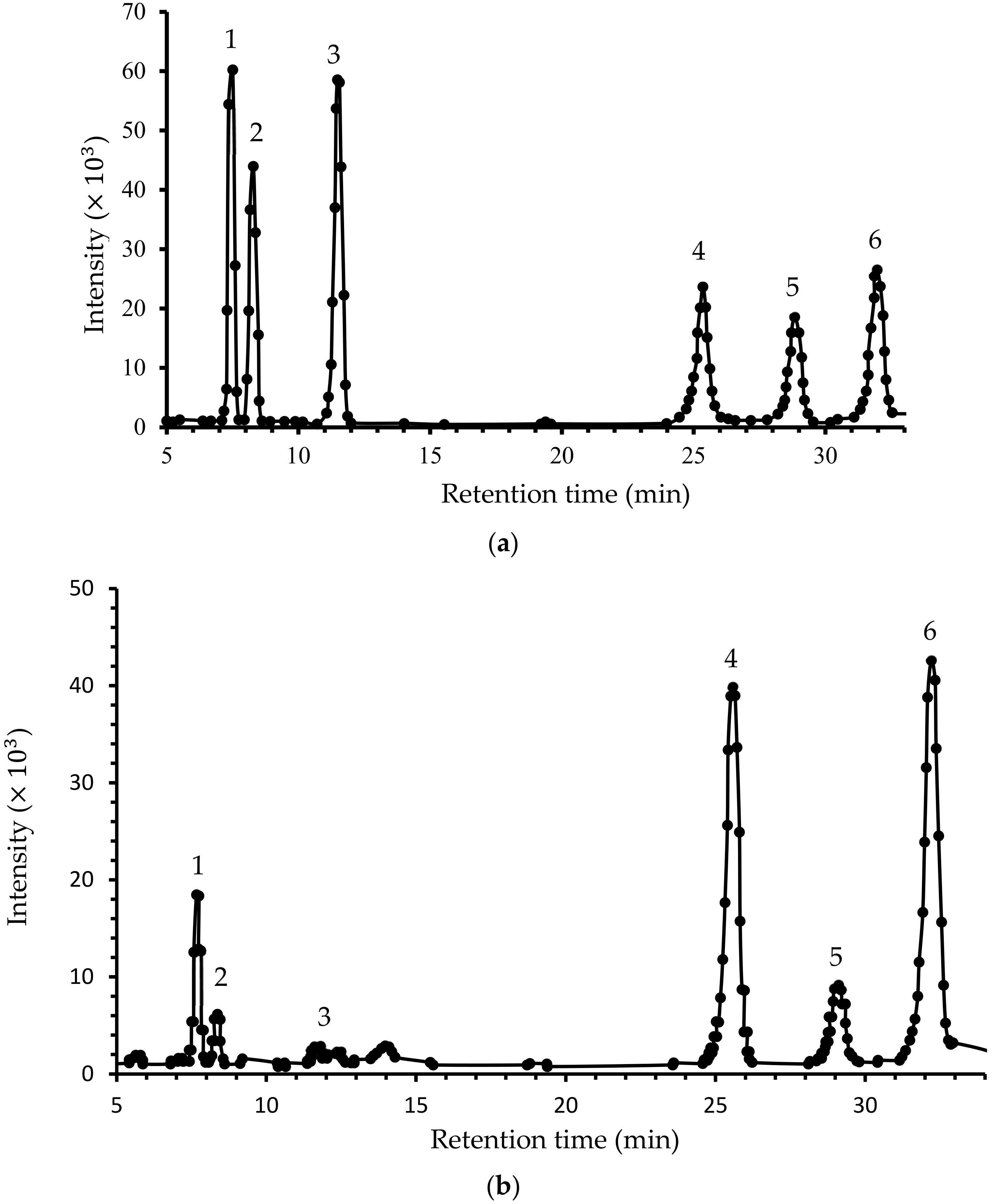
3.3. Monosaccharaides Composition
3.4. FTIR Analysis
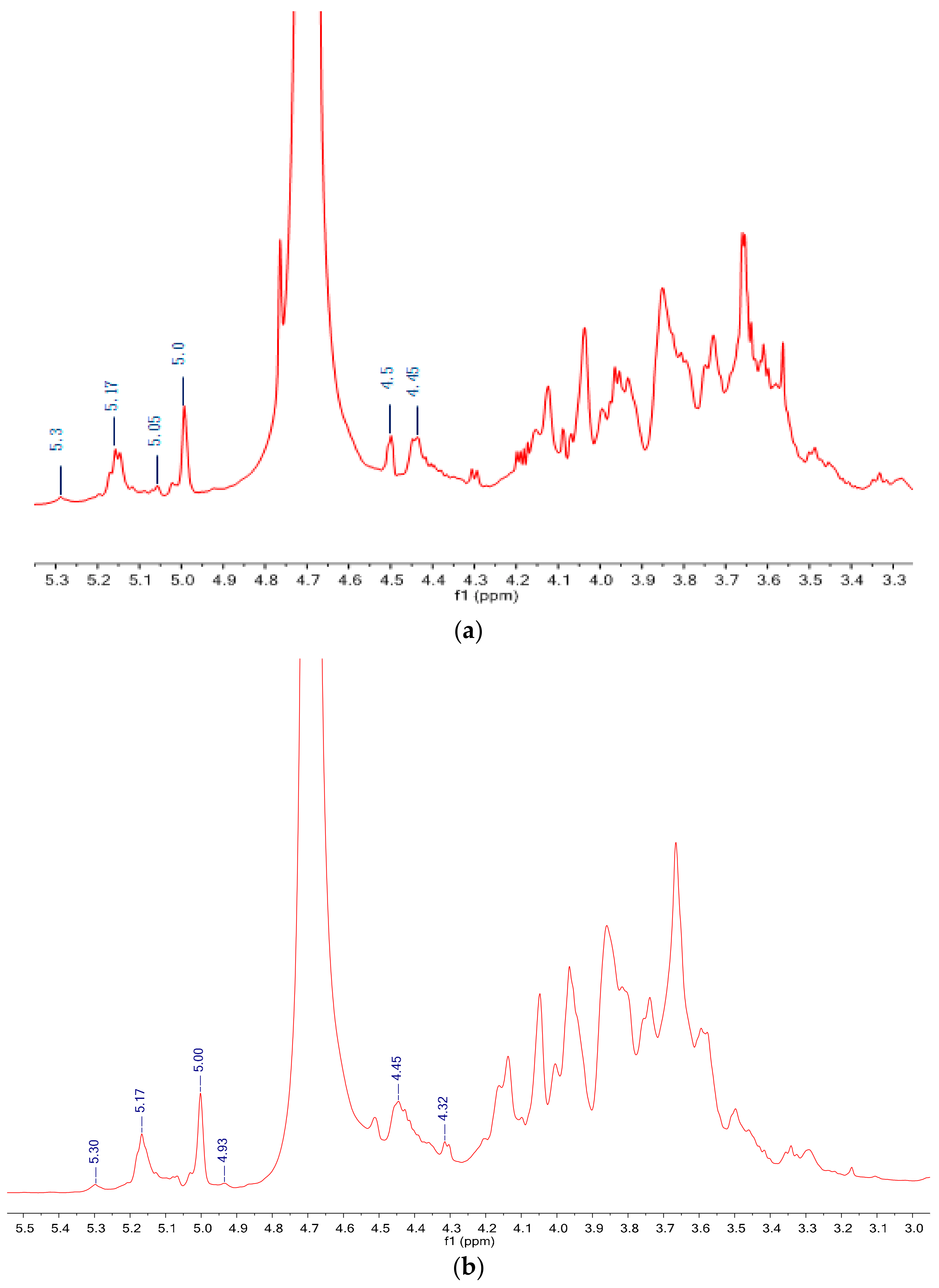
3.5. NMR Spectra Analysis
3.6. LP Molecular Weight and Conformation
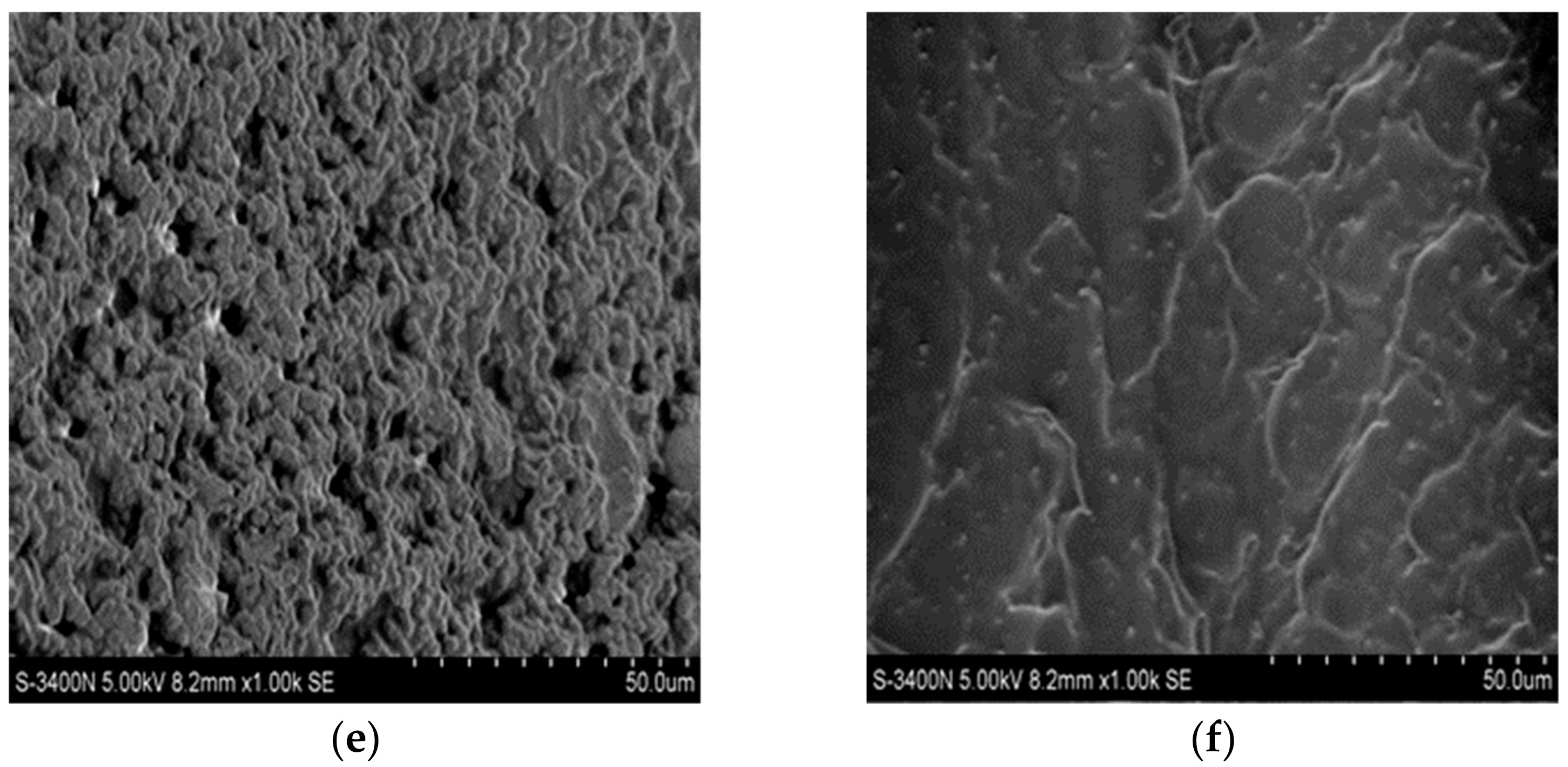
3.7. LP Surface Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, R.; Heinrich, M.; Weckerle, C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byambasuren, S.E.; Wang, J.; Gaudel, G. Medicinal value of wolfberry (Lycium barbarum L.). J. Med. Plants Stud. 2019, 7, 90–97. [Google Scholar]
- Tang, W.-M.; Chan, E.; Kwok, C.-Y.; Lee, Y.-K.; Wu, J.-H.; Wan, C.-W.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Deng, Q.; Zhou, W.; Zhang, Y. Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 2021, 229, 107921. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Biologically active compounds from goji (Lycium barbarum L.) leaves aqueous extracts: Purification and concentration by membrane processes. Biomolecules 2020, 10, 935. [Google Scholar] [CrossRef]
- Wang, C.; Chang, S.; Inbaraj, B.S.; Chen, B. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010, 120, 184–192. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum leaves: Isolation, characterization and splenocyte proliferation activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef]
- Kwok, S.S.; Bu, Y.; Lo, A.C.; Chan, T.C.; So, K.F.; Lai, J.S. A systematic review of potential therapeutic use of Lycium barbarum polysaccharides in disease. BioMed Res. Int. 2019, 2019, 1–18. [Google Scholar] [CrossRef]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Huang, Q.; Zhao, K.; Shang, P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 2013, 54, 16–23. [Google Scholar] [CrossRef]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Lin, S.; Al-Wraikat, M.; Niu, L.; Zhou, F.; Zhang, Y.; Wang, M.; Ren, J.; Fan, J.; Zhang, B.; Wang, L. Degradation enhances the anticoagulant and antiplatelet activities of polysaccharides from Lycium barbarum L. leaves. Int. J. Biol. Macromol. 2019, 133, 674–682. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Fu, L.; Al-Wraikat, M.; Lin, S.; Lu, P.; Shan, L.; Fan, J.; Zhang, B. Degradation of polysaccharides from Lycium barbarum L. leaves improves bioaccessibility and gastrointestinal transport of endogenous minerals. Int. J. Biol. Macromol. 2020, 143, 76–84. [Google Scholar] [CrossRef]
- Al-Wraikat, M.; Hou, C.; Zhao, G.; Lu, H.; Zhang, H.; Lei, Y.; Ali, Z.; Li, J. Degraded polysaccharide from Lycium barbarum L. leaves improve wheat dough structure and rheology. LWT 2021, 145, 111372. [Google Scholar] [CrossRef]
- Banerjee, A.; Halder, U.; Bandopadhyay, R. Preparations and Applications of Polysaccharide Based Green Synthesized Metal Nanoparticles: A State-of-the-Art. J. Clust. Sci. 2017, 28, 1803–1813. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. A Novel Green Preparation of Ag/RGO Nanocomposites with Highly Effective Anticancer Performance. Polymers 2021, 13, 3350. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A. Facile green synthesis of ZnO-RGO nanocomposites with enhanced an-ticancer efficacy. Methods 2021, 199, 28–36. [Google Scholar] [CrossRef]
- Ren, L.; Li, J.; Xiao, Y.; Zhang, Y.; Fan, J.; Zhang, B.; Wang, L.; Shen, X. Polysaccharide from Lycium barbarum L. leaves enhances absorption of endogenous calcium, and elevates cecal calcium transport protein levels and serum cytokine levels in rats. J. Funct. Foods 2017, 33, 227–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhao, M.; Qi, H. Free-radical degradation by Fe2+/Vc/H2O2 and antioxidant activity of polysaccharide from Tremella fuciformis. Carbohydr. Polym. 2014, 112, 578–582. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, J.; Jin, W.; Zhang, H.; Zhang, Q. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydr. Polym. 2012, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Wang, C.; Yu, T.; Wu, Q.; Li, Y.; Lv, Z.; Fan, J.; Wang, L.; Zhang, B. Endogenous calcium attenuates the immunomodulatory activity of a polysaccharide from Lycium barbarum L. leaves by altering the global molecular conformation. Int. J. Biol. Macromol. 2019, 123, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-Y.; Nie, S.-P.; Li, J.; Li, C.; Cui, S.; Xie, M.-Y. Mechanism of Interactions between Calcium and Viscous Polysaccharide from the Seeds of Plantago asiatica L. J. Agric. Food Chem. 2012, 60, 7981–7987. [Google Scholar] [CrossRef] [PubMed]
- Budny, J.; Fornal, J.; Obuchowski, W. Analysis of correlations between contents of protein fractions in wheat endosperm models and their mechanical resistance. J. Cereal Sci. 2016, 71, 10–18. [Google Scholar] [CrossRef]
- Guo, M.M.; Tang, H.G.; Yao, H.J.; Guo, J.Z. Study on the Isolation of Polysaccharide from Purple Sweet Potato by DEAE-Cellulose 52. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2013; Volume 690, pp. 1286–1291. [Google Scholar]
- Rover, M.R.; Johnston, P.A.; Lamsal, B.P.; Brown, R.C. Total water-soluble sugars quantification in bio-oil using the phenol–sulfuric acid assay. J. Anal. Appl. Pyrolysis 2013, 104, 194–201. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Mo, X.; Qi, H. Degradation and the antioxidant activity of polysaccharide from Enteromorpha linza. Carbohydr. Polym. 2013, 92, 2084–2087. [Google Scholar] [CrossRef]
- Akinyele, I.O.; Shokunbi, O.S. Comparative analysis of dry ashing and wet digestion methods for the determination of trace and heavy metals in food samples. Food Chem. 2015, 173, 682–684. [Google Scholar] [CrossRef]
- Ieggli, C.V.S.; Bohrer, D.; Do Nascimento, P.C.; De Carvalho, L.M.; Garcia, S.C. Determination of sodium, potassium, calcium, magnesium, zinc, and iron in emulsified egg samples by flame atomic absorption spectrometry. Talanta 2010, 80, 1282–1286. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Han, M.M.; Zhang, Z.Y.; Sun, Y.N.; Gao, Y.; Xu, D.D. Analysis on monosaccharide composition of white Hypsizygus marmoreus polysaccharides by pre-column derivatization HPLC. Food Sci. Technol. 2013, 38, 286–288. [Google Scholar]
- Stojilkovic, S.S.; Reinhart, J.; Catt, K.J. Gonadotropin-releasing hormone receptors: Structure and signal transduction pathways. Endocr. Rev. 1994, 15, 462–499. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.-M.; Qin, G.-Y. Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal. Food Chem. 2017, 234, 314–322. [Google Scholar] [CrossRef]
- Guo, R.; Cao, N.; Wu, Y.; Wu, J. Optimized extraction and molecular characterization of polysaccharides from Sophora alopecuroides L. seeds. Int. J. Biol. Macromol. 2016, 82, 231–242. [Google Scholar] [CrossRef]
- Hirsh, J. Heparin. N. Engl. J. Med. 1991, 324, 1565–1574. [Google Scholar]
- Zeng, H.L.; Zhang, Y.; Liu, J.; Zheng, B.D. Molar mass distribution and chain conformation of polysaccharides from Fortunella margarita (Lour.) Swingle. Chin. J. Struct. Chem. 2014, 33, 1245–1252. [Google Scholar]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332 Pt 2, 507. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Xie, J.-H.; Jin, M.-L.; Morris, G.; Zha, X.-Q.; Chen, H.-Q.; Yi, Y.; Ming-Liang, J.; Wang, Z.-J.; Gao, J.; Nie, S.-P.; et al. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit. Rev. Food Sci. Nutr. 2015, 56 (Suppl. S1), S60–S84. [Google Scholar] [CrossRef]
- Chen, R.-Z.; Tan, L.; Jin, C.-G.; Lu, J.; Chang, Q.-Q.; Wang, K. Extraction, isolation, characterization and antioxidant activity of polysaccharides from Astragalus membranaceus. Ind. Crop. Prod. 2015, 77, 434–443. [Google Scholar] [CrossRef]
- Wang, K.-P.; Wang, J.; Li, Q.; Zhang, Q.-L.; You, R.-X.; Cheng, Y.; Luo, L.; Zhang, Y. Structural differences and conformational characterization of five bioactive polysaccharides from Lentinus edodes. Food Res. Int. 2014, 62, 223–232. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsu, B.Y.; Chen, B.H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. Int. J. Biol. Macromol. 2010, 47, 445–453. [Google Scholar] [CrossRef]
- Inoue, A.; Kodama, N.; Nanba, H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol. Pharm. Bull. 2002, 25, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [Green Version]



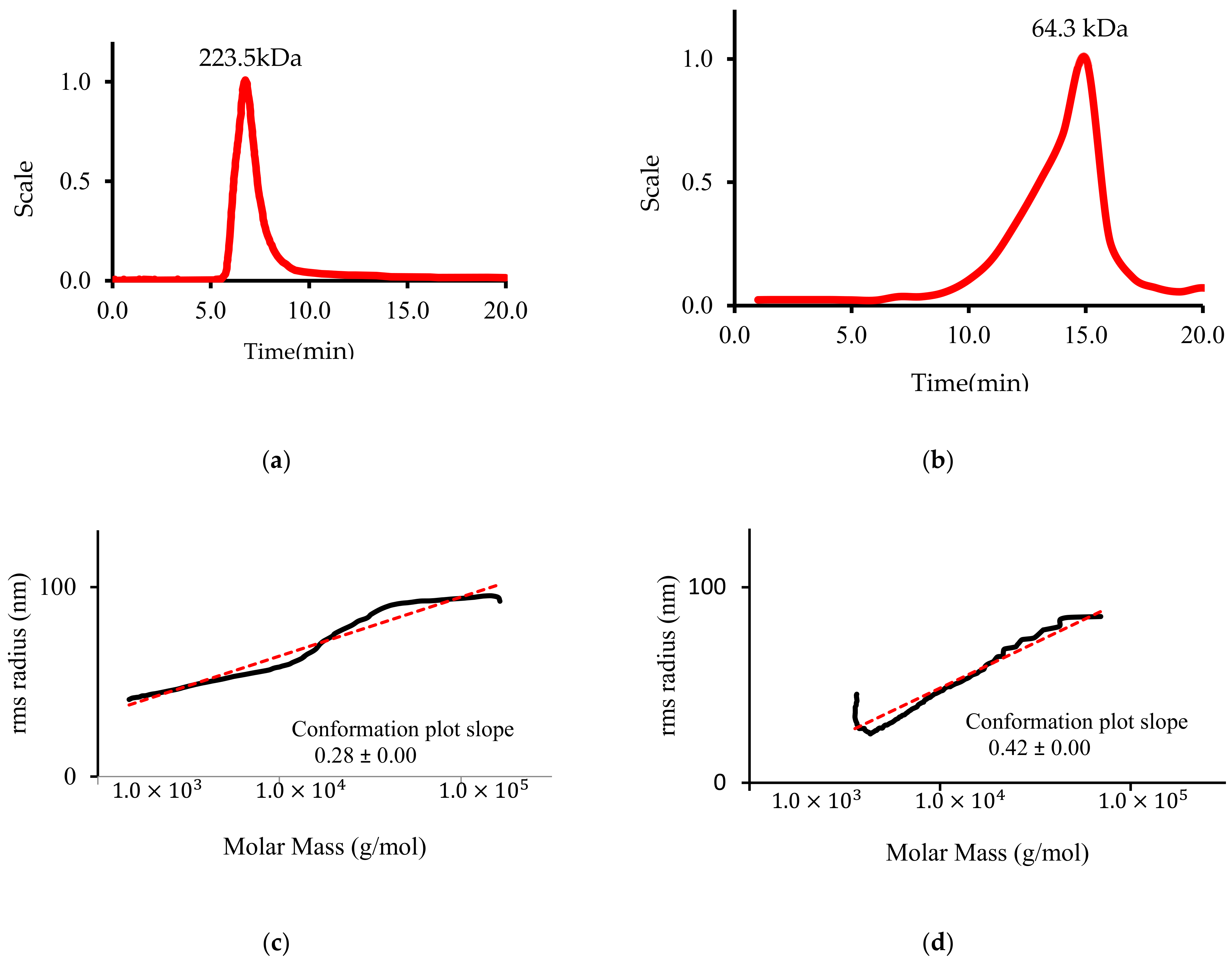

| Content | CLP | DLP |
|---|---|---|
| Mw (kDa) | 223.5 ± 1.13 | 64.3 ± 2.03 |
| Uronic Acid (%) | 13.7 ± 1.7 | 15.2 ± 0.7 |
| Protein (%) | 2.5 ± 0.1 | 2.67 ± 0.36 |
| Total Sugar (%) | 94.0 ± 7.3 | 89.2 ± 7.9 |
| Mg (mg/g) | 3.96 ± 0.22 a | 3.63 ± 0.40 a |
| Fe (mg/g) | 2.29 ± 0.32 b | 1.96 ± 0.17 a |
| Zn (mg/g) | 1.51 ± 0.16 a | 1.42 ± 0.35 b |
| Ca (mg/g) | 8.80 ± 1.1 a | 7.5 ± 0.2 b |
| H2O2 (mM) | Vc (mM) | Mw (kDa) | |
|---|---|---|---|
| CLP | - | - | 223.5 ± 1.13 a |
| DLP | 20 | 20 | 187.7 ± 0.53 a |
| 40 | 40 | 144.9 ± 2.40 b | |
| 60 | 60 | 96.26 ± 1.71 c | |
| 80 | 80 | 64.3 ± 2.03 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wraikat, M.; Liu, Y.; Wu, L.; Ali, Z.; Li, J. Structural Characterization of Degraded Lycium barbarum L. Leaves’ Polysaccharide Using Ascorbic Acid and Hydrogen Peroxide. Polymers 2022, 14, 1404. https://doi.org/10.3390/polym14071404
Al-Wraikat M, Liu Y, Wu L, Ali Z, Li J. Structural Characterization of Degraded Lycium barbarum L. Leaves’ Polysaccharide Using Ascorbic Acid and Hydrogen Peroxide. Polymers. 2022; 14(7):1404. https://doi.org/10.3390/polym14071404
Chicago/Turabian StyleAl-Wraikat, Majida, Yun Liu, Limei Wu, Zeshan Ali, and Jianke Li. 2022. "Structural Characterization of Degraded Lycium barbarum L. Leaves’ Polysaccharide Using Ascorbic Acid and Hydrogen Peroxide" Polymers 14, no. 7: 1404. https://doi.org/10.3390/polym14071404