Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Elastomer matrix: natural rubber RSS I (NR) provided by Torimex Chemicals Sp. z o.o. (Konstantynów Łódzki, Poland).
- Cross-linking system: sulphur (Siarkopol, Tarnobrzeg, Poland), stearin (POCH, Gliwice, Poland), 2-mercaptobenzothiazole (MBT) (Sigma-Aldrich, Poznań, Poland), micro-sized zinc oxide (ZnO) (Huta Będzin, Będzin, Poland).
- Dried plants: Mentha piperita L. (MP) provided by ManuTea (Chałupki, Poland) and Urtica dioica L. (CN) provided by Ziołowy Zakątek (Koryciny, Poland).
2.2. Preparation of Freeze-Dried Extracts
2.3. Preparation of Rubber Mixtures and Vulcanizates
- Plastification of natural rubber in Brabender measuring mixer N50 (Brabender Technologie GmBH & Co. KG, Duisburg, Germany) for 4 min with a rotational speed of 40 rpm and a temperature range of 40–60 °C.
- Addition of freeze-dried extracts to the plasticized natural rubber under the same conditions.
- Mixing with the cross–linking system using a two–roll mill at room temperature.
- Preparation of separate mixture without freeze-dried extracts as a reference sample.
2.4. Methods Used for Freeze-Dried Extracts
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. UV–VIS Diffuse Reflectance Spectroscopy
2.4.3. Near–Infrared Spectroscopy (NIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Total Phenolic Content (TPC)
2.4.6. Antioxidant Activity
- DPPH
- ABTS
2.5. Methods Used for Elastomer Vulcanizates
2.5.1. Rheometric Properties
2.5.2. Aging Processes
2.5.3. Cross–Linking Density
2.5.4. Barrier Properties
2.5.5. Mechanical Properties
2.5.6. Color Stability
3. Results
3.1. Characteristic of Freeze-Dried Extracts
3.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
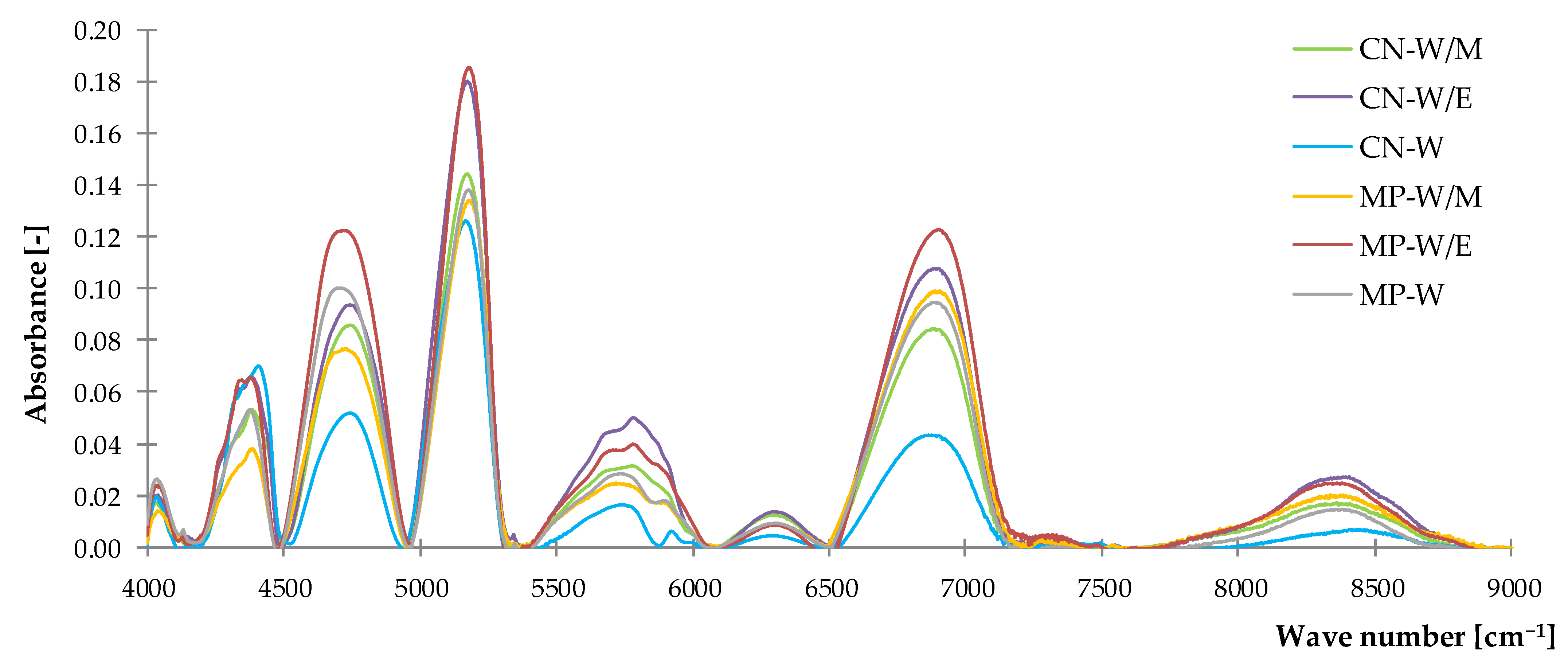
3.1.2. Near–Infrared Spectroscopy (NIR)
3.1.3. UV–VIS Diffuse Reflectance Spectroscopy
3.1.4. Thermal Stability
3.1.5. Total Phenolic Content (TPC) and Antioxidant Activity
3.2. Characteristic of Vulcanizates
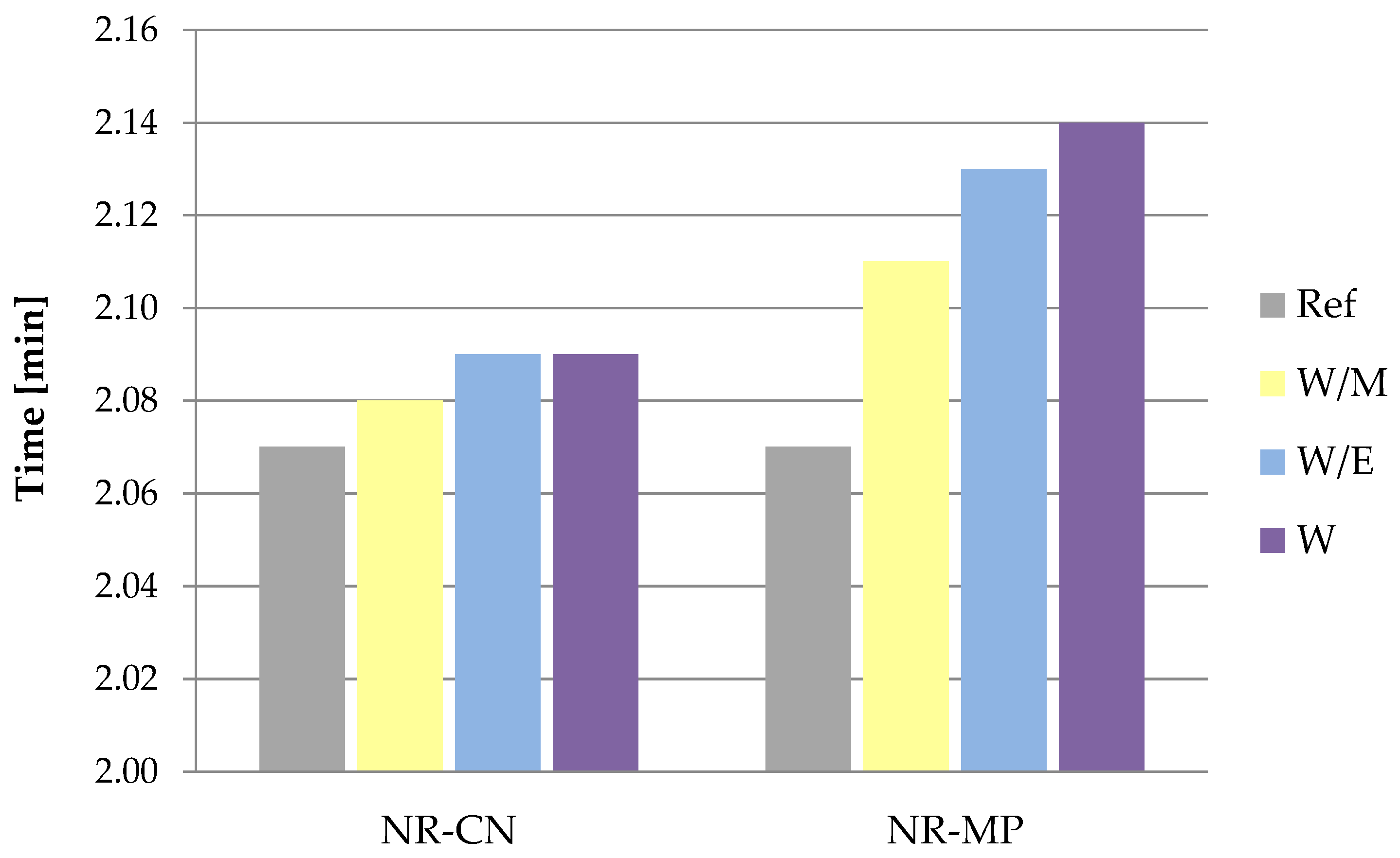
3.2.1. Rheometric Properties
3.2.2. Cross–Linking Density
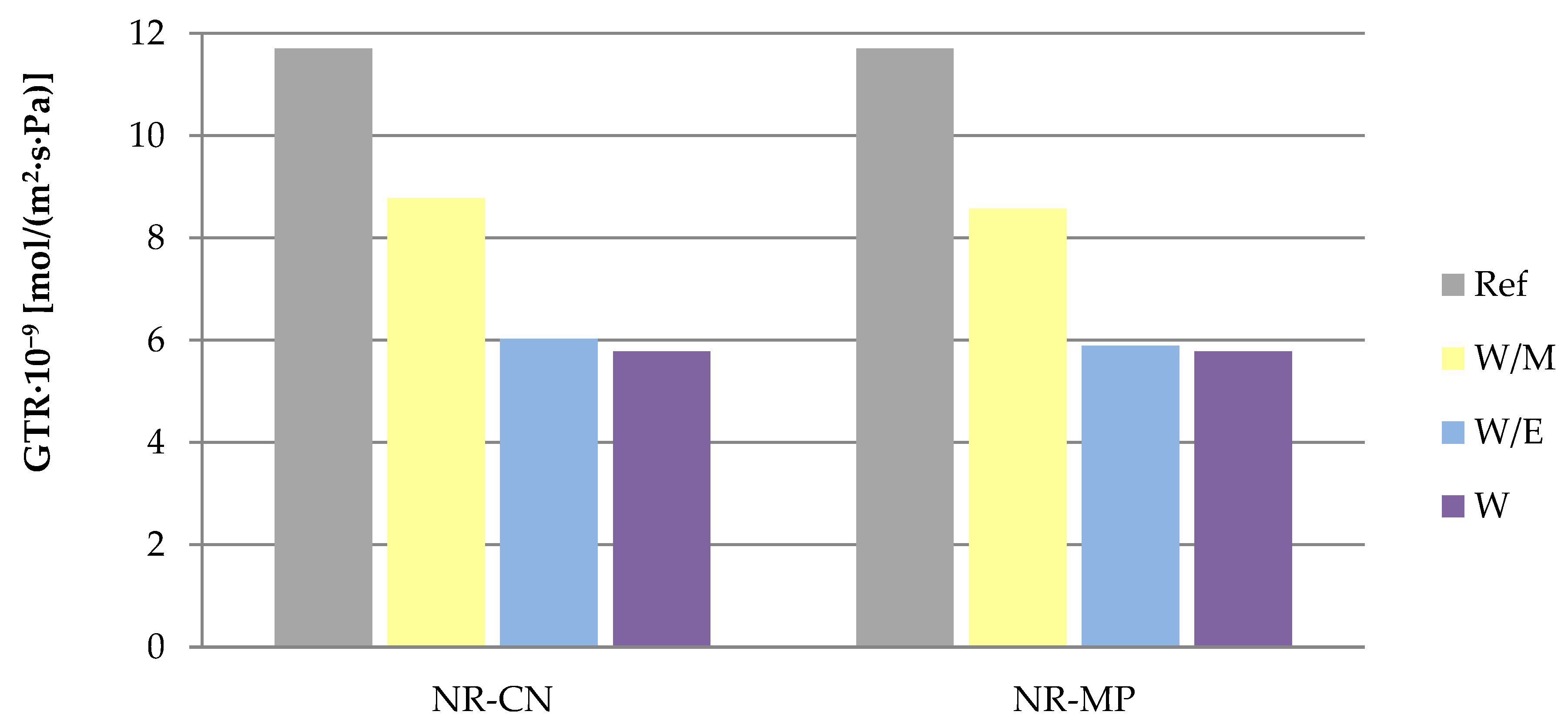
3.2.3. Barrier Properties
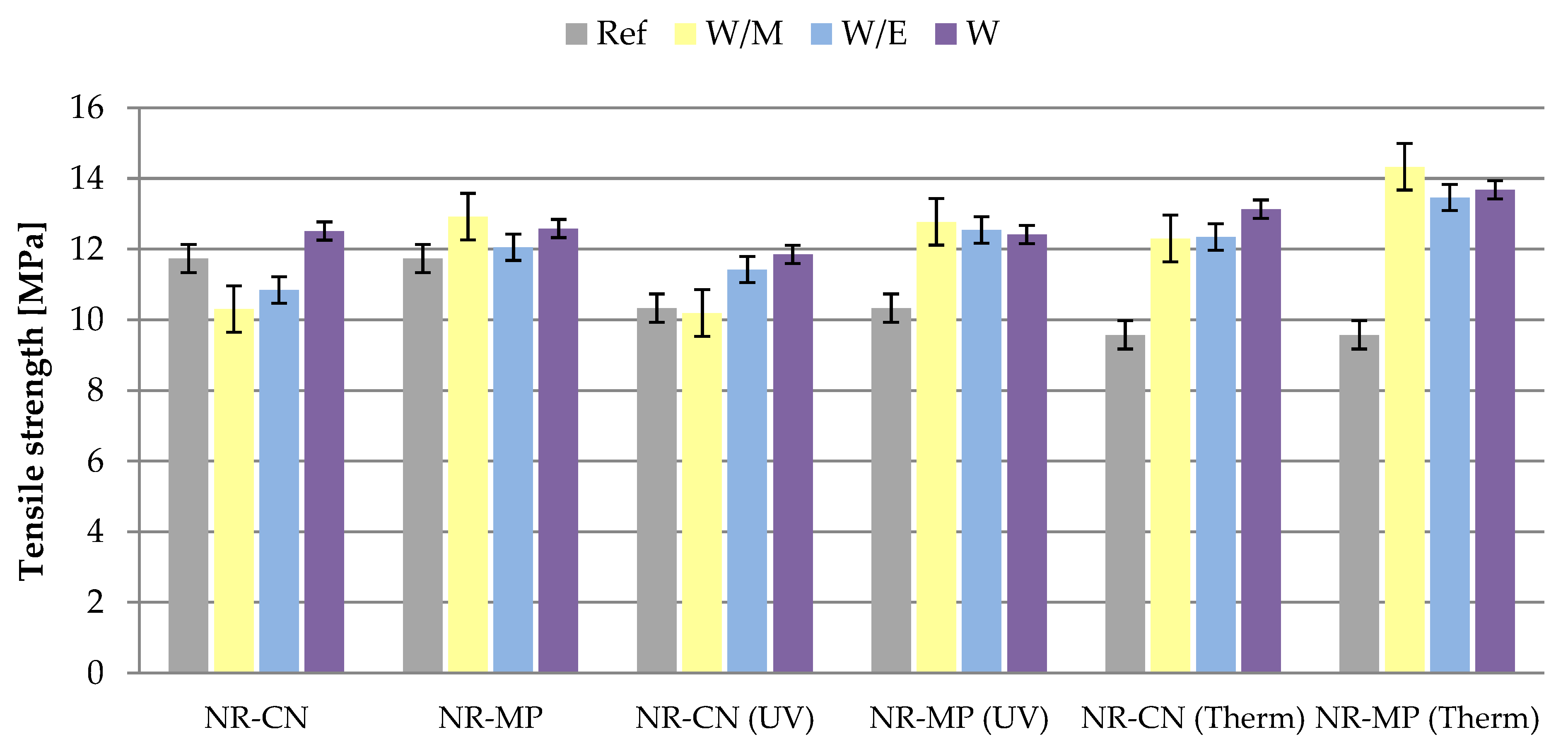
3.2.4. Mechanical Properties
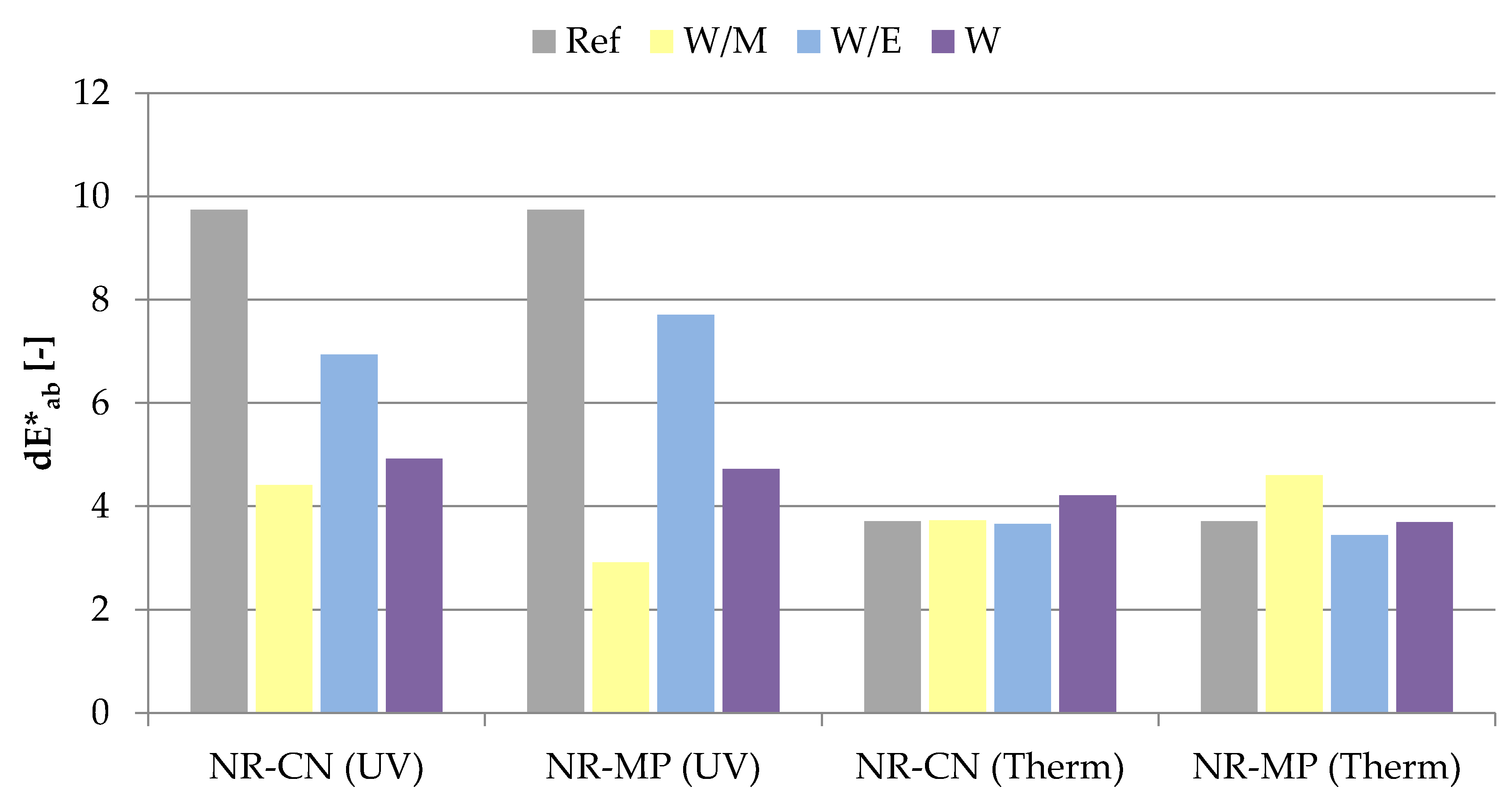
3.2.5. Color Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Li, Q.; Liu, R.; Niu, Y.; Pan, Y.; Zhai, Y.; Mei, Q. Medicinal Herbs in the Prevention and Treatment of Osteoporosis. Am. J. Chin. Med. 2014, 42, 1–22. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.; Pan, Z.; Leng, Y.; Lv, J.; Li, B. The effects of herbal medicine on epilepsy. Oncotarget 2017, 8, 48385–48397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahromi, B.; Pirvulescu, I.; Candido, K.; Knezevic, N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- Cadar, R.-L.; Amuza, A.; Dumitras, D.; Mihai, M.; Pocol, C. Analysing Clusters of Consumers Who Use Medicinal and Aromatic Plant Products. Sustainability 2021, 13, 8648. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Pluháčková, H.; Gregor, T.; Boško, R.; Běláková, S.; Svoboda, Z.; Benešová, K. Fortification of Beer with Extracts of the Selected Czech Medicinal Herbs and Plants. Kvas. Prum. 2020, 66, 314–319. [Google Scholar] [CrossRef]
- Mousa, M.A.; Khalid, N.T.; Hamood, E.K. Reduction of acrylamide content of bread by some herbs and plants. Biochem. Cell. Arch. 2019, 19, 2819–2822. [Google Scholar] [CrossRef]
- Dillard, C.J.; Bruce German, J. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices—A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaprakasha, G.; Selvi, T.; Sakariah, K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Alasmary, F.A.; Awaad, A.S.; Kamal, M.; Alqasoumi, S.I.; Zain, M.E. Antitumor activity of extract and isolated compounds from Drechslera rostrata and Eurotium tonophilum. Saudi Pharm. J. 2018, 26, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-C.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.-A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Tonutti, I.; Liddle, P. Aromatic plants in alcoholic beverages. A review. Flavour Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Tokuyama-Nakai, S.; Kimura, H.; Hirabayashi, Y.; Ishihara, T.; Jisaka, M.; Yokota, K. Constituents of flavonol O-glycosides and antioxidant activities of extracts from seeds, sprouts, and aerial parts of Polygonum tinctorium Lour. Heliyon 2019, 5, e01317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.W.; Pandey, P.; Khan, F.; Souayeh, B.; Farhan, M. Study to Investigate the Potential of Combined Extract of Leaves and Seeds of Moringa oleifera in Groundwater Purification. Int. J. Environ. Res. Public Health 2020, 17, 7468. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Mesas, C.; Martínez, R.; Ortíz, R.; Galisteo, M.; López-Jurado, M.; Cabeza, L.; Perazzoli, G.; Melguizo, C.; Porres, J.; Prados, J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients 2021, 13, 566. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Da Silva, J.A.T. Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits. 3 Biotech 2015, 5, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa Vanegas, A.M.; Naranjo, J.P.; Diez, A.F.; Ocampo, O.; Monsalve, Z.L. Antibacterial and larvicidal activity against Aedes aegypti L. of extracts from Ambrosia peruviana willd (Altamisa). Rev. Cuba. Plantas Med. 2017, 22, 1–11. [Google Scholar]
- Al-Judaibi, A.; Al-Yousef, F. Antifungal effect of ethanol plant extract on Candida SP. Am. J. Agric. Biol. Sci. 2014, 9, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.; Singh, B.; Jha, K.K. Ethanolic whole plant extract of farsetia jacquemontii showed antipyretic & analgesic potential in mice. Acta Pharm. Sci. 2020, 58, 228. [Google Scholar] [CrossRef] [Green Version]
- Omar, G.; Abdallah, L.; Barakat, A.; Othman, R.; Bourinee, H. In vitro haemostatic efficacy of aqueous, methanol and ethanol plant extracts of three medicinal plant species in Palestine. Braz. J. Biol. 2020, 80, 763–768. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Komes, D.; Durgo, K.; Vojvodić, A.; Bušić, A. Nettle (Urtica dioica L.) extracts as functional ingredients for production of chocolates with improved bioactive composition and sensory properties. J. Food Sci. Technol. 2015, 52, 7723–7734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesbahzadeh, B.; Akbari, M.; Kor, N.M. The effects of different levels of peppermint alcoholic extract on body-weight gain and blood biochemical parameters of adult male Wistar rats. Electron. Physician 2015, 7, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Łukomska, A.; Baranowska-Bosiacka, I.; Goschorska, M.; Dec, K.; Wolska, J.; Janda, K.; Piotrowska, K.; Kupnicka, P.; Kapczuk, P.; et al. The influence of extracts from the seeds of the common nettle (Urtica dioica L.) on the activity of antioxidative enzymes in macrophages incubated with sodium fluoride. Fluoride 2018, 51, 65–76. [Google Scholar]
- Pramila, D.M.; Xavier, R.; Marimuthu, K.; Kathiresan, S.; Khoo, M.L.; Senthilkumar, M.; Sathya, K.; Sreeramanan, S. Phytochemical analysis and antimicrobial potential of methanolic leaf extract of peppermint (Mentha piperita: Lamiaceae). J. Med. Plants Res. 2012, 6, 331–335. [Google Scholar] [CrossRef]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.-R.; Seigneuret, J.-M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. Nettle (Urtica dioica L.) as a source of antioxidant and anti-aging phytochemicals for cosmetic applications. Comptes Rendus. Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grauso, L.; De Falco, B.; Lanzotti, V.; Motti, R. Stinging nettle, Urtica dioica L.: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2020, 19, 1341–1377. [Google Scholar] [CrossRef]
- Kosolapov, V.M.; Cherniavskih, V.I.; Zarudny, V.A.; Mazur, K.; Konieczna, A.; Tseiko, L.; Dumacheva, E.V.; Dumachev, D.V. Observations on the Productivity of Breeding Specimens of Urtica dioica L. from European Russian Ecotopes in Comparison with the Breeding Variety under Field Crop Conditions. Agronomy 2021, 12, 76. [Google Scholar] [CrossRef]
- Banjarnahor, S.D.S.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; Salih, Z.A.; Mukhtar, R.M.; Ali, A.O. Extraction and Characterization of Peppermint (Mentha piperita) Essential Oil and its Assessment as Antioxidant and Antibacterial. Gezira J. Eng. Appl. Sci. 2018, 13. [Google Scholar]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Jan, K.N.; Zarafshan, K.; Singh, S. Stinging nettle (Urtica dioica L.): A reservoir of nutrition and bioactive components with great functional potential. J. Food Meas. Charact. 2017, 11, 423–433. [Google Scholar] [CrossRef]
- Sehari, M.; Kouadria, M.; Amirat, M.; Sehari, N.; Hassani, A. Phytochemistry and antifungal activity of plant extracts from Nettle (Urtica dioica L.). Ukr. J. Ecol. 2020, 10, 1–6. [Google Scholar]
- Zhang, H.; Li, N.; Li, K.; Li, P. Protective effect of Urtica dioica methanol extract against experimentally induced urinary calculi in rats. Mol. Med. Rep. 2014, 10, 3157–3162. [Google Scholar] [CrossRef] [Green Version]
- Rita, P.; Animesh, D.K. An updated overview on peppermint (Mentha piperita L.). Int. Res. J. Pharm. 2011, 2, 1–10. [Google Scholar]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015, 22, 2440. [Google Scholar]
- Sústriková, A.; Salamon, I. Essential oil of peppermint (Mentha × piperita L.) from fields in Eastern Slovakia. Hortic. Sci. 2011, 31, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, S.C.C.; Menezes, A.P.P.; Barbalho, S.M.; Guiguer, É.L. Properties of Mentha piperita: A Brief Review. World J. Pharm. Med. Res. 2017, 3, 309–313. [Google Scholar]
- Singh, R.; Shushni, M.A.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Sarikhani, M.; Deylam, M.; Alizadeh, E.; Hejazy, M.; Alizadeh-Salteh, S.; Moeini, H.; Firouzamandi, M. Anti-aging effects of peppermint (Mentha piperita L.) and Shirazi thyme (Zataria multiflora Boiss.) plant extracts. Food Biosci. 2021, 41, 100930. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Benzaid, C.; Tichati, L.; Djeribi, R.; Rouabhia, M. Evaluation of the Chemical Composition, the Antioxidant and Antimicrobial Activities of Mentha × piperita Essential Oil against Microbial Growth and Biofilm Formation. J. Essent. Oil Bear. Plants 2019, 22, 335–346. [Google Scholar] [CrossRef]
- Vora, J.; Srivastava, A.; Modi, H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked 2018, 13, 128–132. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Efenberger-Szmechtyk, M.; Nowak, A.; Strzelec, K. Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes. Materials 2020, 13, 4903. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Common Nettle (Urtica dioica L.) as an Active Filler of Natural Rubber Biocomposites. Materials 2021, 14, 1616. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strąkowska, A.; Strzelec, K.; Szynkowska, M.I. The use of rye, oat and triticale straw as fillers of natural rubber composites. Polym. Bull. 2018, 75, 4607–4626. [Google Scholar] [CrossRef] [Green Version]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo fiber and its reinforced composites: Structure and properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Article modified nanoclays/straw fillers as functional additives of natural rubber biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Strzelec, K. Thermoplastic Elastomeric Composites Filled with Lignocellulose Bioadditives. Part 1: Morphology, Processing, Thermal and Rheological Properties. Materials 2020, 13, 1598. [Google Scholar] [CrossRef] [Green Version]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Hardinnawirda, K.; Aisha, I.S. Effect of Rice Husks as Filler in Polymer Matrix Composites. J. Mech. Eng. Sci. 2012, 2, 181–186. [Google Scholar] [CrossRef]
- Dong, Y.; Ghataura, A.; Takagi, H.; Haroosh, H.J.; Nakagaito, A.N.; Lau, K.-T. Polylactic acid (PLA) biocomposites reinforced with coir fibres: Evaluation of mechanical performance and multifunctional properties. Compos. Part A Appl. Sci. Manuf. 2014, 63, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Bokobza, L. Natural Rubber Nanocomposites: A Review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frida, E.; Bukit, N.; Ginting, E.M.; Bukit, B.F. The effect of carbon black composition in natural rubber compound. Case Stud. Therm. Eng. 2019, 16, 100566. [Google Scholar] [CrossRef]
- Low, D.; Supramaniam, J.; Soottitantawat, A.; Charinpanitkul, T.; Tanthapanichakoon, W.; Tan, K.; Tang, S. Recent Developments in Nanocellulose-Reinforced Rubber Matrix Composites: A Review. Polymer 2021, 13, 550. [Google Scholar] [CrossRef]
- Ansari, A.H.; Jakarni, F.M.; Muniandy, R.; Hassim, S.; Elahi, Z. Natural rubber as a renewable and sustainable bio-modifier for pavement applications: A review. J. Clean. Prod. 2021, 289, 125727. [Google Scholar] [CrossRef]
- Zainudin, Z.; Baharulrazi, N.; Hajjar, S.; Man, C. Natural rubber derivatives for adhesives applications: A review. Chem. Eng. Trans. 2021, 83, 493–498. [Google Scholar] [CrossRef]
- Ansari, A.H.; Jakarni, F.M.; Muniandy, R.; Hassim, S. A review on the application of natural rubber as asphalt modifier. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1075, 012031. [Google Scholar] [CrossRef]
- Vijayaram, T.R. SA Technical Review on Rubber. Int. J. Des. Manuf. Technol. 2009, 3, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Ginting, E.M.; Bukit, N.; Frida, E.; Bukit, B.F. Microstructure and thermal properties of natural rubber compound with palm oil boilers ash for nanoparticle filler. Case Stud. Therm. Eng. 2020, 17, 17. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. The potential application of cereal straw as a bio-filler for elastomer composites. Polym. Bull. 2020, 77, 2021–2038. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasan, D.P.; Sujith, A.; Rajesh, C. Cure characteristics and mechanical properties of biocomposites of natural rubber reinforced with chicken feather fibre: Effect of fibre loading, alkali treatment, bonding and vulcanizing systems. Mater. Today Commun. 2019, 20, 100555. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-Magnesium Hydroxycarbonate/Azo Dye Hybrids as Novel Multifunctional Colorants for Elastomer Composites. Polymer 2018, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Maciejewska, M.; Sowińska, A.; Kucharska, J. Organic Zinc Salts as Pro-Ecological Activators for Sulfur Vulcanization of Styrene–Butadiene Rubber. Polymer 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Rybiński, P.; Maniukiewicz, W. Novel eco-friendly hybrid pigment with improved stability as a multifunctional additive for elastomer composites with reduced flammability and pH sensing properties. Dye. Pigment. 2021, 186, 108965. [Google Scholar] [CrossRef]
- Doroshenko, I.; Pogorelov, V.; Sablinskas, V. Infrared Absorption Spectra of Monohydric Alcohols. Dataset Pap. Chem. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sadowska, U.; Matwijczuk, A.; Niemczynowicz, A.; Dróżdż, T.; Żabiński, A. Spectroscopic examination and chemometric analysis of essential oils obtained from peppermint herb (Mentha piperita L.) and caraway fruit (Carum carvi L.) subjected to pulsed electric fields. Processes 2019, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Metrohm NIR Spectroscopy: A Guide to Near-Infrared Spectroscopic Analysis of Industrial Manufacturing Processes. Available online: https://pdf.directindustry.com/pdf/metrohm/monograph/15372-781663-_3.html (accessed on 24 February 2022).
- Schulz, H.; Engelhardt, U.H.; Wegent, A.; Drews, H.-H.; Lapczynski, S. Application of Near-Infrared Reflectance Spectroscopy to the Simultaneous Prediction of Alkaloids and Phenolic Substances in Green Tea Leaves. J. Agric. Food Chem. 1999, 47, 5064–5067. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of phenolic compounds of grape skins during ripening by NIR spectroscopy. LWT 2011, 44, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Valdivieso, D.; Font, R.; Blanco-Díaz, M.T.; Moreno-Rojas, J.M.; Gómez, P.; Alonso-Moraga, Á.; Del Río-Celestino, M. Application of near-infrared reflectance spectroscopy for predicting carotenoid content in summer squash fruit. Comput. Electron. Agric. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Davey, M.W.; Saeys, W.; Hof, E.; Ramon, H.; Swennen, R.L.; Keulemans, J. Application of Visible and Near-Infrared Reflectance Spectroscopy (Vis/NIRS) to Determine Carotenoid Contents in Banana (Musa spp.) Fruit Pulp. J. Agric. Food Chem. 2009, 57, 1742–1751. [Google Scholar] [CrossRef]
- Brenna, O.V.; Berardo, N. Application of Near-Infrared Reflectance Spectroscopy (NIRS) to the Evaluation of Carotenoids Content in Maize. J. Agric. Food Chem. 2004, 52, 5577–5582. [Google Scholar] [CrossRef]
- Beć, K.B.; Huck, C.W. Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, Y.; Morisawa, Y. Principles and Characteristics of NIR Spectroscopy. In Near-Infrared Spectroscopy; Springer: Singapore, 2020. [Google Scholar]
- Ali, M.; Emsley, A.; Herman, H.; Heywood, R. Spectroscopic studies of the ageing of cellulosic paper. Polymer 2001, 42, 2893–2900. [Google Scholar] [CrossRef]
- Pratiwi, R.A.; Bayu, A.; Nandiyanto, D. How to Read and Interpret UV-VIS Spectrophotometric Results in Determining the Structure of Chemical Compounds. Indones. J. Educ. Res. Technol. 2021, 2, 1–20. [Google Scholar] [CrossRef]
- Ernawati; Suprayitno, E.; Hardoko; Yanuhar, U. Extraction of bioactive compounds fruit from Rhizophora mucronata using sonication method. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012122. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Remote Sensing of Environment Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Zaghdoudi, K.; Ngomo, O.; Vanderesse, R.; Arnoux, P.; Myrzakhmetov, B.; Frochot, C.; Guiavarc, H.Y. Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; Macêdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Da Costa, E.M.; Filho, J.M.B.; do Nascimento, T.G.; Macêdo, R.O. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 2002, 392–393, 79–84. [Google Scholar] [CrossRef]
- Ferreira, L.M.B.; Kobelnik, M.; Regasini, L.O.; Dutra, L.A.; da Silva Bolzani, V.; Ribeiro, C.A. Synthesis and evaluation of the thermal behavior of flavonoids: Thermal decomposition of flavanone and 6-hydroxyflavanone. J. Therm. Anal. Calorim. 2017, 127, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brñić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Fu, Z.-F.; Tu, Z.-C.; Zhang, L.; Wang, H.; Wen, Q.-H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Klimek-Szczykutowicz, M.; El-Ansary, D.O.; Mahmoud, E.A. Polyphenol profile and antimicrobial and cytotoxic activities of natural Mentha x piperita and Mentha longifolia populations in Northern Saudi Arabia. Processes 2020, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Khanzada, B.; Akhtar, N.; Okla, M.K.; Alamri, S.A.; Al-Hashimi, A.; Baig, M.W.; Rubnawaz, S.; AbdElgawad, H.; Hirad, A.H.; Haq, I.-U.; et al. Profiling of Antifungal Activities and In Silico Studies of Natural Polyphenols from Some Plants. Molecules 2021, 26, 7164. [Google Scholar] [CrossRef]
- Pavlić, B.; Kaplan, M.; Bera, O.; Olgun, E.O.; Canli, O.; Milosavljević, N.; Antić, B.; Zeković, Z. Microwave-assisted extraction of peppermint polyphenols—Artificial neural networks approach. Food Bioprod. Process. 2019, 118, 258–269. [Google Scholar] [CrossRef]
- Figueroa Pérez, M.G.F.; Rocha-Guzmán, N.E.; Mercado-Silva, E.; Loarca-Piña, G.; Reynoso-Camacho, R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014, 156, 273–278. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çinar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments fromwild nettle leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Garofulić, I.E.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive compounds in wild nettle (Urtica dioica L) leaves and stalks: Polyphenols and pigments upon seasonal and habitat variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2014, 131, 131. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.; Valentova, H.; Ilavsky, M.; Radovanović, B.; Budinski-Simendić, J. Curing Characteristics and Dynamic Mechanical Behaviour of Reinforced Acrylonitrile-Butadiene/Chlorosulfonated Polyethylene Rubber Blends. Mater. Sci. Forum 2005, 494, 475–480. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komethi, M.; Othman, N.; Ismail, H.; Sasidharan, S. Comparative study on natural antioxidant as an aging retardant for natural rubber vulcanizates. J. Appl. Polym. Sci. 2012, 124, 1490–1500. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, 44. [Google Scholar] [CrossRef] [PubMed]



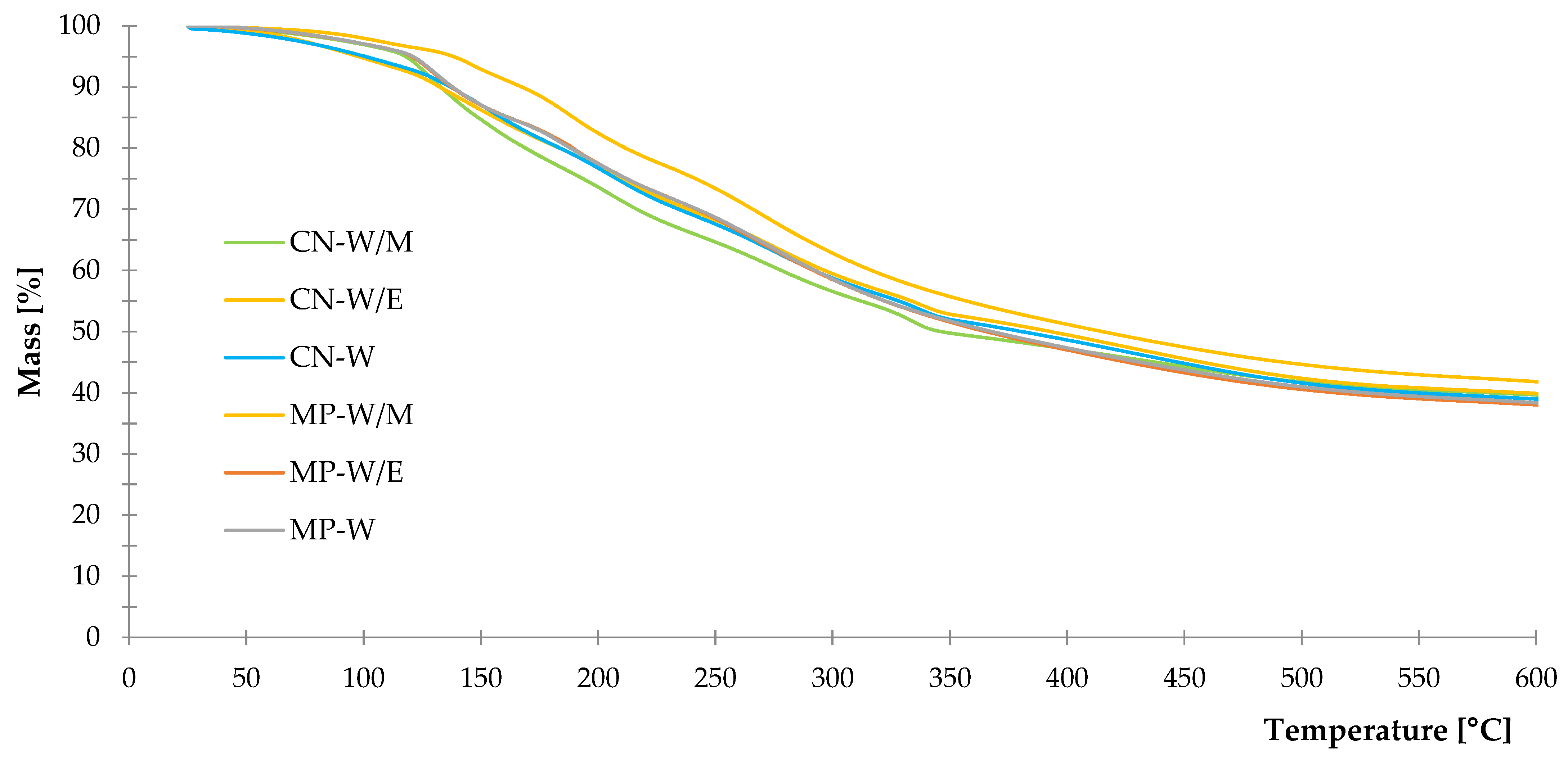



| Sample Name | Extract | NR | Stearin | ZnO | MBT | Sulfur |
|---|---|---|---|---|---|---|
| (phr 1) | ||||||
| Reference Sample (Ref) | 0 | 100 | 1 | 5 | 2 | 2 |
| NR-CN-W/M 2 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-CN-W/E 3 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-CN-W 4 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-MP-W/M 5 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-MP-W/E 6 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-MP-W 7 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| Peak Assignments and Type of Vibration | Wave Number [cm−1] |
|---|---|
| v (O–H) phenols & alcohols, –C=Ow (overtone) and v (=C–Hvw) | 3600–3000 |
| vs (C–H) aliphatic and vas (–C–Hm, –CH3, –CH2) | 2970–2800 |
| v (C=O) | 1730–1690 |
| v (C=C) in aromatic rings | 1680–1550 |
| vvw (–C=C–, cis-) and d (–OH) | 1675–1648 |
| v (C=C) aryl, dvw(–CH2) and (–CH3) bending (scissoring) or vvw (–C–H) bending (rocking) | 1600–1500 |
| v (C–C) aliphatic | 1500 |
| sv (C=C) aromatic | 1441 |
| (O–H) bending | 1390–1310 |
| v (–C–H, –CH3) | 1372–1337 |
| vm (–C–O) or dm (–CH2–), vw,m,vw (–C–H, –CH3) | 1285/1244 |
| v (–C–O), v (–C–H), v (–O–H) | 1056 |
| vm,vw (–C–O), | 1044/1023 |
| v (C–O), (C–C) | 1020–1030 |
| v (O–C–O) | 1090–1020 |
| vm (–C–O) | 1091 |
| Peak Assignments and Type of Vibration | Wave Number [cm−1] |
|---|---|
| s (–CHn) | 4034–4040 |
| s (–C–H) | 4375–4408 |
| s (–O–H) | 4531–4890 |
| s (–O–H) | 5093–5233 |
| s (–C–H) first overtone | 5551–5928 |
| s (–O–H) | 6670–7068 |
| s (–C–H) second overtone | 8093–8550 |
| Sample | Δm25–150 °C (%) | Δm150–350 °C (%) | Δm350–600 °C (%) | R600 (%) |
|---|---|---|---|---|
| CN-W/M | 15.4 | 34.9 | 10.0 | 39.7 |
| CN-W/E | 13.8 | 33.4 | 12.9 | 39.9 |
| CN-W | 13.0 | 35.0 | 13.0 | 39.0 |
| MP-W/M | 7.1 | 37.2 | 13.9 | 41.8 |
| MP-W/E | 13.0 | 35.5 | 13.5 | 38.0 |
| MP-W | 13.0 | 35.3 | 13.3 | 38.4 |
| Sample | TPC (µgGAE/mL) | Antioxidant Activity (mgTE/mL) | |
|---|---|---|---|
| ABTS | DPPH | ||
| MP-W/M | 6206.2 ± 41.8 a | 11.75 ± 0.06 a | 9.12 ± 0.23 a |
| MP-W/E | 5208.2 ± 276.9 b | 9.67 ± 0.05 b | 8.89 ± 0.94 a,b |
| MP-W | 540.5 ± 15.7 e | 1.45 ± 0.03 e | 2.30 ± 0.04 c |
| CN-W/M | 1282.4 ± 54.9 d | 1.85 ± 0.01 d | 2.25 ± 0.13 c |
| CN-W/E | 2972.5 ± 115.0 c | 5.47 ± 0.02 c | 8.53 ± 0.06 b |
| CN-W | 61.2 ± 5.2 f | 0.09 ± 0.01 f | 0.20 ± 0.02 d |
| Sample | νe × 105 (mol/cm3) | ||
|---|---|---|---|
| Unaged | UV | Therm | |
| Ref | 1.63 ± 0.01 | 1.98 ± 0.03 | 1.74 ± 0.02 |
| NR-CN-W/M | 1.23 ± 0.04 | 1.75 ± 0.04 | 1.36 ± 0.03 |
| NR-CN-W/E | 1.19 ± 0.04 | 1.47 ± 0.03 | 1.33 ± 0.03 |
| NR-CN-W | 0.93 ± 0.03 | 1.49 ± 0.02 | 1.26 ± 0.01 |
| NR-MP-W/M | 1.60 ± 0.05 | 1.83 ± 0.05 | 1.63 ± 0.04 |
| NR-MP-W/E | 1.24 ± 0.04 | 1.49 ± 0.05 | 1.37 ± 0.03 |
| NR-MP-W | 1.16 ± 0.04 | 1.36 ± 0.04 | 1.33 ± 0.02 |
| Sample | Eb (%) | EbUV (%) | EbTherm (%) |
|---|---|---|---|
| Ref | 649.15 ± 4.48 | 527.74 ± 6.41 | 695.57 ± 8.31 |
| NR-CN-W/M | 646.47 ± 5.22 | 595.44 ± 4.14 | 748.33 ± 7.91 |
| NR-CN-W/E | 655.38 ± 5.23 | 621.50 ± 4.22 | 740.60 ± 6.34 |
| NR-CN-W | 645.40 ± 6.22 | 541.56 ± 2.49 | 731.39 ± 2.58 |
| NR-MP-W/M | 639.47 ± 7.18 | 528.49 ± 5.65 | 770.43 ± 9.98 |
| NR-MP-W/E | 668.26 ± 6.89 | 648.95 ± 5.37 | 744.90 ± 7.68 |
| NR-MP-W | 619.08 ± 5.81 | 639.61 ± 4.47 | 736.87 ± 5.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Efenberger-Szmechtyk, M.; Strzelec, K. Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates. Polymers 2022, 14, 1460. https://doi.org/10.3390/polym14071460
Masłowski M, Aleksieiev A, Miedzianowska J, Efenberger-Szmechtyk M, Strzelec K. Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates. Polymers. 2022; 14(7):1460. https://doi.org/10.3390/polym14071460
Chicago/Turabian StyleMasłowski, Marcin, Andrii Aleksieiev, Justyna Miedzianowska, Magdalena Efenberger-Szmechtyk, and Krzysztof Strzelec. 2022. "Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates" Polymers 14, no. 7: 1460. https://doi.org/10.3390/polym14071460
APA StyleMasłowski, M., Aleksieiev, A., Miedzianowska, J., Efenberger-Szmechtyk, M., & Strzelec, K. (2022). Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates. Polymers, 14(7), 1460. https://doi.org/10.3390/polym14071460







