High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications
Abstract
:1. Introduction
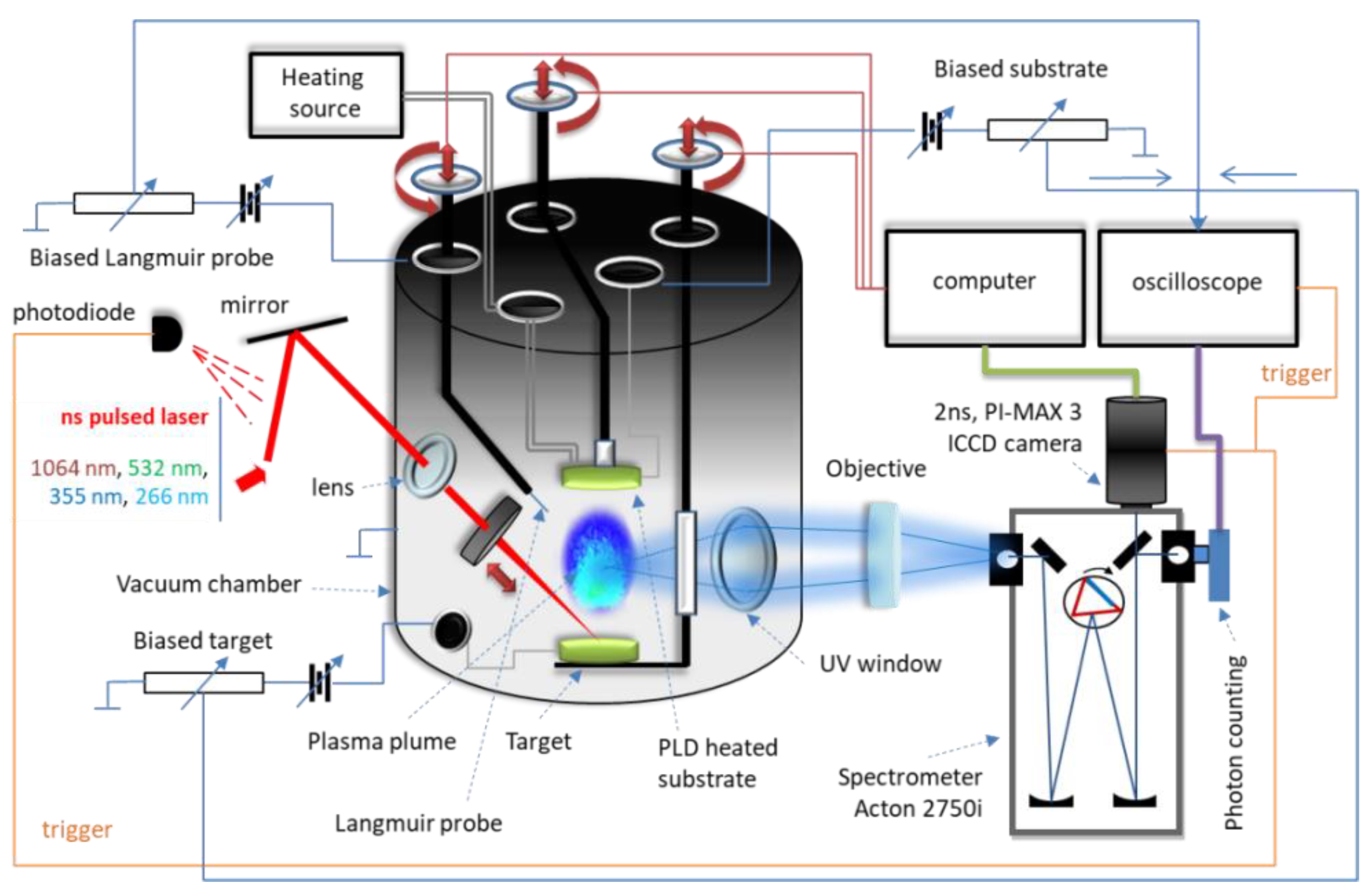
2. Materials and Methods
3. Results and Discussion
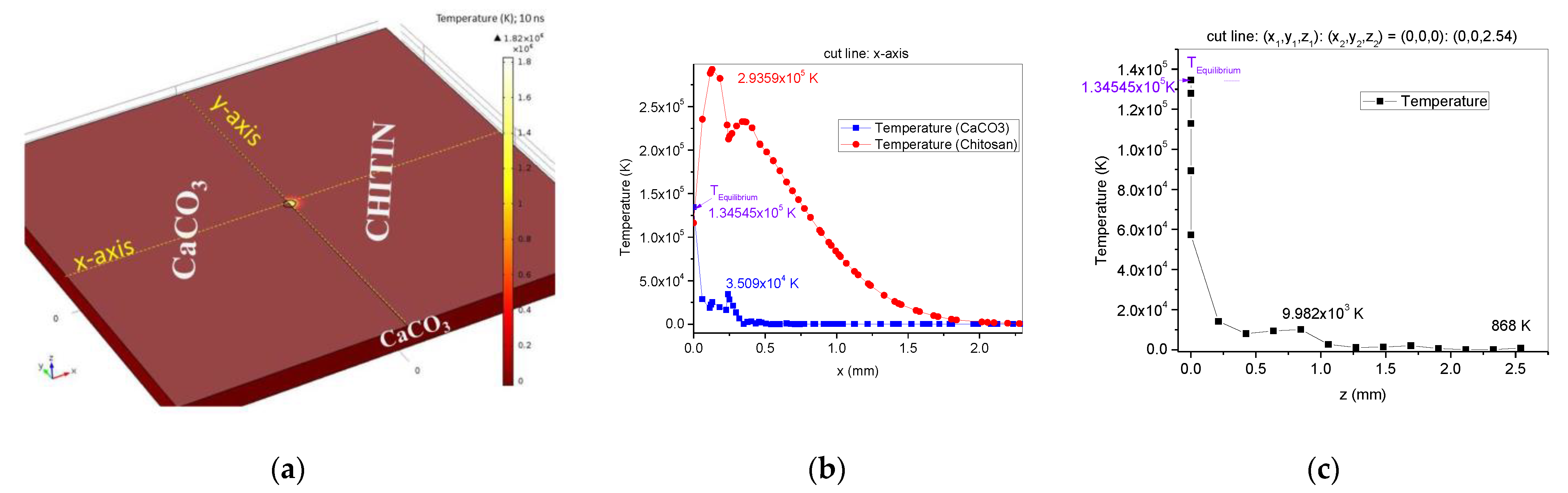
3.1. Numerical Simulation in COMSOL
3.2. Fourier-Transform Infrared Spectroscopy Analysis of Oyster Shell and Deposited Thin Film
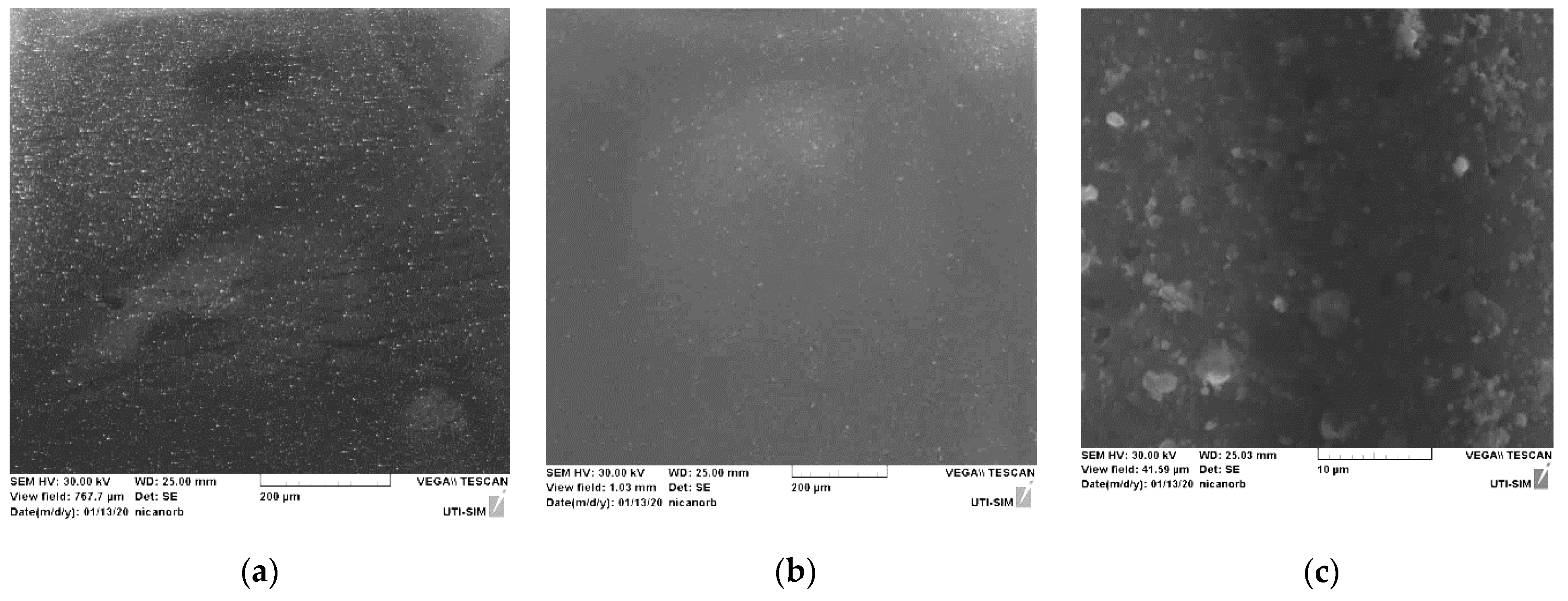
3.3. Elemental Composition Analysis with Energy-Dispersive X-ray Spectroscopy Coupled with Scanning Electron Microscopy (SEM–EDX)
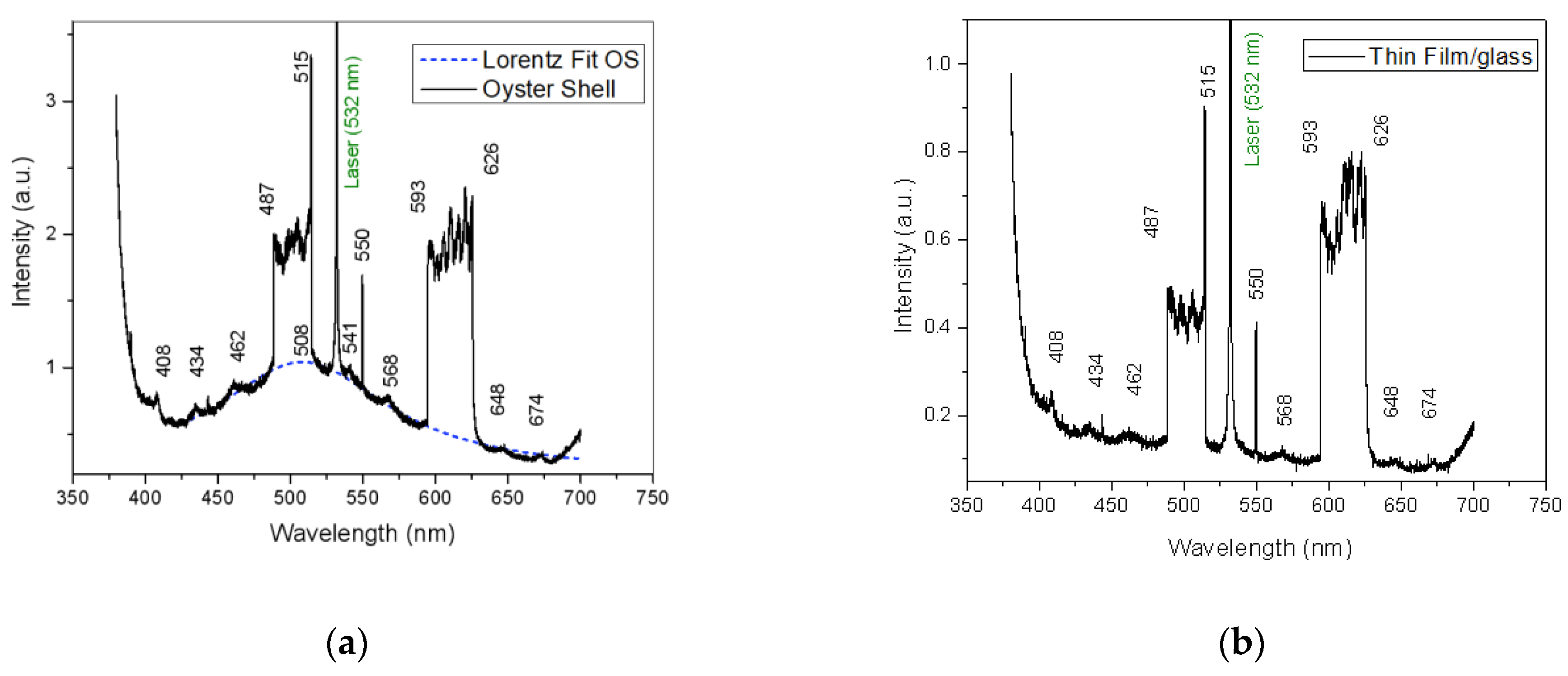
3.4. Laser-Induced Fluorescence
3.5. UV-VIS Thin Film Spectra Analysis
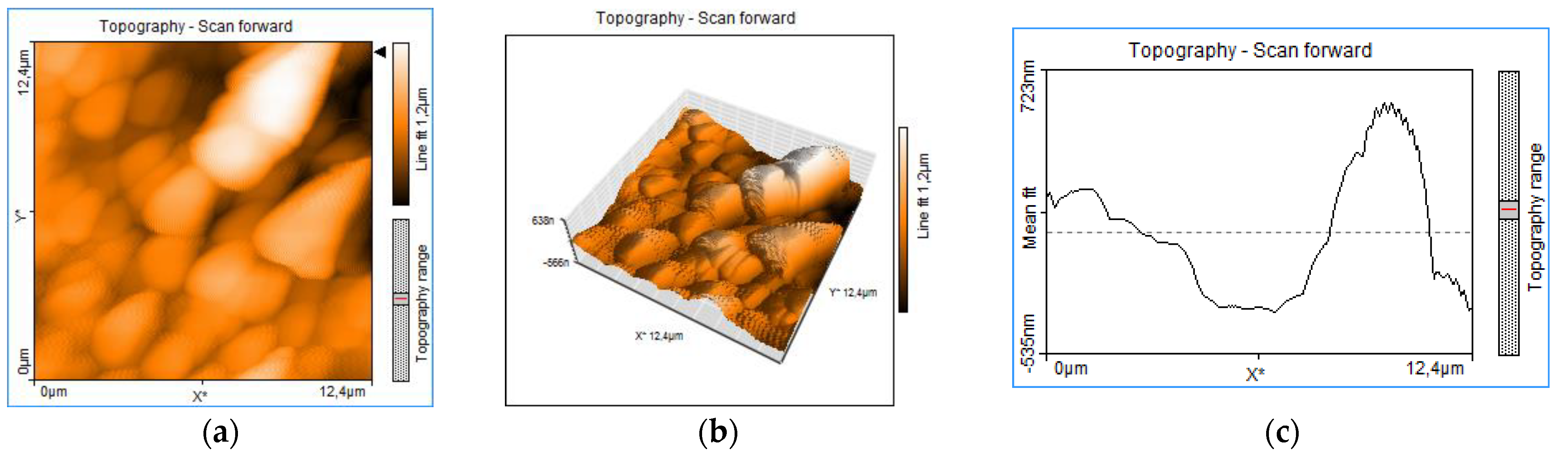
3.6. Atomic Force Microscopy Thin Film Analysis
3.7. Sorption Test of Thin Film of Chitosan
- ➢
- Oyster shell target: ; ; adsorbed
- ➢
- Ablation Plume: ; ; ; ; ; ; ; ; ;
- ➢
- Thin film resulting from deposition on the substrate: ; ; adsorbed ; adsorbed ; adsorbed
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stricker-Krongrad, A.H.; Alikhassy, Z.; Matsangos, N.; Sebastian, R.; Marti, G.; Lay, F.; Harmon, J.W. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. EPlasty 2018, 18, e14. [Google Scholar]
- Hafner, A.; Lovrić, J.; Pepić, I.; Filipović-Grčić, J. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J. Microencapsul. 2011, 28, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 3, 20–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Geng, X.; Jiang, H.; Li, J.; Huang, J. Synthesis and characteristics of chitin and chitosan with the (2-hydroxy-3-trimethylammonium)propyl functionality, and evaluation of their antioxidant activity in vitro. Carbohydr. Polym. 2012, 89, 486–491. [Google Scholar] [CrossRef]
- Gokila, S.; Gomathi, T.; Sudha, P.N.; Anil, S. Removal of the heavy metal ion chromiuim(VI) using Chitosan and Alginate nanocomposites. Int. J. Biol. Macromol. 2017, 104, 1459–1468. [Google Scholar] [CrossRef]
- Akinyeye, O.J. Effect of Chitosan Powder Prepared from Snail Shells to Remove Lead (II) Ion and Nickel (II) Ion from Aqueous Solution and Its Adsorption Isotherm Model. Am. J. Appl. Chem. 2016, 4, 146. [Google Scholar] [CrossRef] [Green Version]
- Dewage, N.B.; Fowler, R.E.; Pittman, C.U.; Mohan, D.; Mlsna, T. Lead (Pb2+) sorptive removal using chitosan-modified biochar: Batch and fixed-bed studies. RSC Adv. 2018, 8, 25368–25377. [Google Scholar] [CrossRef] [Green Version]
- Al-Manhel, A.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Yasmeen, S.; Kabiraz, M.K.; Saha, B.; Qadir, G.; Masum, S. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int. Res. J. Pure Appl. Chem. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Shao, Z.; Zhou, P. Preparation and Characterization of Chitosan/Cu(II) Affinity Membrane for Urea Adsorption. J. Appl. Polym. Sci. 2003, 90, 1108–1112. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.P.; Thierry, B.; Gauthier, C. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Ahyat, N.M.; Mohamad, F.; Ahmad, A.; Azmi, A.A. Chitin and chitosan extraction from Portunus pelagicus. Malays. J. Anal. Sci. 2017, 21, 770–777. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; De Oliveira Lima, V.A.; De Farias Rached, R.Í.; Lima, E.P.N.; Da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- John, A.T. Chemical Composition of the Edible Oyster Shell Crassostrea Madrasensis (Preston 1916). J. Mar. Biol. Aquac. 2016, 2, 1–4. [Google Scholar] [CrossRef] [Green Version]
- De Moura, C.M.; de Moura, J.M.; Soares, N.M.; de Almeida Pinto, L.A. Evaluation of molar weight and deacetylation degree of chitosan during chitin deacetylation reaction: Used to produce biofilm. Chem. Eng. Process. Process Intensif. 2011, 50, 351–355. [Google Scholar] [CrossRef]
- Fauzi, I.; Arcana, I.M.; Wahyuningrum, D. Synthesis and Characterization of Solid Polymer Electrolyte from N-Succinyl Chitosan and Lithium Perchlorate. Adv. Mater. Res. 2014, 896, 58–61. [Google Scholar] [CrossRef]
- Aziz, S.B. Morphological and Optical Characteristics of Chitosan(1−x):Cux (4 ≤ x ≤ 12) Based Polymer Nano-Composites: Optical Dielectric Loss as an Alternative Method for Tauc’s Model. Nanomaterials 2017, 7, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocean, A.; Pelin, V.; Cazacu, M.M.; Cocean, I.; Sandu, I.; Gurlui, S.; Iacomi, F. Thermal effects induced by laser ablation in non-homogeneous limestone covered by an impurity layer. Appl. Surf. Sci. 2017, 424, 324–329. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Gurlui, S.; Iacomi, F. Study of the pulsed laser deposition phenomena by means of Comsol Multiphysics. Univ. Politeh. Buchar. Sci. Bull. Ser. A Appl. Math. Phys 2017, 79, 263–274. [Google Scholar]
- Optical Constants of CaCO3 (Calcium Carbonate, Calcite). Available online: http://refractiveindex.info/?shelf=main&book=CaCO3&page= (accessed on 31 January 2022).
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater. 2012, 35, 175–183. [Google Scholar] [CrossRef]
- Cocean, I.; Cocean, A.; Postolachi, C.; Pohoata, V.; Cimpoesu, N.; Bulai, G.; Iacomi, F.; Gurlui, S. Alpha keratin amino acids BEHVIOR under high FLUENCE laser interaction. Medical applications. Appl. Surf. Sci. 2019, 488, 418–426. [Google Scholar] [CrossRef]
- Cocean, A.; Cocean, I.; Cimpoesu, N.; Cocean, G.; Cimpoesu, R.; Postolachi, C.; Popescu, V.; Gurlui, S. Laser Induced Method to Produce Curcuminoid-Silanol Thin Films for Transdermal Patches Using Irradiation of Turmeric Target. Appl. Sci. 2021, 11, 4030. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Anal. Chem. 1952, 24, 1253–1294. [Google Scholar] [CrossRef]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data, 4th ed.; Revised and Enlarged Edition; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93810-1. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Andersen, F.A.; Brecevic, L.; Beuter, G.; Dell’Amico, D.B.; Calderazzo, F.; Bjerrum, N.J.; Underhill, A.E. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Bőkea, H.; Akkurtb, S.; Őzdemirb, S.; Gőktȕrkc, E.H.; Saltikd, E.N.C. Quantification of CaCO3–CaSO3·0.5H2O–CaSO4·2H2O mixtures by FTIR analysis and its ANN mode. Mater. Lett. 2004, 58, 723–726. [Google Scholar] [CrossRef] [Green Version]
- Israel, F.P. Light on Dark Matter; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Gates, B.C.; Knozinger, H. (Eds.) Advances in Catalysis; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 46. [Google Scholar]
- Davidson, G.; Carpenter, J.H. Spectroscopic Properties of Inorganic and Organometallic Compunds; Royal Society of Chemistry: London, UK, 1993; Volume 26. [Google Scholar]
- Tsuboi, Y.; Kimoto, N.; Kabeshita, M.; Itaya, A. Pulsed laser deposition of collagen and keratin. J. Photochem. Photobiol. A Chem. 2001, 145, 209–214. [Google Scholar] [CrossRef]
- Rabasovic, D.; Pantelic, D.V.; Jelenkovic, B.M.; Curcic, S.B.; Rabasovic, M.S.; Vrbica, M.D.; Lazovic, V.M.; Curcic, B.P.M.; Krmpot, A.J. Nonlinear microscopy of chitin and chitinous structures: A case study of two cave-dwelling insects. J. Biomed. Opt. 2015, 20, 016010. [Google Scholar] [CrossRef] [PubMed]
- Konwar, A.; Gogoi, N.; Majumdar, G.; Chowdhury, D. Green chitosan–carbon dots nanocomposite hydrogel film with superior properties. Carbohydr. Polym. 2015, 115, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Haze, O.; Dinnocenzo, J.P.; Farid, S.; Farid, R.S.; Gould, I.R. Bonded Exciplexes. A New Concept in Photochemical Reactions. J. Org. Chem. 2007, 72, 6970–6981. [Google Scholar] [CrossRef]
- Liang, J.; Nguyen, Q.L.; Matsika, S. Exciplexes and conical intersections lead to fluorescence quenching in π-stacked dimers of 2-aminopurine with natural purine nucleobases. Photochem. Photobiol. Sci. 2013, 12, 1387–1400. [Google Scholar] [CrossRef] [Green Version]
- Demchenko, A.P. Introduction to Fluorescence Sensing; Springer International Publishing Ag: New Nork, NY, USA, 2009; Volume 26, 590p, ISBN 978-1-4020-9002-8. [Google Scholar]
- Aziz, S.B.; Abidin, Z.H.Z.; Arof, A.K. Influence of silver ion reduction on electrical modulus parameters of solid polymer electrolyte based on chitosan-silver triflate electrolyte membrane. Express Polym. Lett. 2010, 4, 300–310. [Google Scholar] [CrossRef]
- Thamilarasan, V.; Sethuraman, V.; Gopinath, K.; Balalakshmi, C.; Govindarajan, M.; Mothana, R.A.; Siddiqui, N.A.; Khaled, J.M.; Benelli, G. Single Step Fabrication of Chitosan Nanocrystals Using Penaeus semisulcatus: Potential as New Insecticides, Antimicrobials and Plant Growth Promoters. J. Clust. Sci. 2018, 29, 375–384. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Malyar, Y.N.; Issaoui, N.; Vasilieva, N.Y.; Karacharov, A.A. Synthesis optimization, DFT and physicochemical study of chitosan sulfates. J. Mol. Struct. 2021, 1245, 131083. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Kazachenko, A.S. Assessment of various density functionals and solvation models to describe acid-base, spectral and complexing properties of thiobarbituric and barbituric acids in aqueous solution. J. Comput. Methods Sci. Eng. 2017, 17, 851–863. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.; Cheng, Y.; Kong, S.; Li, S.; Li, C.; Yang, L. Investigation of the Effects of Molecular Parameters on the Hemostatic Properties of Chitosan. Molecules 2018, 23, 3147. [Google Scholar] [CrossRef] [Green Version]
- Pogorielov, M.V.; Sikora, V.Z. Chitosan as a Hemostatic Agent: Current State. Eur. J. Med. Ser. B 2015, 2, 24–33. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef] [PubMed]
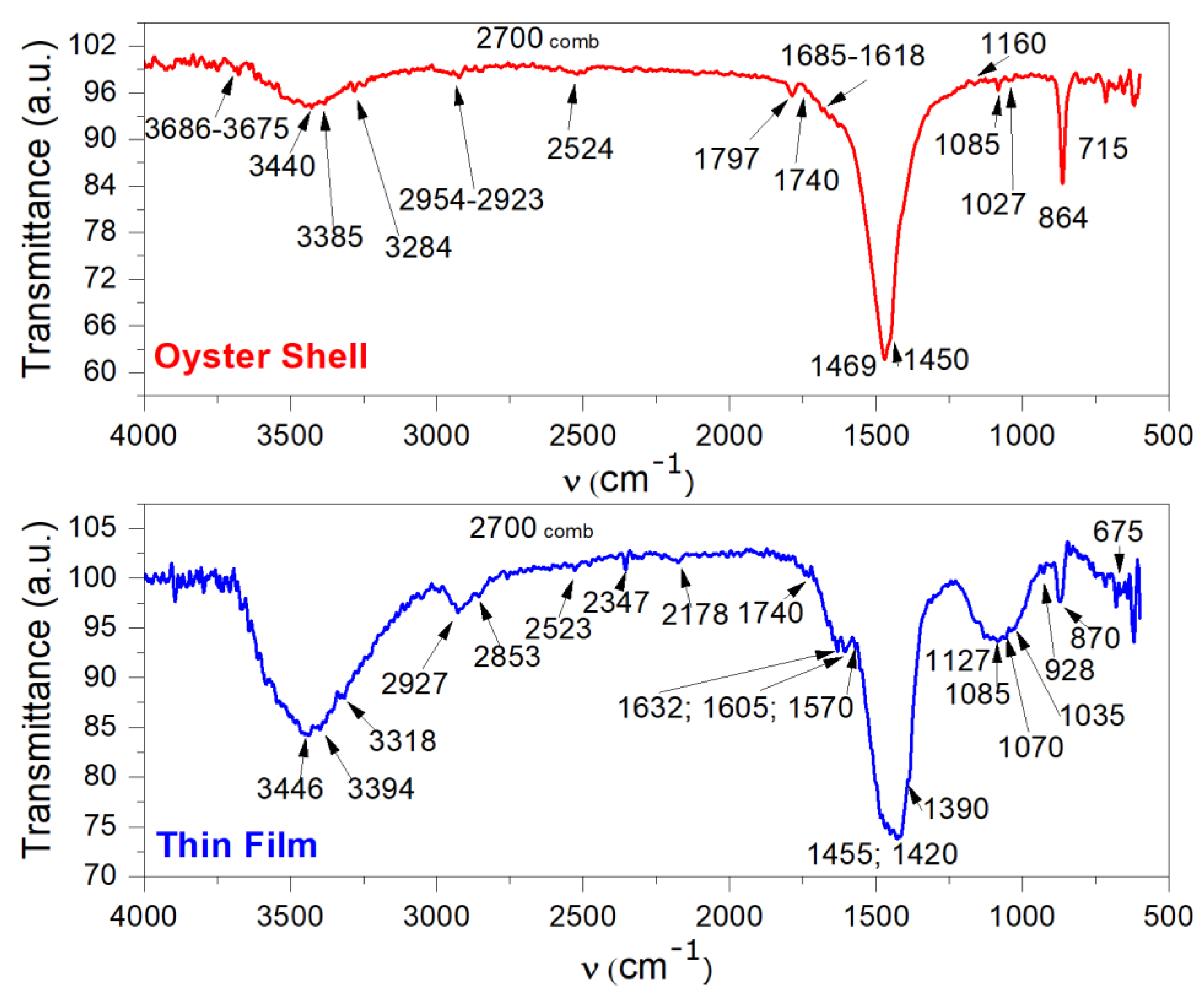
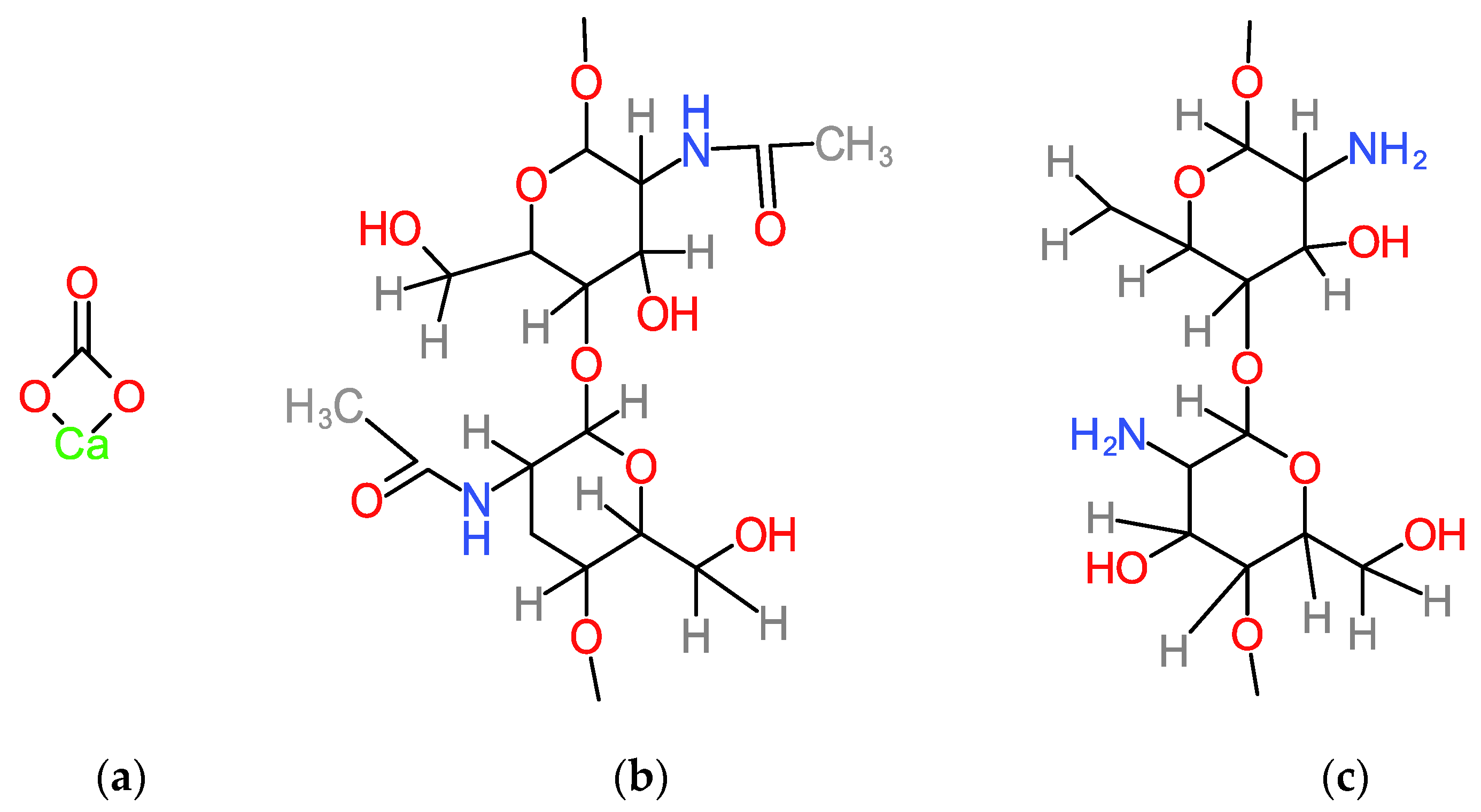


| Component | Functional Groups | Wavenumber (cm−1) | Observation and References |
|---|---|---|---|
| CaCO3 | 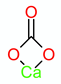 | 2524 weak | Chelates 2539 [28] 3200–2500 [29] |
| C=O | 1797 weak | C=O stretching vibration in calcium carbonate 1796 [30] 1785 [28] 1793 [31] | |
| 1450 very strong, narrow | stretching mode of carbonate; asymmetric stretch bands 1453 [32] 1425 [30] 1430 [28] | ||
| 1085 weak, narrow; 864 strong, narrow 715 weak | 1085 symmetric stretch of carbonate ions and 864; 720 out-of-plane bending modes 1085; 876 [30] 1453; 873 [32] 877; 715 [28] 871; 711 [31] | ||
| Lattice modes | 1469 strong | Lattice modes [30] | |
| Chitin | 3686; 3675 sharp; 3440 broad, numerous bands | 3650–3200 Free OH; 3550–3450 H–bonded OH; 3500–3200 polymer OH; broad, often numerous bands [29] | |
| 3440; 3385 broad medium | 3500–3100 amides with 3180: stretch in secondary amides (2°); 1560–1510 (overlapped on the 1469 band:
deformation vibration in secondary amides (2°): –NHC=O stretch symmetric, H–bonded, also specific to polypeptides [29] 1567 [7] | ||
| –C=O | 1685–1618 multiple peaks | 1685 –C=O stretching in primary amides –NHC=O; multiple picks assessed to different other components as impurities in the natural biocomposite [28] 1659 [7] | |
| –NHC=O | 1469 very strong; 864; 715 | 1465 NH in plane deformation vibration ~800; ~700 NH out of plane deformation vibration [29] | |
| 2954; 2923 Comb at ~2700 | 3000–2840 stretching vibrations 2900–2850 cyclohexanes, weak, comb at ~2700 [29] 2924; 2853 [7] | ||
(alcoholic) | 3284 medium | Corresponds to (3335, 1350 characteristic bands, the shift to 3284 due to the influence of the other functional groups, including to the oxygen contained in the oxane ring, and the 1350 band overlaps on the 1469 band) [29] 1378 [7] | |
| 1085 weak | 1075–1000 stretching vibrations in [28] 1084 [7] | ||
| 1160 | 1170–1115 stretching symmetric vibrations bridge [29] 1157 [7] | ||
 oxane (oxacyclohexane) in glucosamine ring | 1085; 1027 864 | 1085; 1027 skeletal vibrations due to stretching; ~1100 asymmetric; ~815 symmetric; shifts caused by the conjugated bonds in the chitin molecule, but also because of intra and intermolecular H-bondings and/or Van der Vaals interactions (among temporary dipoles and induced dipoles) [29] 1084; 892 [7] 1075; 1025 [12] | |
| Remnant Water | 3675 weak | free (water 3657) [29] | |
| Formaldehyde | H2C=O | 1740 | 1720/1500, bands for adsorbed formaldehyde [33] Adsorbed formaldehyde on the oyster shell may be the formaldehyde naturally produced by the oyster as its cells metabolism. The 1500 band in the spectrum overlaps on 1469 band. |
| Component | Functional Groups | Wavenumber (cm−1) | Observations References |
|---|---|---|---|
| Chitosan | 3446 strong, broad, numerous bands | 3650–3200 Free OH; 3550–3450 H–bonded OH; 3500–3200 polymer OH; broad, often numerous bands; In the same range with [29] 3385.92 [8] 3478.68 [12] 3478.68 [12] | |
| 3446; 3394 overlapped with the 3446 band; 1632, 1605; 1570 medium, partially overlaps with 1455–1420 bands | 3650–3200; 3550–3450; 3500–3300; stretch in amines and 1650–1590 medium to weak for deformation vibration in amines: [29] 3385.92; 1635.60; 1545.60 [8] 3360–3440; 1629.85; 1529.55 [12] 1656.88; 1571.05 [12] 1661, 1559 [7] | ||
| 2927; 2853; comb at ~2700 | 3000–2840 stretching vibrations 2900–2850 cyclohexanes, weak, comb at ~2700 [29] 2939.52 [8] 2924.13 [12] 2924; 2853 [7] | ||
(alcoholic) | 3318 medium | 3318 band partially overlaps with the band at 3446 and a band at 1350 can be assessed as overlapping on the bands in the range 1455–1420 Corresponds to  (3335, 1350 characteristic bands, the shift to higher wavenumbers is due to the influence of the other functional groups, including oxygen contained in the oxane ring) [29] 1387.7 [8] 1378.16 [12] 1377 [7] | |
| 1070 weak | 1075–1000 stretching vibrations in [29] 1075.33; 1025.18 [12]; 1072; 1022 [7] | ||
| 1127 | 1170–1115 stretching symmetric vibrations bridge [29] 1025.18 [12] 1118 [7] | ||
 oxane (oxacyclohexane) in glucosamine ring | 1085 870 | skeletal vibrations due to stretching; ~1100 asymmetric; ~815 symmetric; shifts caused by the conjugated bonds in the chitosan molecule, but also because of intra and intermolecular H-bonds and/or Van der Waals interactions (among temporary dipoles and induced dipoles) [29] 1096; 893.6 [8] 1075.33 [12] 1072: 816 [7] 1075; 1025 [12] | |
| Acetates and acetic acid resulted from chitin deacetylation | (carboxyl) (carboxylate) | 3446 strong, numerous bands; 2524 weak; 1632 medium; 1570 medium 1455; 1420 928 weak | 3550–2500 variable intensity, “hairy beard” aspect of the band stretching vibrations; 1670–1650 –C=O stretching vibrations, intramolecular H-bonded; 1610–1550 stretching asymmetric vibrations with a 20 cm−1 shift; 1450–1400 stretching symmetric vibrations; ~925 acetates deformation vibration [29] |
| Adsorbed gas phase | Adsorbed CO | 2178 weak | 2200–2100 range, adsorbed CO on different metal oxydes (metals ionic state) used as catalysts [34] 2178 [34,35] |
| Adsorbed CO2 | 2377 weak; 675 weak | 2347 and 660 CO2 molecule adsorbed [33] | |
| Formaldehyde | H2C=O | 1740 | 1720/1500, specific bands for adsorbed formaldehyde [33] Adsorbed formaldehyde on the thin film could be sourced by the oyster shell, but may also be new molecules of formaldehyde resulted during laser induced deacetylation. The 1500 band in the spectrum overlaps on the 1455 band. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocean, G.; Cocean, A.; Postolachi, C.; Garofalide, S.; Bulai, G.; Munteanu, B.S.; Cimpoesu, N.; Cocean, I.; Gurlui, S. High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications. Polymers 2022, 14, 1537. https://doi.org/10.3390/polym14081537
Cocean G, Cocean A, Postolachi C, Garofalide S, Bulai G, Munteanu BS, Cimpoesu N, Cocean I, Gurlui S. High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications. Polymers. 2022; 14(8):1537. https://doi.org/10.3390/polym14081537
Chicago/Turabian StyleCocean, Georgiana, Alexandru Cocean, Cristina Postolachi, Silvia Garofalide, Georgiana Bulai, Bogdanel Silvestru Munteanu, Nicanor Cimpoesu, Iuliana Cocean, and Silviu Gurlui. 2022. "High-Power Laser Deposition of Chitosan Polymers: Medical and Environmental Applications" Polymers 14, no. 8: 1537. https://doi.org/10.3390/polym14081537






