Effect of Thiophene Insertion on X-Shaped Anthracene-Based Hole-Transporting Materials in Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Method
2.2. Electrochemical Characterization
2.3. Optical Characterization
2.4. Thermal Characterization
2.5. Device Fabrication and Characterization
2.6. Mobility Measurements
2.7. Synthesis
2.7.1. Synthesis of N2,N2,N6,N6,N9,N9,N10,N10-Octakis(4-methoxyphenyl)anthracene-2,6,9,10-tetraamine (X1)
2.7.2. 5-(6,9,10-tris(5-(bis(4-Methoxyphenyl)amino)thiophen-2-yl)anthracen-2-yl)-N,N-bis(4-methoxyphenyl)thiophen-2-amine (X2)
3. Results and Discussion
3.1. Synthesis Materials
3.2. Optical Properties
3.3. Electrochemical Properties
3.4. Thermal Properties
3.5. Theoretical Approach
3.6. Hole-Transporting Properties
3.7. Steady-State Photoluminescence (PL)
3.8. Morphology and Water-Resisting Capability
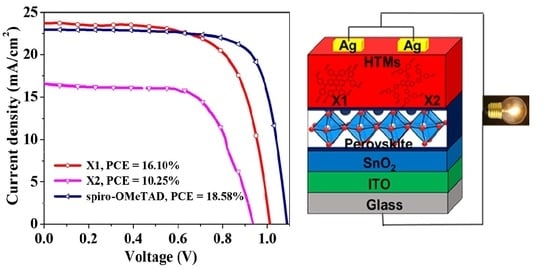
3.9. Application as HTMs in Perovskite Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, H.; Chen, C.; Li, H.; Tian, Y.; Li, Q.; Cheng, M. Passivation functionalized phenothiazine-based hole transport material for highly efficient perovskite solar cell with efficiency exceeding 22%. Chem. Eng. J. 2021, 410, 128328. [Google Scholar] [CrossRef]
- Sathiyan, G.; Sivakumar, E.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for a highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Pham, H.D.; Xianqiang, L.; Li, W.; Manzhos, S.; Kyaw, A.K.K.; Sonar, P. Organic interfacial materials for perovskite-based optoelectronic devices. Energy Environ. Sci. 2019, 12, 1177–1209. [Google Scholar] [CrossRef]
- Zhang, J.; Morbidoni, M.; Huang, K.; Feng, S.; McLachlan, M.A. Environmentally friendly, aqueous processed ZnO as an efficient electron transport layer for low temperature processed metal–halide perovskite photovoltaics. Inorg. Chem. Front. 2018, 5, 84–89. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-transport materials for perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Duan, L.; Chen, Y.; Zong, X.; Liu, R.; Sun, Z.; Liang, M.; Xue, S. Facile synthesis of triphenylamine-based hole-transporting materials for planar perovskite solar cells. J. Power Sources 2019, 435, 226767. [Google Scholar] [CrossRef]
- Zhao, L.; Qiu, L.; Xia, D.; Liu, S.; Yi, X.; Fan, J.; Yang, Y. Cyclooctatetrathiophene-Cored Three-Dimensional Hole Transport Material Enabling Over 19% Efficiency of Perovskite Solar Cells. ACS Appl. Energy Mater. 2019, 2, 8173–8180. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; Li, Z.; Zhang, X.; Shen, L.; Guo, W. Efficient 4, 4′, 4″-tris (3-methylphenylphenylamino) triphenylamine (m-MTDATA) Hole Transport Layer in Perovskite Solar Cells Enabled by Using the Nonstoichiometric Precursors. Adv. Funct. Mater. 2018, 28, 1803126. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Z.; Han, Y.; Zuo, Z.; Liu, B.; Yu, M.; Huang, W. Diarylfluorene-based nano-molecules as dopant-free hole-transporting materials without post-treatment process for flexible pin type perovskite solar cells. Nano Energy 2018, 46, 241–248. [Google Scholar] [CrossRef]
- Kung, P.K.; Li, M.H.; Lin, P.Y.; Chiang, Y.H.; Chan, C.R.; Guo, T.F.; Chen, P. A review of inorganic hole transport materials for perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1800882. [Google Scholar] [CrossRef]
- Qin, P.; Paek, S.; Dar, M.I.; Pellet, N.; Ko, J.; Grätzel, M.; Nazeeruddin, M.K. Perovskite solar cells with 12.8% efficiency by using conjugated quinolizino acridine based hole transporting material. J. Am. Chem. Soc. 2014, 136, 8516–8519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fu, K.; Hagfeldt, A.; Grätzel, M.; Mhaisalkar, S.G.; Grimsdale, A.C. A simple 3,4-ethylenedioxythiophene based hole-transporting material for perovskite solar cells. Angew. Chem. 2014, 126, 4169–4172. [Google Scholar] [CrossRef]
- Krishna, A.; Sabba, D.; Li, H.; Yin, J.; Boix, P.P.; Soci, C.; Grimsdale, A.C. Novel hole transporting materials based on triptycene core for high efficiency mesoscopic perovskite solar cells. Chem. Sci. 2014, 5, 2702–2709. [Google Scholar] [CrossRef] [Green Version]
- Christians, J.A.; Fung, R.C.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, S.; Miguel, O.; Grande, H.J.; Gonzalez-Pedro, V.; Sánchez, R.S.; Barea, E.M.; Tena-Zaera, R. Organo-metal halide perovskite-based solar cells with CuSCN as the inorganic hole selective contact. J. Mater. Chem. A 2014, 2, 12754–12760. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, C.; Yang, X.; Huang, J.; Zhang, F.; Xu, B.; Sun, L. Novel small molecular materials based on phenoxazine core unit for efficient bulk heterojunction organic solar cells and perovskite solar cells. Chem. Mater. 2015, 27, 1808–1814. [Google Scholar] [CrossRef]
- Chen, H.; Bryant, D.; Troughton, J.; Kirkus, M.; Neophytou, M.; Miao, X.; McCulloch, I. One-step facile synthesis of a simple hole transport material for efficient perovskite solar cells. Chem. Mater. 2016, 28, 2515–2518. [Google Scholar] [CrossRef] [Green Version]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.P.; Nazeeruddin, M.K. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Hua, Y.; Liu, P.; Wang, L.; Ruan, C.; Sun, L. Tailor-making low-cost spiro [fluorene-9,9′-xanthene]-based 3D oligomers for perovskite solar cells. Chem 2017, 2, 676–687. [Google Scholar] [CrossRef] [Green Version]
- Ge, Q.Q.; Shao, J.Y.; Ding, J.; Deng, L.Y.; Zhou, W.K.; Chen, Y.X.; Zhong, Y.W. A Two-Dimensional Hole-Transporting Material for High-Performance Perovskite Solar Cells with 20% Average Efficiency. Angew. Chem. Int. Ed. 2018, 57, 10959–10965. [Google Scholar] [CrossRef] [PubMed]
- Chiykowski, V.A.; Cao, Y.; Tan, H.; Tabor, D.P.; Sargent, E.H.; Aspuru-Guzik, A.; Berlinguette, C.P. Precise Control of Thermal and Redox Properties of Organic Hole-Transport Materials. Angew. Chem. Int. Ed. 2018, 57, 15529–15533. [Google Scholar] [CrossRef] [PubMed]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; de Villers, B.J.T.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Vaitukaityte, D.; Wang, Z.; Malinauskas, T.; Magomedov, A.; Bubniene, G.; Jankauskas, V.; Snaith, H.J. Efficient and Stable Perovskite Solar Cells Using Low-Cost Aniline-Based Enamine Hole-Transporting Materials. Adv. Mater. 2018, 30, 1803735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Ma, X.J.; Wang, Y.K.; Li, Y.; Gao, C.H.; Wang, Z.K.; Liao, L.S. Hole-Transporting Materials Incorporating Carbazole into Spiro-Core for Highly Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1807094. [Google Scholar] [CrossRef]
- Shen, C.; Wu, Y.; Zhang, H.; Li, E.; Zhang, W.; Xu, X.; Zhu, W.H. Semi-Locked Tetrathienylethene as a Building Block for Hole-Transporting Materials: Toward Efficient and Stable Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 3784–3789. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Park, S.; Im, S.H.; Son, H.J. Development of Dopant-Free Donor−Acceptor-type Hole Transporting Material for Highly Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 39511–39518. [Google Scholar] [CrossRef]
- Li, Y.; Scheel, K.R.; Clevenger, R.G.; Shou, W.; Pan, H.; Kilway, K.V.; Peng, Z. Highly Efficient and Stable Perovskite Solar Cells Using a Dopant-Free Inexpensive Small Molecule as the Hole-Transporting Material. Adv. Energy Mater. 2018, 8, 1801248. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, D.; Yu, Z.; Ma, W.; Li, H.B.; Yang, X.; Liu, F.; Hagfeldt, A.; Sun, L. Molecular Engineering of Copper Phthalocyanines: A Strategy in Developing Dopant-Free Hole-Transporting Materials for Efficient and Ambient-Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803287. [Google Scholar] [CrossRef]
- Molina-Ontoria, A.; Zimmermann, I.; Garcia-Benito, I.; Gratia, P.; Roldán-Carmona, C.; Aghazada, S.; Martín, N. Benzotrithiophene-Based Hole-Transporting Materials for 18.2% Perovskite Solar Cells. Angew. Chem. 2016, 128, 6378–6382. [Google Scholar] [CrossRef] [Green Version]
- Schwarzburg, K.; Willig, F. Diffusion impedance and space charge capacitance in the nanoporous dye-sensitized electrochemical solar cell. J. Phys. Chem. B 2003, 107, 3552–3555. [Google Scholar] [CrossRef]
- Nishimura, H.; Ishida, N.; Shimazaki, A.; Wakamiya, A.; Saeki, A.; Scott, L.T.; Murata, Y. Hole-transporting materials with a two-dimensionally expanded π-system around an azulene core for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 15656–15659. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.H.; Daik, R.; Lim, E.L.; Yap, C.C.; Ibrahim, M.A.; Ludin, N.A.; Teridi, M.A.M. A review of organic small molecule-based hole-transporting materials for meso-structured organic–inorganic perovskite solar cells. J. Mater. Chem. A 2016, 4, 15788–15822. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Cheng, T.; Chen, W.; Tan, Z.A.; Yu, T.; Dai, S. Tetraphenylmethane-arylamine hole-transporting materials for perovskite solar cells. ChemSusChem 2017, 10, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Ghadari, R.; Li, M.; Zhou, Z.A.; Ding, Y.; Dai, S. Tetraphenylethylene-Arylamine Derivatives as Hole Transporting Materials for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 12322–12330. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yen, J.H.; Chung, C.L.; Chen, C.P. Methoxy groups on bifluorenylidene-based hole transporting materials result in highly efficient and stable dopant-free inverted perovskite solar cells. Sol. Energy 2019, 179, 371–379. [Google Scholar] [CrossRef]
- Urieta-Mora, J.; Garcia-Benito, I.; Zimmermann, I.; Arago, J.; Molina-Ontoria, A.; Orti, E.; Nazeeruddin, M.K. Tetrasubstituted thieno [3,2-b] thiophenes as hole-transporting materials for perovskite solar cells. J. Org. Chem. 2019, 85, 224–233. [Google Scholar] [CrossRef]
- Lai, K.W.; Chang, C.C.; Chu, C.W. Benzodithiophene-based small molecules with various termini as hole transporting materials in efficient planar perovskite solar cells. Org. Electron. 2021, 89, 106010. [Google Scholar] [CrossRef]
- Cho, A.N.; Chakravarthi, N.; Kranthiraja, K.; Reddy, S.S.; Kim, H.S.; Jin, S.H.; Park, N.G. Acridine-based novel hole transporting material for high efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 7603–7611. [Google Scholar] [CrossRef]
- Yao, H.; Wu, T.; Wu, B.; Zhang, H.; Wang, Z.; Sun, Z.; Liang, M. The triple π-bridge strategy for tailoring indeno [2,1-b] carbazole-based HTMs enables perovskite solar cells with efficiency exceeding 21%. J. Mater. Chem. A 2021, 9, 8598–8606. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, J.; Song, Z.; Dong, Z.; Bao, Q.; Shrestha, N.; Tang, W. Dithieno [3, 2-b:2′, 3′-d] pyrrol-Cored hole transport material enabling over 21% efficiency dopant-free perovskite solar cells. Adv. Funct. Mater. 2019, 29, 1904300. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Shen, C.; Zhang, D.; Liu, S.; Wu, Y.; Zhu, W.H. A coplanar π-extended quinoxaline based hole-transporting material enabling over 21% efficiency for dopant-free perovskite solar cells. Angew. Chem. Int. Ed. 2021, 60, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Fu, Q.; Zong, X.; Dong, Y.; Zhang, W.; Wu, Q.; Xue, S. Coplanar phenanthro [9, 10-d] imidazole based hole-transporting material enabling over 19%/21% efficiency in inverted/regular perovskite solar cells. Chem. Eng. J. 2021, 421, 129823. [Google Scholar] [CrossRef]
- Akin, S.; Bauer, M.; Uchida, R.; Arora, N.; Jacopin, G.; Liu, Y. Cyclopentadithiophene-based hole-transporting material for highly stable perovskite solar cells with stabilized efficiencies approaching 21%. ACS Appl. Energy Mater. 2020, 3, 7456–7463. [Google Scholar] [CrossRef]
- Deng, Z.; He, M.; Zhang, Y.; Ullah, F.; Ding, K.; Liang, J.; Chen, C.C. Design of Low Crystallinity Spiro-Typed Hole Transporting Material for Planar Perovskite Solar Cells to Achieve 21.76% Efficiency. Chem. Mater. 2020, 33, 285–297. [Google Scholar] [CrossRef]
- Park, J.H.; Chung, D.S.; Lee, D.H.; Kong, H.; Jung, I.H.; Park, M.J.; Cho, N.S. New anthracene thiophene-based copolymers that absorb across the entire UV-vis spectrum for application in organic solar cells. Chem. Commun. 2010, 46, 1863–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.Y.; Yun, H.J.; Kim, S.O.; Lee, G.B.; Cha, H.J.; Park, C.E.; Kwon, S.K.; Kim, Y.H. Novel Alkoxyanthracene Donor and Benzothiadiazole Acceptor for Organic Thin Film Transistor and Bulk Heterojunction Organic Photovoltaic Cells. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1306–1314. [Google Scholar] [CrossRef]
- Shih, P.-I.; Chuang, C.-Y.; Chien, C.-H.; Diau, E.W.-G.; Shu, C.-F. Highly efficient non-doped blue-light-emitting diodes based on an anthracene derivative end capped with tetraphenylethylene groups. Adv. Funct. Mater. 2007, 17, 3141–3146. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W.; Jiang, H.; Gao, Y.; Jiang, X.; Lin, H.; Zhao, W.; Hao, J. C-9 Fluorenyl substituted anthracenes: A promising new family of blue luminescent materials. Org. Lett. 2010, 12, 3874–3877. [Google Scholar] [CrossRef]
- Wee, K.-R.; Han, W.-S.; Kim, J.-E.; Kim, A.-L.; Kwon, S.; Kang, S.O. Asymmetric anthracene-based blue host materials: Synthesis and electroluminescence properties of 9-(2-naphthyl)-10-arylanthracenes. J. Mater. Chem. 2011, 21, 1115–1123. [Google Scholar] [CrossRef]
- Chung, D.S.; Park, J.W.; Park, J.H.; Moon, D.; Kim, G.H.; Lee, D.H.; Shim, H.K.; Kwon, S.K.; Park, C.E. High mobility organic single crystal transistors based on soluble triisopropylsilylethynyl anthracene derivatives. J. Mater. Chem. 2010, 20, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Bae, S.Y.; Kim, K.H.; Cho, M.J.; Lee, K.; Kim, Z.H.; Choi, D.H.; Chung, D.S.; Park, C.E. High-mobility anthracene-based X-shaped conjugated molecules for thin film transistors. Chem. Commun. 2009, 5290–5292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.; Yang, X.; Yang, C.; Li, S.; Cheng, M.; Hagfeldt, A.; Sun, L. Molecular design of anthracene-bridged metal-free organic dyes for efficient dye-sensitized solar cells. J. Phys. Chem. C 2010, 114, 9101–9110. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Singh, P.; Baheti, A.; Hsu, Y.-C.; Ho, K.-C.; Lin, J.T. Electro-optical properties of new anthracene based organic dyes for dye-sensitized solar cells. Dyes Pigments 2011, 91, 33–43. [Google Scholar] [CrossRef]
- Marrocchi, A.; Silvestri, F.; Seri, M.; Facchetti, A.; Taticchi, A.; Marks, T.J. Conjugated anthracene derivatives as donor materials for bulk heterojunction solar cells: Olefinic versus acetylenic spacers. Chem. Commun. 2009, 1380–1382. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Ghadari, R.; Jin, S.; Yu, T.; Chen, W.; Dai, S. Anthracene–arylamine hole transporting materials for perovskite solar cells. Chem. Commun. 2017, 53, 9558–9561. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.D.; Hu, H.; Wong, F.L.; Lee, C.S.; Chen, W.C.; Feron, K.; Sonar, P. Acene-based organic semiconductors for organic light-emitting diodes and perovskite solar cells. J. Mater. Chem. C 2018, 6, 9017–9029. [Google Scholar] [CrossRef]
- Singh, A.; Abate, S.Y.; Pavan Kumar, C.; Wu, W.T.; Hsiao, J.C.; Wu, F.L.; Tao, Y.T. Bis (diphenylamine)-Tethered Carbazolyl Anthracene Derivatives as Hole-Transporting Materials for Stable and High-Performance Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 10752–10764. [Google Scholar] [CrossRef]
- Liu, C.C.; Cai, W.Z.; Guan, X.; Duan, C.H.; Xue, Q.F.; Ying, L.; Huang, F.; Cao, Y. Synthesis of donor-acceptor copolymers based on anthracene derivatives for polymer solar cells. Polym. Chem. 2013, 4, 3949–3958. [Google Scholar] [CrossRef]
- Chandrasekaran, D.; Chiu, Y.L.; Yu, C.K.; Yen, Y.S.; Chang, Y.J. Polycyclic Arenes Dihydrodinaphthopentacene-based Hole-Transporting Materials for Perovskite Solar Cells Application. Chem. Asian J. 2021, 16, 3719–3728. [Google Scholar] [CrossRef]
- Hsu, C.-P. The Electronic Couplings in Electron Transfer and Excitation Energy Transfer. Acc. Chem. Res. 2009, 42, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Zhou, P.; Wu, Y.; Xu, Y.; Zhi, Y.; Zhu, W. Isomeric Organic Semiconductors Containing Fused-Thiophene Cores: Molecular Packing and Charge Transport. Phys. Chem. Chem. Phys. 2018, 20, 13171–13177. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, P.G.; Almassio, M.F.; Bruno, M.; Garay, R.O. Mild persubstitution of di-and tetrabrominated arenes with arylthiolate nucleophiles. Tetrahedron Lett. 2010, 51, 6730–6733. [Google Scholar] [CrossRef]
- Li, Y.; Scudiero, L.; Ren, T.; Dong, W.J. Synthesis and characterizations of benzothiadiazole-based fluorophores as potential wavelength-shifting materials. J. Photochem. Photobiol. A Chem. 2012, 231, 51–59. [Google Scholar] [CrossRef]
- Leliège, A.; Grolleau, J.; Allain, M.; Blanchard, P.; Demeter, D.; Rousseau, T.; Roncali, J. Small D–π–A Systems with o-Phenylene-Bridged Accepting Units as Active Materials for Organic Photovoltaics. Chem. Eur. J. 2013, 19, 9948–9960. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Ivaturi, A.; Robertson, N. SFX as a low-cost ‘Spiro’ hole-transport material for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 4855–4863. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Zhou, X.; Kong, F.; Sun, Y.; Huang, Y.; Zhang, X.; Ghadari, R. Benzothiadiazole-based hole transport materials for high-efficiency dopant-free perovskite solar cells: Molecular Planarity effect. J. Energy Chem. 2020, 44, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Chen, C.; Wu, C.; Ding, X.; Zheng, M.; Li, H.; Li, G.; Lu, H.; Cheng, M. Fluorine-Substituted Benzotriazole Core Building Block-Based Highly Efficient Hole-Transporting Materials for Mesoporous Perovskite Solar Cells. Sol. RRL 2020, 4, 1900362. [Google Scholar] [CrossRef]
- Lee, S.; Park, K.H.; Lee, J.H.; Back, H.; Sung, M.J.; Lee, J.; Lee, K. Achieving thickness-insensitive morphology of the photoactive layer for printable organic photovoltaic cells via side chain engineering in nonfullerene acceptors. Adv. Energy Mater. 2019, 9, 1900044. [Google Scholar] [CrossRef]
- Swick, S.M.; Zhu, W.; Matta, M.; Aldrich, T.J.; Harbuzaru, A.; Lopez Navarrete, J.T.; Ponce Ortiz, R.; Kohlstedt, K.L.; Schatz, G.C.A.; Facchetti, F.S.; et al. Closely Packed, Low Reorganization Energy π-extended Post fullerene Acceptors for Efficient Polymer Solar Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8341–E8348. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| HTM | λmax a (Soln) (ε × 10−5 M−1 cm−1) (nm) | λmax b (Film) (nm) | λem a (Soln) (nm) | E0–0 c (ev) | HOMO/ LUMO (eV) | Td/Tg d (°C) | µh e (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|---|
| X1 | 524 (1.49) | 540 | 564 | 2.28 | −5.03/−2.76 | 424/135 | 2.8 × 10−4 |
| X2 | 486 (3.12) | 525 | 589 | 2.31 | −4.94/−2.63 | 422/95 | 3.1 × 10−4 |
| Spiro-OMeTAD [60] | 385 (5.24) | 396 | 430 | 3.04 | −5.15/−2.11 | 422/129 | 3.8 × 10−4 |
| HTM | Scan Direction | VOC (V) (Average) a | JSC (mA/cm2) (Average) a | FF (Average) a | PCE (%) Best | PCE (%) (Average) a | |
|---|---|---|---|---|---|---|---|
| X1 | Undoped | FS | 0.909 (0.882 ± 0.040) | 22.228 (21.752 ± 0.379) | 47.006 (42.445 ± 3.012) | 9.43 | 8.098 ± 0.827 |
| Undoped | RS | 0.911 (0.900 ± 0.011) | 22.325 (21.883 ± 0.297) | 45.804 (40.643 ± 3.458) | 9.25 | 7.966 ± 0.825 | |
| Doped | FS | 1.005 (0.941 ± 0.113) | 23.799 (22.147 ± 1.609) | 65.269 (62.612 ± 6.389) | 15.51 | 12.926 ± 2.091 | |
| Doped | RS | 1.012 (0.974 ± 0.019) | 23.721 (22.714 ± 0.705) | 67.543 (62.744 ± 4.351) | 16.10 | 13.793 ± 1.209 | |
| X2 | Undoped | FS | 0.303 (0.152 ± 0.145) | 5.523 (4.332 ± 0.859) | 35.943 (18.622 ± 6.842) | 0.59 | 0.279 ± 0.281 |
| Undoped | RS | 0.029 (0.018 ± 0.006) | 4.837 (4.095 ± 0.371) | 24.156 (22.161 ± 5.711) | 0.03 | 0.018 ± 0.009 | |
| Doped | FS | 0.933 (0.901 ± 0.534) | 16.432 (14.137 ± 1.109) | 52.763 (55.412 ± 1.878) | 8.08 | 7.005 ± 1.659 | |
| Doped | RS | 0.936 (0.831 ± 0.676) | 16.568 (14.842 ± 1.223) | 66.165 (58.326 ± 1.229) | 10.25 | 7.126 ± 1.407 | |
| Spiro-OMeTAD | Doped | FS | 1.079 (1.066 ± 0.014) | 23.022 (22.646 ± 0.401) | 73.056 (71.189 ± 1.502) | 18.03 | 17.001 ± 0.603 |
| Doped | RS | 1.088 (1.074 ± 0.012) | 22.934 (22.608 ± 0.371) | 75.012 (72.298 ± 1.747) | 18.58 | 17.104 ± 0.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, D.; Chiu, W.-H.; Lee, K.-M.; Liao, J.-M.; Chou, H.-H.; Yen, Y.-S. Effect of Thiophene Insertion on X-Shaped Anthracene-Based Hole-Transporting Materials in Perovskite Solar Cells. Polymers 2022, 14, 1580. https://doi.org/10.3390/polym14081580
Chandrasekaran D, Chiu W-H, Lee K-M, Liao J-M, Chou H-H, Yen Y-S. Effect of Thiophene Insertion on X-Shaped Anthracene-Based Hole-Transporting Materials in Perovskite Solar Cells. Polymers. 2022; 14(8):1580. https://doi.org/10.3390/polym14081580
Chicago/Turabian StyleChandrasekaran, Dharuman, Wei-Hao Chiu, Kun-Mu Lee, Jian-Ming Liao, Hsien-Hsin Chou, and Yung-Sheng Yen. 2022. "Effect of Thiophene Insertion on X-Shaped Anthracene-Based Hole-Transporting Materials in Perovskite Solar Cells" Polymers 14, no. 8: 1580. https://doi.org/10.3390/polym14081580
APA StyleChandrasekaran, D., Chiu, W.-H., Lee, K.-M., Liao, J.-M., Chou, H.-H., & Yen, Y.-S. (2022). Effect of Thiophene Insertion on X-Shaped Anthracene-Based Hole-Transporting Materials in Perovskite Solar Cells. Polymers, 14(8), 1580. https://doi.org/10.3390/polym14081580