Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
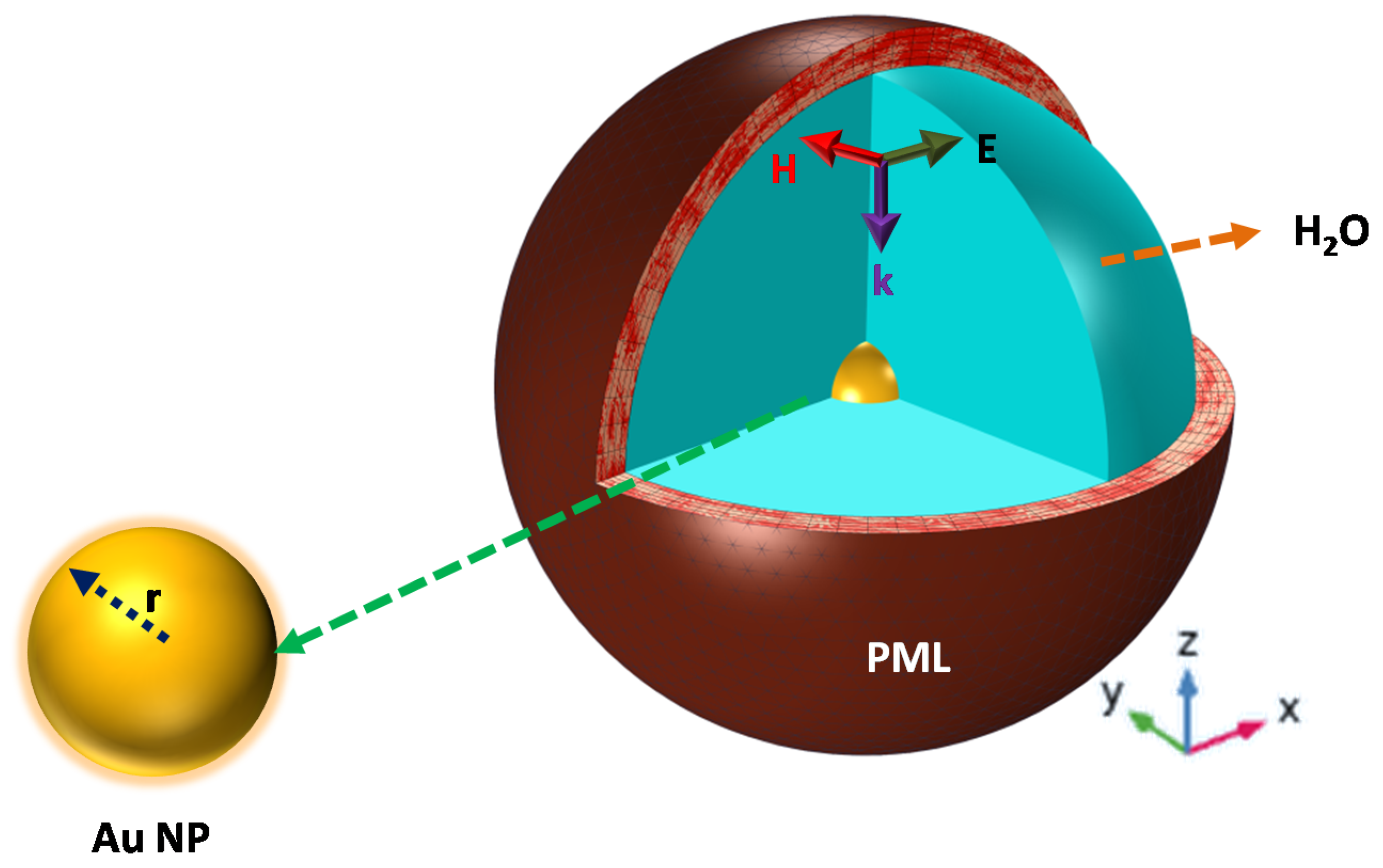
2.1. Computational Modeling
2.2. Extinction Spectrum Setup
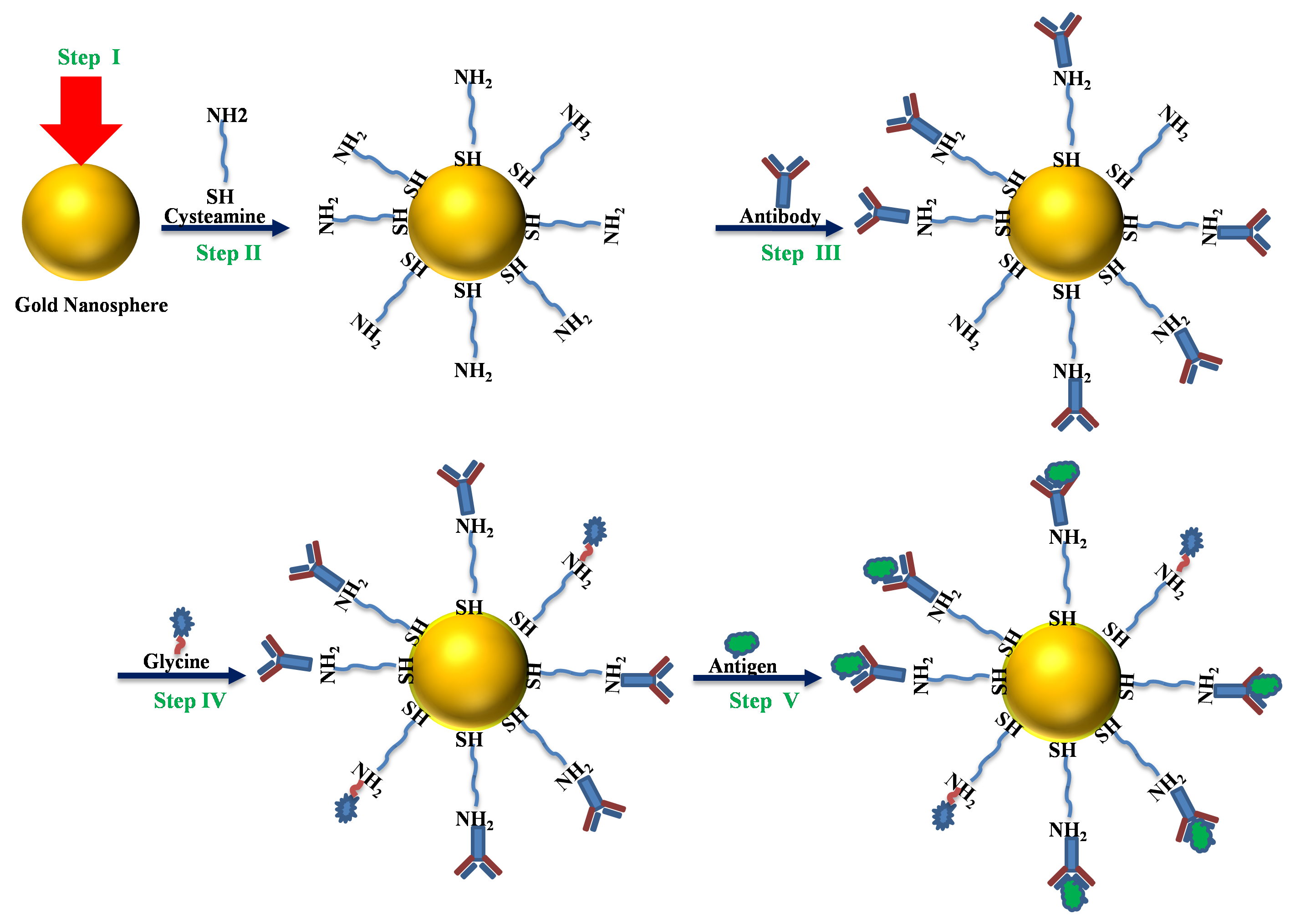
2.3. Preparation of Sensing Elements
3. Results
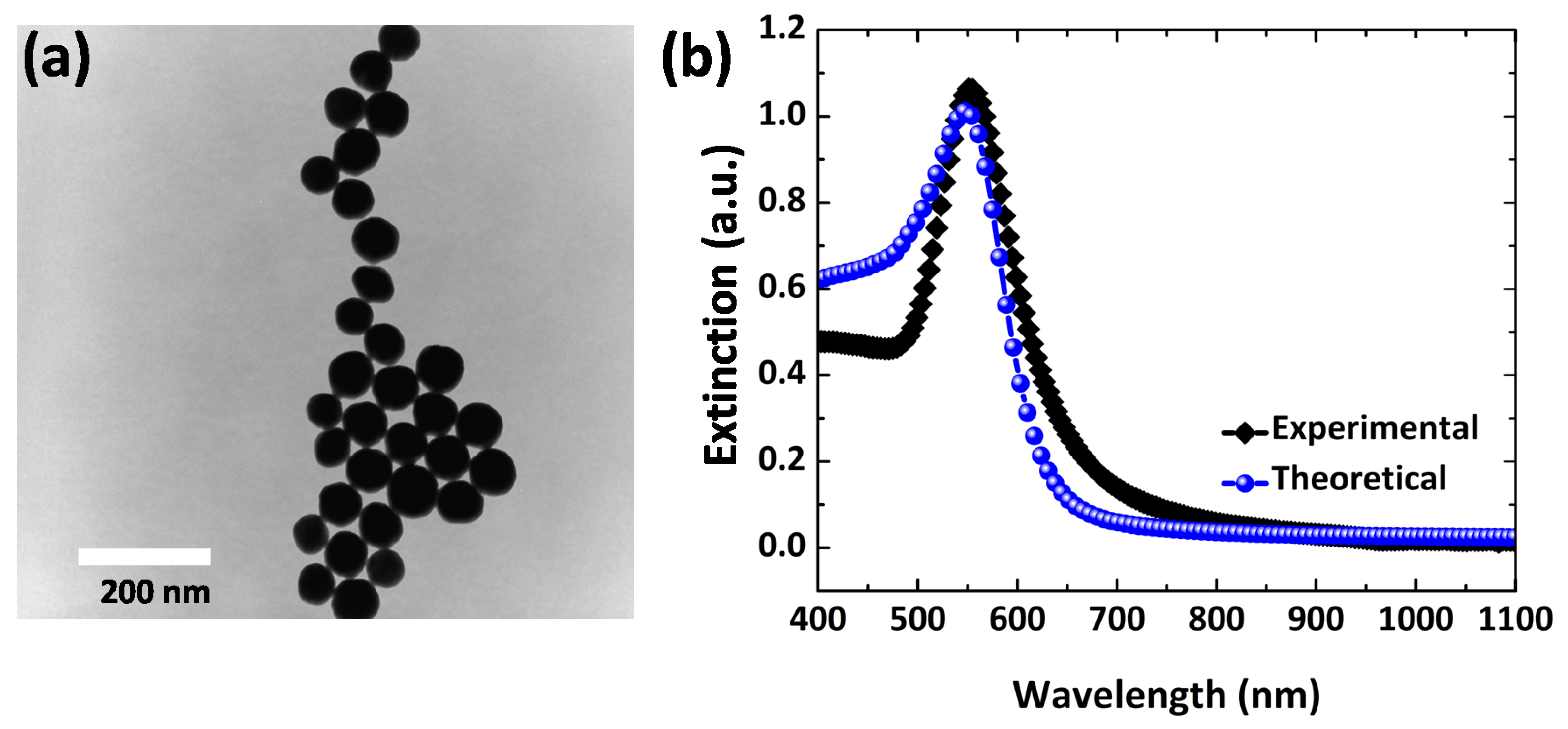
3.1. Optical Properties of Au Nanoparticles
3.2. Localized Field Enhancement
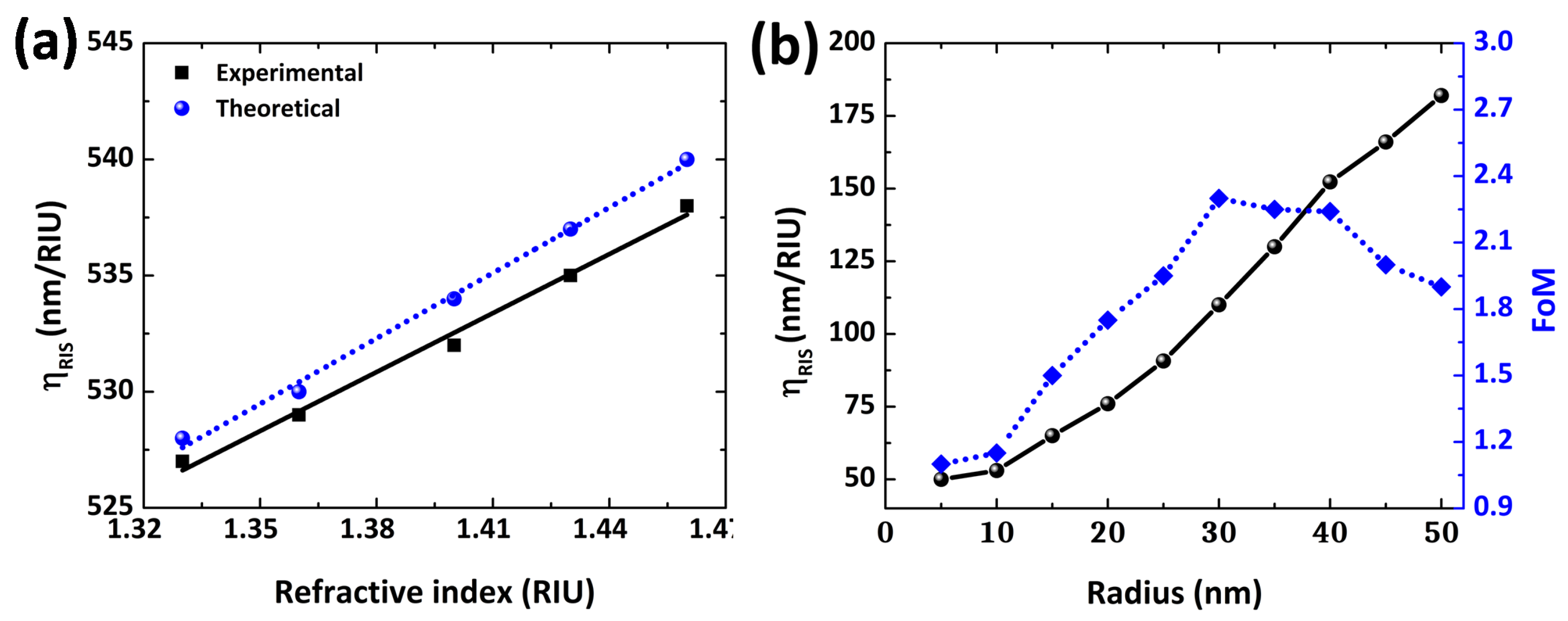
3.3. Refractive Index-Based Sensing for Au NPs
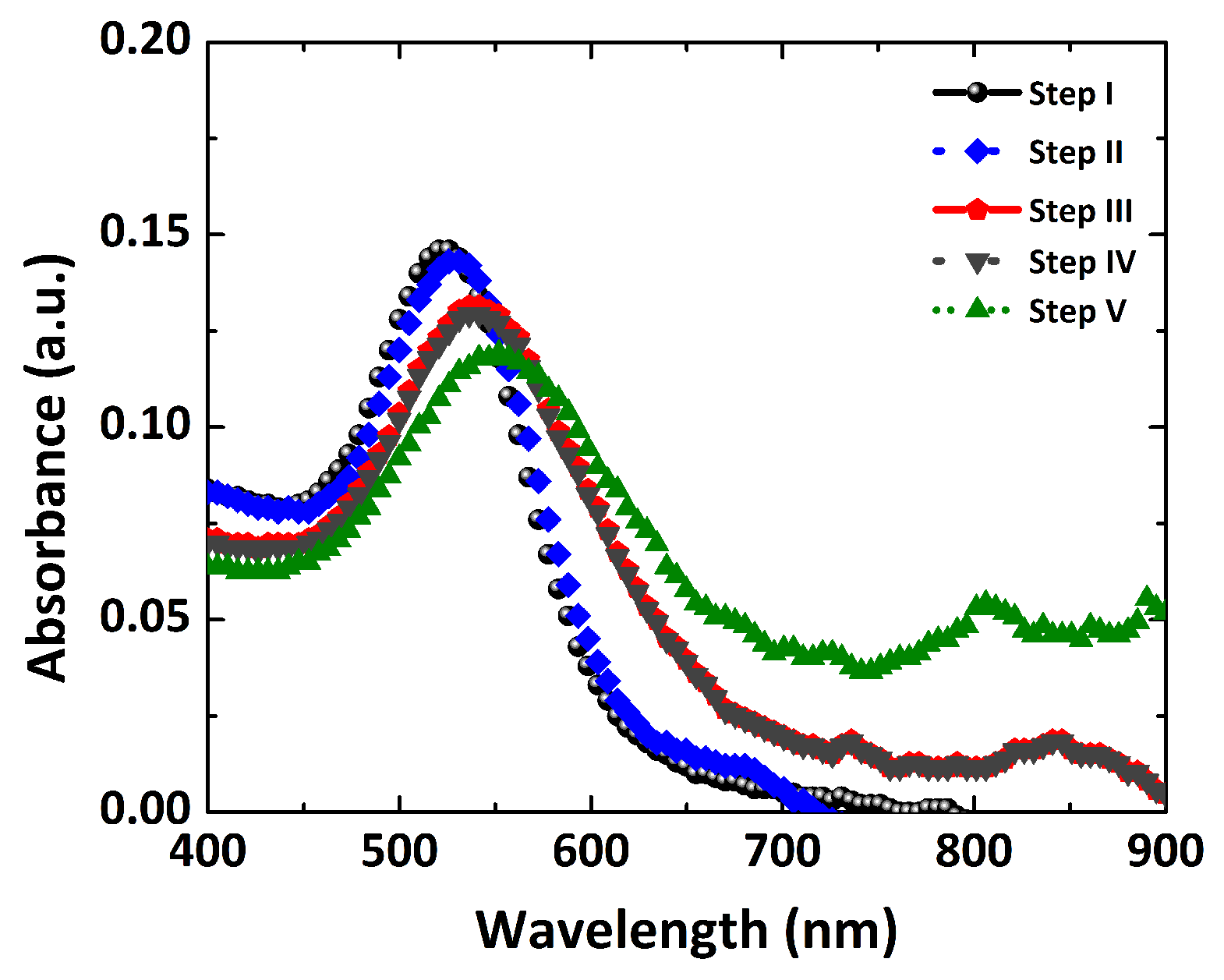
3.4. Molecular Sensing and Dengue Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farooq, S.; Rativa, D.; de Araujo, R.E. High performance gold dimeric nanorods for plasmonic molecular sensing. IEEE Sens. J. 2021, 21, 13184–13191. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Albella, P.; Alcaraz de la Osa, R.; Moreno, F.; Maier, S.A. Electric and magnetic field enhancement with ultralow heat radiation dielectric nanoantennas: Considerations for surface-enhanced spectroscopies. ACS Photonics 2014, 1, 524–529. [Google Scholar] [CrossRef]
- Mahmood, H.Z.; Jilani, A.; Farooq, S.; Javed, Y.; Jamil, Y.; Iqbal, J.; Ullah, S.; Wageh, S. Plasmon-Based Label-Free Biosensor Using Gold Nanosphere for Dengue Detection. Crystals 2021, 11, 1340. [Google Scholar] [CrossRef]
- Farooq, S.; Rativa, D.; de Araujo, R.E. Orientation Effects on Plasmonic Heating of Near-Infrared Colloidal Gold Nanostructures. Plasmonics 2020, 15, 1507–1515. [Google Scholar] [CrossRef]
- Tezcan, T.; Hsu, C.H. High-sensitivity SERS based sensing on the labeling side of glass slides using low branched gold nanoparticles prepared with surfactant-free synthesis. RSC Adv. 2020, 10, 34290–34298. [Google Scholar] [CrossRef]
- Farooq, S.; Vital, C.V.; Gómez-Malagón, L.A.; de Araujo, R.E.; Rativa, D. Thermo-optical performance of iron-doped gold nanoshells-based nanofluid on direct absorption solar collectors. Solar Energy 2020, 208, 1181–1188. [Google Scholar] [CrossRef]
- Farooq, S.; de Araujo, R.E. Identifying high performance gold nanoshells for singlet oxygen generation enhancement. Photodiagn. Photodyn. Ther. 2021, 35, 102466. [Google Scholar] [CrossRef]
- Ribeiro, M.S.; de Melo, L.S.; Farooq, S.; Baptista, A.; Kato, I.T.; Nunez, S.C.; de Araujo, R.E. Photodynamic inactivation assisted by localized surface plasmon resonance of silver nanoparticles: In vitro evaluation on Escherichia coli and Streptococcus mutans. Photodiagn. Photodyn. Ther. 2018, 22, 191–196. [Google Scholar] [CrossRef]
- Conteduca, D.; Barth, I.; Pitruzzello, G.; Reardon, C.P.; Martins, E.R.; Krauss, T.F. Dielectric nanohole array metasurface for high-resolution near-field sensing and imaging. Nat. Commun. 2021, 12, 3293. [Google Scholar] [CrossRef]
- Moore, K.A.; Pate, K.M.; Soto-Ortega, D.D.; Lohse, S.; van der Munnik, N.; Lim, M.; Jackson, K.S.; Lyles, V.D.; Jones, L.; Glassgow, N.; et al. Influence of gold nanoparticle surface chemistry and diameter upon Alzheimer’s disease amyloid-β protein aggregation. J. Biol. Eng. 2017, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Charles, D.E.; Ledwith, D.M.; Aherne, D.; Cunningham, S.; Voisin, M.; Blau, W.J.; Gun’ko, Y.K.; Kelly, J.M.; Brennan-Fournet, M.E. Wash-free highly sensitive detection of C-reactive protein using gold derivatised triangular silver nanoplates. RSC Adv. 2014, 4, 29022–29031. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Kwak, C.H.; Heo, N.S.; Oh, S.Y.; Lee, H.; Lee, G.W.; Vilian, A.E.; Han, Y.K.; Kim, W.S.; et al. Rapid and label-free bioanalytical method of alpha fetoprotein detection using LSPR chip. J. Cryst. Growth 2017, 469, 131–135. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 18. [Google Scholar] [CrossRef]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Hernández, M.E.; Goicoechea, J.; Rivero, P.J.; Arregui, F.J. In Situ Synthesis of Gold Nanoparticles in Layer-by-Layer Polymeric Coatings for the Fabrication of Optical Fiber Sensors. Polymers 2022, 14, 776. [Google Scholar] [CrossRef]
- Camara, A.R.; Gouvêa, P.M.; Dias, A.C.M.; Braga, A.M.; Dutra, R.F.; de Araujo, R.E.; Carvalho, I.C. Dengue immunoassay with an LSPR fiber optic sensor. Optics Express 2013, 21, 27023–27031. [Google Scholar] [CrossRef]
- Abdul Rahman, S.; Saadun, R.; Azmi, N.E.; Ariffin, N.; Abdullah, J.; Yusof, N.A.; Sidek, H.; Hajian, R. Label-free dengue detection utilizing PNA/DNA hybridization based on the aggregation process of unmodified gold nanoparticles. J. Nanomater. 2014, 2014, 839286. [Google Scholar] [CrossRef]
- Matsunaga, K.i.; Kimoto, M.; Lim, V.W.; Vasoo, S.; Leo, Y.S.; Sun, W.; Hirao, I. Competitive ELISA for a serologic test to detect dengue serotype-specific anti-NS1 IgGs using high-affinity UB-DNA aptamers. Sci. Rep. 2021, 11, 18000. [Google Scholar] [CrossRef]
- Cucunawangsih, C.; Pratama, N.; Lugito, N.P.H.; Kurniawan, A. Immunoglobulin G (IgG) to IgM ratio in secondary adult dengue infection using samples from early days of symptoms onset. BMC Infect. Dis. 2015, 15, 276. [Google Scholar] [CrossRef] [Green Version]
- Eivazzadeh-Keihan, R.; Pashazadeh-Panahi, P.; Mahmoudi, T.; Chenab, K.K.; Baradaran, B.; Hashemzaei, M.; Radinekiyan, F.; Mokhtarzadeh, A.; Maleki, A. Dengue virus: A review on advances in detection and trends–from conventional methods to novel biosensors. Microchim. Acta 2019, 186, 329. [Google Scholar] [CrossRef]
- Zacheo, A.; Hodek, J.; Witt, D.; Mangiatordi, G.F.; Ong, Q.K.; Kocabiyik, O.; Depalo, N.; Fanizza, E.; Laquintana, V.; Denora, N.; et al. Multi-sulfonated ligands on gold nanoparticles as virucidal antiviral for Dengue virus. Sci. Rep. 2020, 10, 9052. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Almuqati, T.; Alhokbany, N.; Ahamad, T.; Naushad, M.; Alshehri, S.M. A clean approach for the reduction of hazardous 4-nitrophenol using gold nanoparticles decorated multiwalled carbon nanotubes. J. Clean. Prod. 2018, 191, 429–435. [Google Scholar] [CrossRef]
- Şologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. [Google Scholar] [CrossRef] [Green Version]
- Lepinay, S.; Staff, A.; Ianoul, A.; Albert, J. Improved detection limits of protein optical fiber biosensors coated with gold nanoparticles. Biosens. Bioelectron. 2014, 52, 337–344. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Yushanov, S.; Crompton, J.S.; Koppenhoefer, K.C. Mie scattering of electromagnetic waves. In Proceedings of the COMSOL Conference, Rotterdam, The Netherlands, 23–25 October 2013; pp. 1–7. [Google Scholar]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Farooq, S.; Rativa, D.; de Araujo, R.E. Optimizing the Sensing Performance of SiO 2-Au Nanoshells. Plasmonics 2019, 14, 1519–1526. [Google Scholar] [CrossRef]
- Khoury, C.G.; Norton, S.J.; Vo-Dinh, T. Investigating the plasmonics of a dipole-excited silver nanoshell: Mie theory versus finite element method. Nanotechnology 2010, 21, 315203. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.B.; Schatz, G.C. Radiative effects in plasmonic aluminum and silver nanospheres and nanorods. J. Phys. D Appl. Phys. 2014, 48, 184004. [Google Scholar] [CrossRef]
- Farooq, S.; de Araujo, R.E. Engineering a localized surface plasmon resonance platform for molecular biosensing. Open J. Appl. Sci. 2018, 8, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Mendoza Herrera, L.J.; Arboleda, D.M.; Schinca, D.C.; Scaffardi, L.B. Determination of plasma frequency, damping constant, and size distribution from the complex dielectric function of noble metal nanoparticles. J. Appl. Phys. 2014, 116, 233105. [Google Scholar] [CrossRef]
- Coronado, E.A.; Schatz, G.C. Surface plasmon broadening for arbitrary shape nanoparticles: A geometrical probability approach. J. Chem. Phys. 2003, 119, 3926–3934. [Google Scholar] [CrossRef]
- Stuart, D.; Haes, A.; Yonzon, C.; Hicks, E.; Van Duyne, R. Biological applications of localised surface plasmonic phenomenae. IEE Proc. Nanobiotechnol. 2005, 152, 13–32. [Google Scholar] [CrossRef]
- Barchiesi, D.; Grosges, T.; Vial, A. Measurement of decay lengths of evanescent waves: The lock-in nonlinear filtering. New J. Phys. 2006, 8, 263. [Google Scholar] [CrossRef]
- Barbillon, G. Determination of evanescent electric field decay length of metallic nanodisks by using localized surface plasmon spectroscopy. J. Mater. Sci. Eng. 2010, 4, 69–74. [Google Scholar]
- Jung, L.S.; Campbell, C.T.; Chinowsky, T.M.; Mar, M.N.; Yee, S.S. Quantitative interpretation of the response of surface plasmon resonance sensors to adsorbed films. Langmuir 1998, 14, 5636–5648. [Google Scholar] [CrossRef]
- Zaman, Q.; Souza, J.; Pandoli, O.; Costa, K.Q.; Dmitriev, V.; Fulvio, D.; Cremona, M.; Aucelio, R.Q.; Fontes, G.; Del Rosso, T. Two-color surface plasmon resonance nanosizer for gold nanoparticles. Optics Express 2019, 27, 3200–3216. [Google Scholar] [CrossRef]
- Basso, C.R.; Tozato, C.C.; Crulhas, B.P.; Castro, G.R.; Junior, J.P.A.; Pedrosa, V.A. An easy way to detect dengue virus using nanoparticle-antibody conjugates. Virology 2018, 513, 85–90. [Google Scholar] [CrossRef]




| NP Size | Experimental Analysis | Thoeretical Analysis | ||
|---|---|---|---|---|
| FoM | FoM | |||
| 2.5 nm | 60 | 1.1 | 50 | 1.2 |
| 30.0 nm | 84 | 2.0 | 83 | 2.3 |
| 50.0 nm | 178 | 1.8 | 185 | 1.9 |
| Nanostructure | Type of Platform | (nm/RIU) | Molecular Identification | Reference |
|---|---|---|---|---|
| Au NPs | solution-based | 110 | 1.5 nM | This work |
| Au NPs | substrate-based | 89 | 1.2 μM | [4] |
| Au NPs | substrate-based | − | 1.54 nM | [17] |
| Au NPs | solution-based | − | 104 μM | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, S.; Wali, F.; Zezell, D.M.; de Araujo, R.E.; Rativa, D. Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis. Polymers 2022, 14, 1592. https://doi.org/10.3390/polym14081592
Farooq S, Wali F, Zezell DM, de Araujo RE, Rativa D. Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis. Polymers. 2022; 14(8):1592. https://doi.org/10.3390/polym14081592
Chicago/Turabian StyleFarooq, Sajid, Faiz Wali, Denise Maria Zezell, Renato E. de Araujo, and Diego Rativa. 2022. "Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis" Polymers 14, no. 8: 1592. https://doi.org/10.3390/polym14081592
APA StyleFarooq, S., Wali, F., Zezell, D. M., de Araujo, R. E., & Rativa, D. (2022). Optimizing and Quantifying Gold Nanospheres Based on LSPR Label-Free Biosensor for Dengue Diagnosis. Polymers, 14(8), 1592. https://doi.org/10.3390/polym14081592







