Amination of Non-Functional Polyvinyl Chloride Polymer Using Polyethyleneimine for Removal of Phosphorus from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
2.3. Comparison of Functional Group Characteristics in PVC and PEI-PVC Fibers
2.4. Evaluation of Phosphorus Adsorption Performance of Adsorbents
2.5. Determination of PEI-PVC Reusability
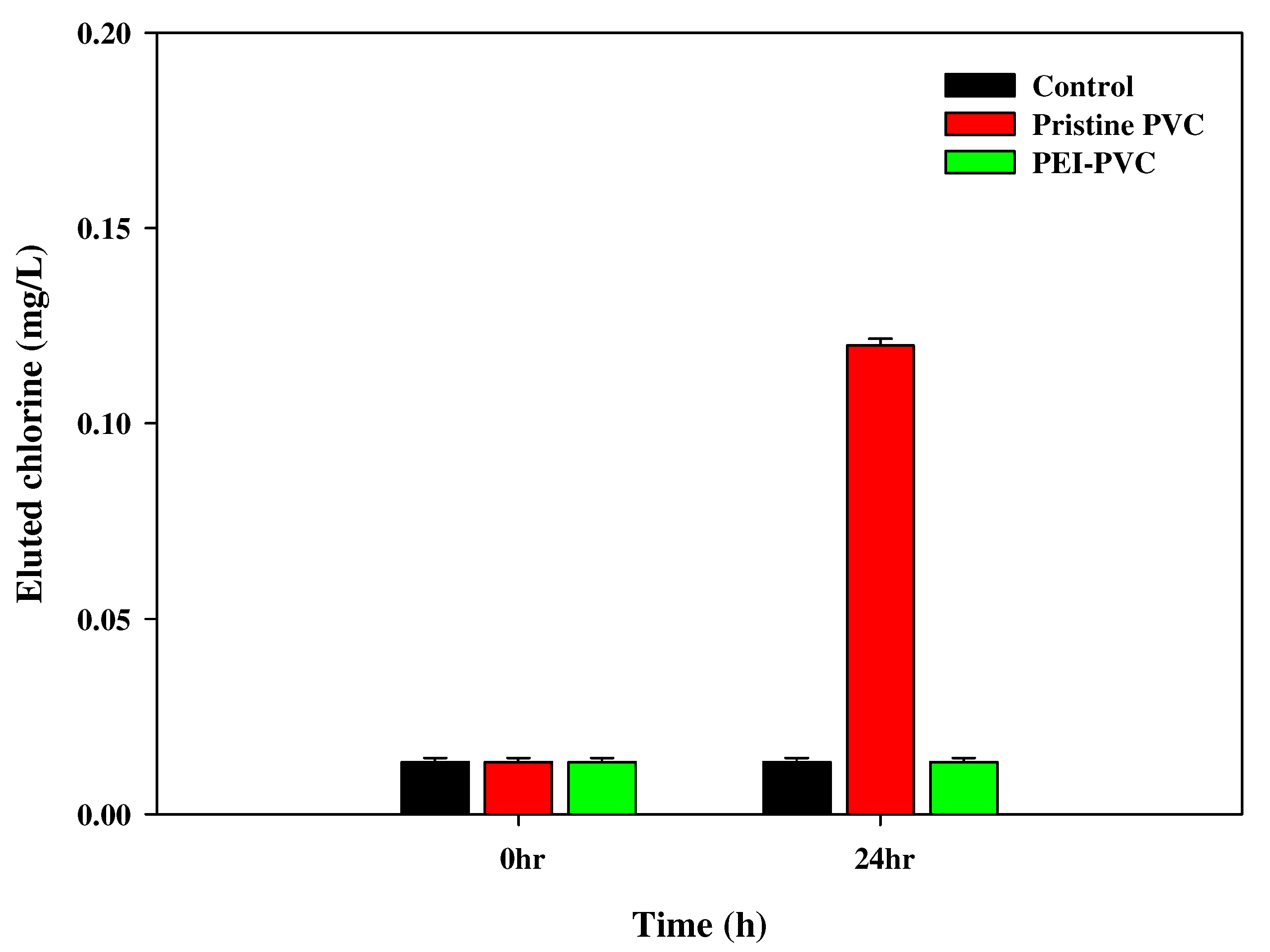
2.6. Measurement of Chlorine Elution from PVC-Based Sorbents
3. Results and Discussion
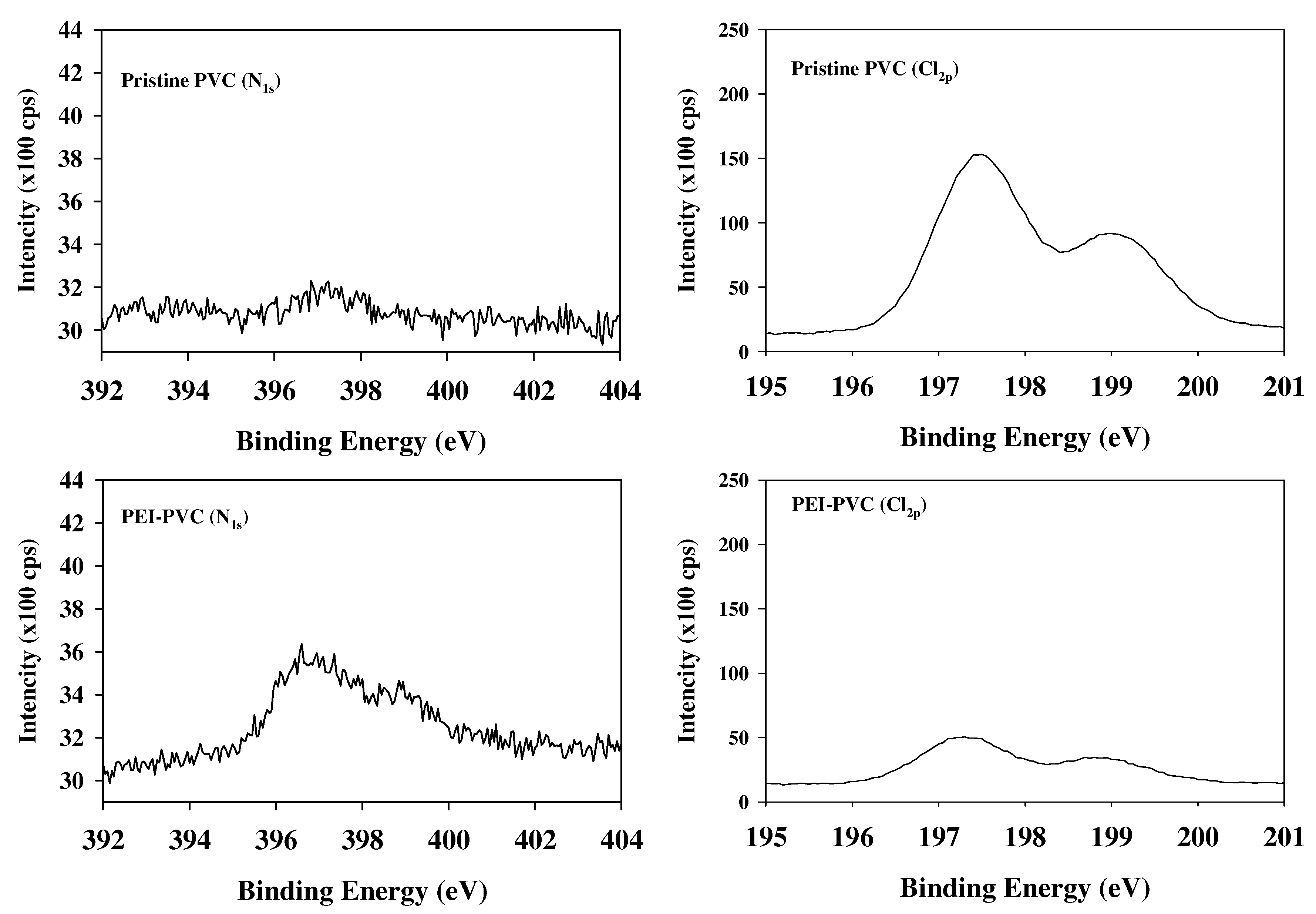
3.1. Change of Functional Group Properties of PVC Sorbent after PEI Reaction
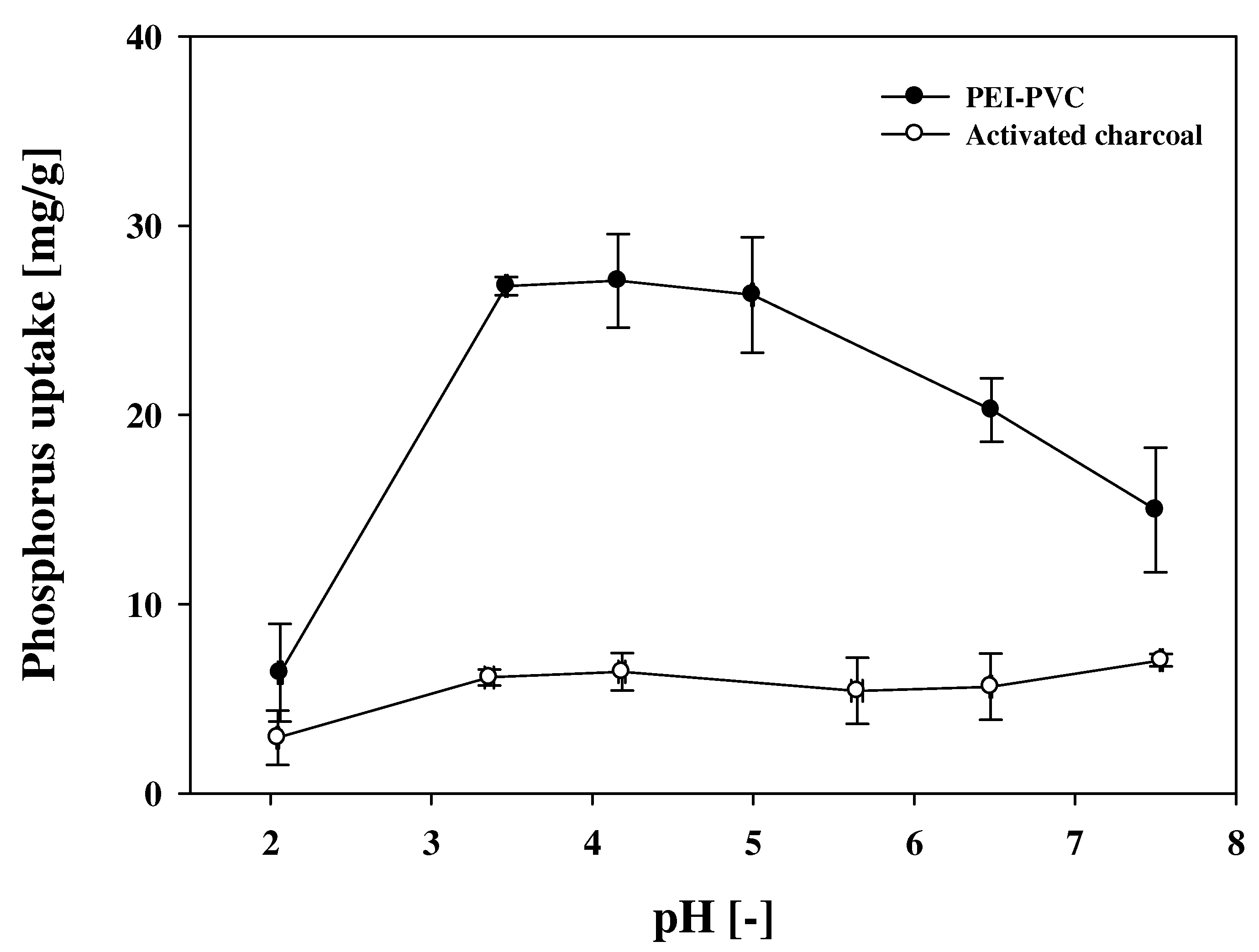
3.2. pH Effect on Phosphorus Adsorption
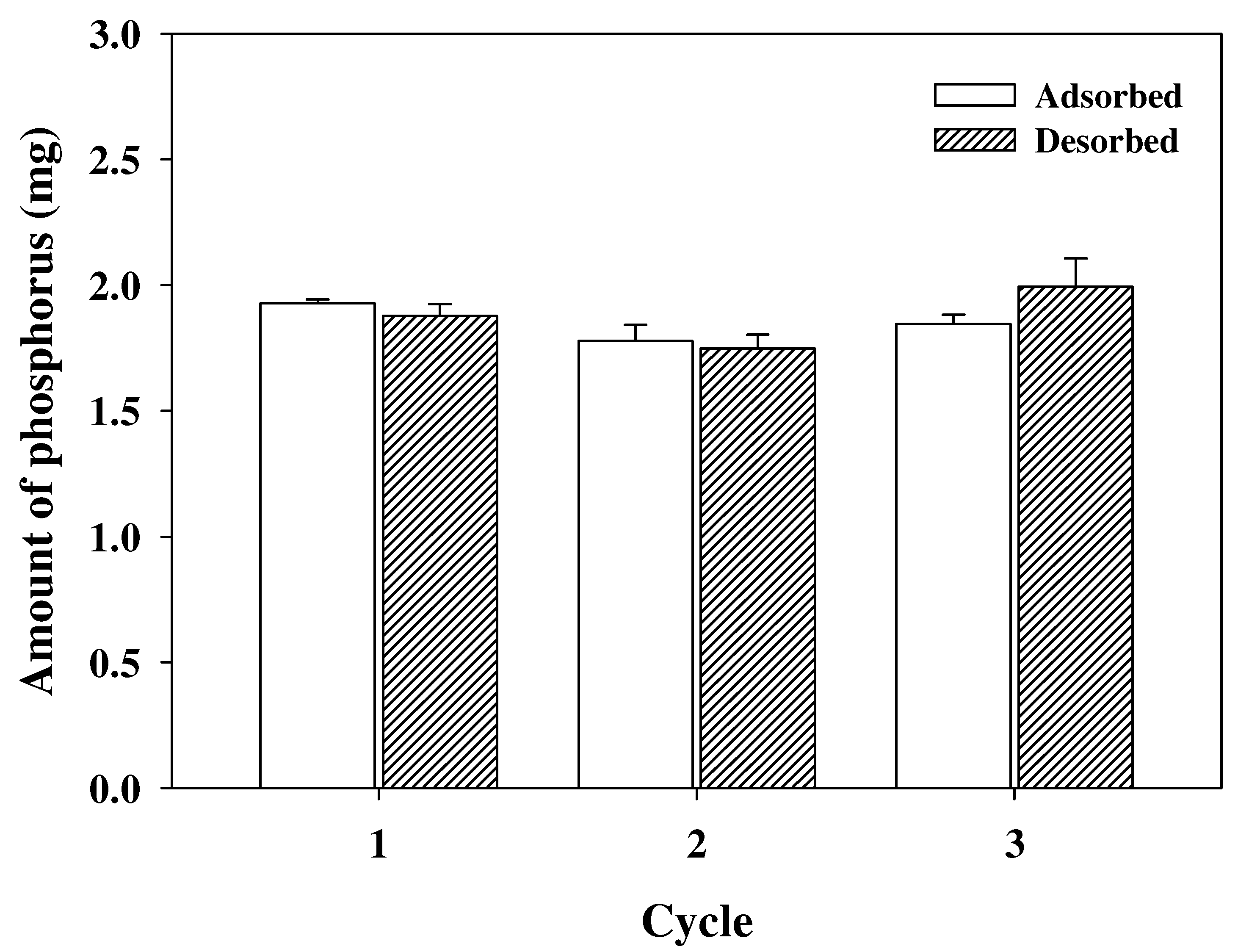
3.3. Reusability of PEI-PVC Fiber
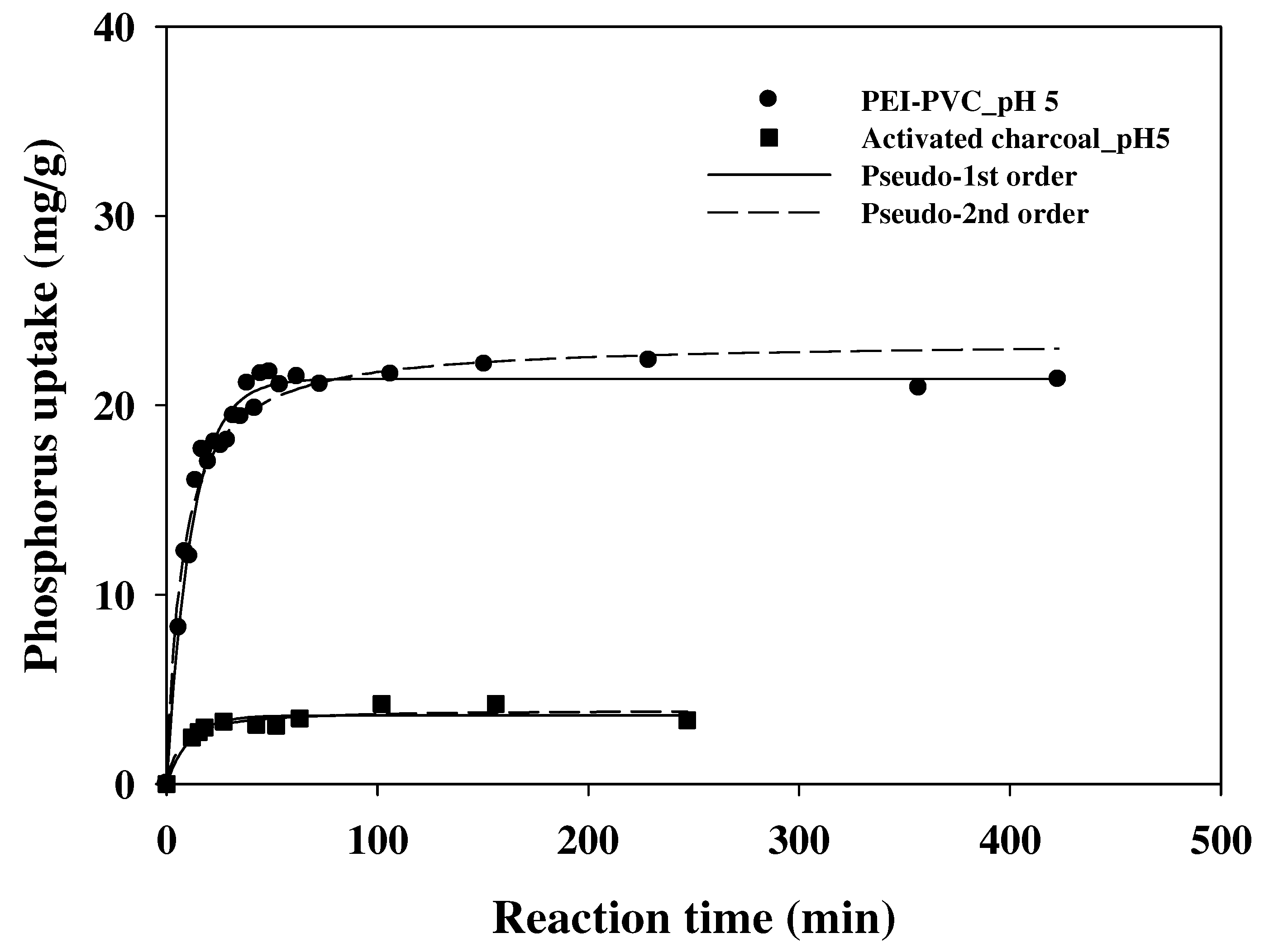
3.4. Adsorption Kinetics
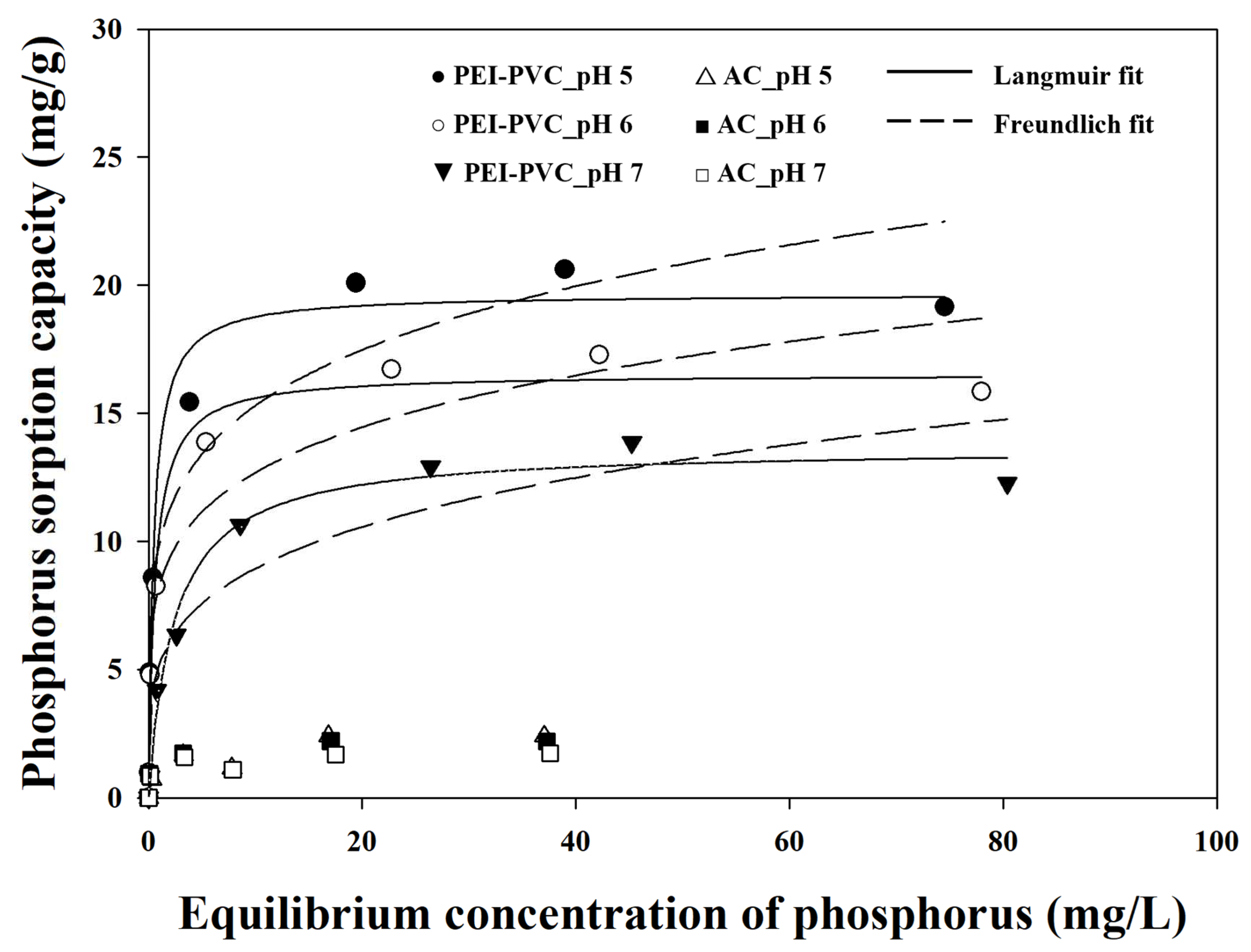
3.5. Maximum Phosphorus Sorption Capacity of PEI-PVC Fiber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Ianaiev, V.; Huff, A.; Zalusky, J.; Ozersky, T.; Katsev, S. Benthic invaders control the phosphorus cycle in the world’s largest freshwater ecosystem. Proc. Natl. Acad. Sci. USA 2021, 118, e2008223118. [Google Scholar] [CrossRef]
- Kim, S.; Park, Y.H.; Lee, J.B.; Kim, H.S.; Choi, Y.-E. Phosphorus adsorption behavior of industrial waste biomass-based adsorbent, esterified polyethylenimine-coated polysulfone-Escherichia coli biomass composite fibers in aqueous solution. J. Hazard. Mater. 2020, 400, 123217. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yang, F. Critical review on soil phosphorus migration and transformation under freezing-thawing cycles and typical regulatory measurements. Sci. Total Environ. 2021, 751, 141614. [Google Scholar] [CrossRef]
- Yindong, T.; Xiwen, X.; Miao, Q.; Jingjing, S.; Yiyan, Z.; Wei, Z.; Mengzhu, W.; Xuejun, W.; Yang, Z. Lake warming intensifies the seasonal pattern of internal nutrient cycling in the eutrophic lake and potential impacts on algal blooms. Water Res. 2021, 188, 116570. [Google Scholar] [CrossRef]
- Xu, C.; Dan, S.F.; Yang, B.; Lu, D.; Kang, Z.; Huang, H.; Zhou, J.; Ning, Z. Biogeochemistry of dissolved and particulate phosphorus speciation in the Maowei Sea, northern Beibu Gulf. J. Hydrol. 2021, 593, 125822. [Google Scholar] [CrossRef]
- Javier, L.; Farhat, N.M.; Vrouwenvelder, J.S. Enhanced hydraulic cleanability of biofilms developed under a low phosphorus concentration in reverse osmosis membrane systems. Water Res. 2021, 10, 100085. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Application of membrane separation processes in phosphorus recovery: A review. Sci. Total Environ. 2021, 767, 144346. [Google Scholar] [CrossRef]
- Guida, S.; Rubertelli, G.; Jefferson, B.; Soares, A. Demonstration of ion exchange technology for phosphorus removal and recovery from municipal wastewater. Chem. Eng. J. 2021, 420, 129913. [Google Scholar] [CrossRef]
- Xia, W.-J.; Guo, L.-X.; Yu, L.-Q.; Zhang, Q.; Xiong, J.-R.; Zhu, X.-Y.; Wang, X.-C.; Huang, B.-C.; Jin, R.-C. Phosphorus removal from diluted wastewaters using a La/C nanocomposite-doped membrane with adsorption-filtration dual functions. Chem. Eng. J. 2021, 405, 126924. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Li, Z.; Yang, X.; Wu, X.; Zhao, C.; Xing, Y.; Yang, R.T. NOx removal with efficient recycling of NO2 from iron-ore sintering flue gas: A novel cyclic adsorption process. J. Hazard. Mater. 2021, 407, 124380. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, S.; Wang, X.; Zhang, H.; Zhan, Y. Removal of phosphate from aqueous solution by a novel Mg(OH)2/ZrO2 composite: Adsorption behavior and mechanism. Colloid Surf. A Physicochem. Eng. Asp. 2019, 561, 301–314. [Google Scholar] [CrossRef]
- Ye, H.; Chen, F.; Sheng, Y.; Sheng, G.; Fu, J. Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep. Purif. Technol. 2006, 50, 283–290. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kizito, S.; Luo, H.; Wu, S.; Ajmal, Z.; Lv, T.; Dong, R. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manag. 2017, 201, 260–267. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Szymczyk, P.; Kuczajowska-Zadrożna, M.; Mielcarek, A. The use of cross-linked chitosan beads for nutrients (nitrate and orthophosphate) removal from a mixture of P-PO4, N-NO2 and N-NO3. Int. J. Biol. Macromol. 2017, 104, 1280–1293. [Google Scholar] [CrossRef]
- Pei, H.-Y.; Ma, C.-X.; Hu, W.-R.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Abdel-Fattah, E.; Alharthi, A.I.; Fahmy, T. Spectroscopic, optical and thermal characterization of polyvinyl chloride-based plasma-functionalized MWCNTs composite thin films. Appl. Phys. A 2019, 125, 475. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, Y.H.; Nam, K.; Kim, S.; Choi, Y.-E. Amination of cotton fiber using polyethyleneimine and its application as an adsorbent to directly remove a harmful cyanobacterial species, Microcystis aeruginosa, from an aqueous medium. Environ. Res. 2021, 197, 111235. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.-E.; Yun, Y.-S. Ruthenium recovery from acetic acid industrial effluent using chemically stable and high-performance polyethylenimine-coated polysulfone-Escherichia coli biomass composite fibers. J. Hazard. Mater. 2016, 313, 29–36. [Google Scholar] [CrossRef]
- Sneddon, G.; McGlynn, J.C.; Neumann, M.S.; Aydin, H.M.; Yiu, H.H.P.; Ganin, A.Y. Aminated poly(vinyl chloride) solid state adsorbents with hydrophobic function for post-combustion CO2 capture. J. Mater. Chem. A 2017, 5, 11864–11872. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.; Joshi, G.M.; Mukherjee, A.; Thomas, P. Electrical properties and thermal degradation of poly(vinyl chloride)/polyvinylidene fluoride/ZnO polymer nanocomposites. Polym. Int. 2016, 65, 1098–1106. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L.; Madejová, J. Near-IR study of the impact of alkyl-ammonium and -phosphonium cations on the hydration of montmorillonite. J. Mol. Struct. 2022, 1256, 132568. [Google Scholar] [CrossRef]
- Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Lamponi, S.; Donati, A.; Rossi, C.; Magnani, A. Plasticizers free polyvinyl chloride membrane for metal ions sequestering. Inorg. Chem. Commun. 2020, 119, 108100. [Google Scholar] [CrossRef]
- Hezma, A.M.; Elashmawi, I.S.; Rajeh, A.; Kamal, M. Change Spectroscopic, thermal and mechanical studies of PU/PVC blends. Phys. B Condens. Matter 2016, 495, 4–10. [Google Scholar] [CrossRef]
- Kim, M.H.; Hwang, C.-H.; Kang, S.B.; Kim, S.; Park, S.W.; Yun, Y.-S.; Won, S.W. Removal of hydrolyzed Reactive Black 5 from aqueous solution using a polyethylenimine–polyvinyl chloride composite fiber. Chem. Eng. J. 2015, 280, 18–25. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, T.; Woo, S.H. Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for Reactive Black 5 from aqueous solutions. Chem. Eng. J. 2011, 166, 168–175. [Google Scholar] [CrossRef]
- Yousif, E.; Hasan, A. Photostabilization of poly(vinyl chloride)—Still on the run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Yun, Y.-S.; Choi, Y.-E. Development of waste biomass based sorbent for removal of cyanotoxin microcystin-LR from aqueous phases. Bioresour. Technol. 2018, 247, 690–696. [Google Scholar] [CrossRef]
- Jang, J.W.; Park, J.J.; Kwan, M.H.; Lim, H. Simultaneous Monitoring Method for Chemical Constituents in Aluminum Etchant. IEEE Sens. J. 2012, 12, 2180–2185. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing diazo dye. Chem. Eng. J. 2012, 187, 79–88. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Harada, H.; Ohto, K.; Kawakita, H. Removal and recovery of phosphorus from water by means of adsorption onto orange waste gel loaded with zirconium. Bioresour. Technol. 2008, 99, 8685–8690. [Google Scholar] [CrossRef]
- Han, J.S.; Min, S.-H.; Kim, Y.-K. Removal of phosphorus using AMD-treated lignocellulosic material. For. Prod. J. 2005, 55, 48–53. [Google Scholar]
- Mezenner, N.Y.; Bensmaili, A. Kinetics and thermodynamic study of phosphate adsorption on iron hydroxide-eggshell waste. Chem. Eng. J. 2009, 147, 87–96. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Liu, J.; Huo, M.; Huo, H.; Yang, W. Removing phosphorus from aqueous solutions using lanthanum modified pine needles. PLoS ONE 2015, 10, e0142700. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple Peels—A Versatile Biomass for Water Purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Equilibrium and kinetic studies of adsorption of phosphate onto ZnCl2 activated coir pith carbon. J. Colloid Interface Sci. 2004, 280, 359–365. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Ghimire, K.N.; Ohta, S.; Harada, H.; Ohto, K.; Kawakita, H. The adsorption of phosphate from an aquatic environment using metal-loaded orange waste. J. Colloid Interface Sci. 2007, 312, 214–223. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, H.; Guo, W.; Zhang, J.; Liang, S.; Tung, K. Feasibility of iron loaded ‘okara’for biosorption of phosphorous in aqueous solutions. Bioresour. Technol. 2013, 150, 42–49. [Google Scholar] [CrossRef]

| Conditions | Experiments | ||
|---|---|---|---|
| pH Effect | Kinetics * | Isotherm | |
| Working volume (L) | 0.03 | 0.08 | 0.03 |
| Weight of adsorbents (g) | 0.03 | 0.08 | 0.03 |
| pH | 2–8 | 5 (4.95–5.05) | 5, 6, and 7 |
| Initial phosphorus concentration (mg/L) | 98.3 | 91.7 | 1–100 |
| Adsorption reaction time (h) | 24 | 8 | 24 |
| Adsorbents | Kinetic Parameters | ||
|---|---|---|---|
| k2 (g/mg·min) | qe (mg/g) | R2 | |
| PEI-PVC | 0.0057 ± 0.0007 | 23.39 ± 0.47 | 0.9585 |
| AC | 0.037 ± 0.013 | 3.94 ± 0.21 | 0.9211 |
| Models | Parameters | pH | ||
|---|---|---|---|---|
| 5 | 6 | 7 | ||
| Freundlich | Kf | 9.83 ± 1.34 | 8.20 ± 1.14 | 5.12 ± 0.97 |
| n | 5.21 ± 1.05 | 5.29 ± 1.10 | 4.14 ± 0.91 | |
| R2 | 0.9192 | 0.9161 | 0.9046 | |
| Langmuir | b | 2.08 ± 0.59 | 1.65 ± 0.35 | 0.42 ± 0.08 |
| qmax, L | 19.66 ± 0.82 | 16.54 ± 0.54 | 13.66 ± 0.51 | |
| R2 | 0.9755 | 0.9843 | 0.9844 | |
| Adsorbent | Maximum Phosphorus Adsorption Capacity (mg/g) | pH Condition | Ref. |
|---|---|---|---|
| Mg(OH)2 | 5.3 | pH 7 | [12] |
| ZrO2 | 21.9 | pH 7 | [12] |
| Palygorskite | 3.7 | pH 7 | [13] |
| Clinoptilolite | 6.6 | pH 5.3 | [14] |
| Zeolite | 8.3 | pH 5.3 | [14] |
| Fe-loaded juniper fiber | 2.31 | pH 6.4 | [33] |
| Iron–hydroxide eggshell | 14.49 | pH 7 | [34] |
| Zirconium ferrite | 13 | pH 7 | [32] |
| Zr(IV)-loaded apple peels | 20.35 | pH 2 | [36] |
| Zr(IV)-loaded orange waste gel | 57 | pH 7 | [32] |
| Zr(IV)-loaded MUROMAC | 43 | pH 7 | [32] |
| Zn(II)-activated coir pith carbon | 5.1 | pH 4 | [37] |
| Cross-linked chitosan by epichlorohydrin | 38.22 | pH 3 | [16] |
| Cross-linked chitosan by sodium citrate | 13.3 | pH 3 | [16] |
| La(III)-loaded orange waste | 13.94 | pH 5–7 | [38] |
| La(III)-modified fine needle | 6.31 | pH 7.1 | [35] |
| Fe(III)-loaded okara | 4.78 | pH 3 | [39] |
| Zr-loaded okara | 14.39 | pH 7 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Park, Y.H.; Choi, Y.-E. Amination of Non-Functional Polyvinyl Chloride Polymer Using Polyethyleneimine for Removal of Phosphorus from Aqueous Solution. Polymers 2022, 14, 1645. https://doi.org/10.3390/polym14091645
Kim S, Park YH, Choi Y-E. Amination of Non-Functional Polyvinyl Chloride Polymer Using Polyethyleneimine for Removal of Phosphorus from Aqueous Solution. Polymers. 2022; 14(9):1645. https://doi.org/10.3390/polym14091645
Chicago/Turabian StyleKim, Sok, Yun Hwan Park, and Yoon-E Choi. 2022. "Amination of Non-Functional Polyvinyl Chloride Polymer Using Polyethyleneimine for Removal of Phosphorus from Aqueous Solution" Polymers 14, no. 9: 1645. https://doi.org/10.3390/polym14091645
APA StyleKim, S., Park, Y. H., & Choi, Y.-E. (2022). Amination of Non-Functional Polyvinyl Chloride Polymer Using Polyethyleneimine for Removal of Phosphorus from Aqueous Solution. Polymers, 14(9), 1645. https://doi.org/10.3390/polym14091645






