Aluminum Diethylphosphinate-Incorporated Flame-Retardant Polyacrylonitrile Separators for Safety of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
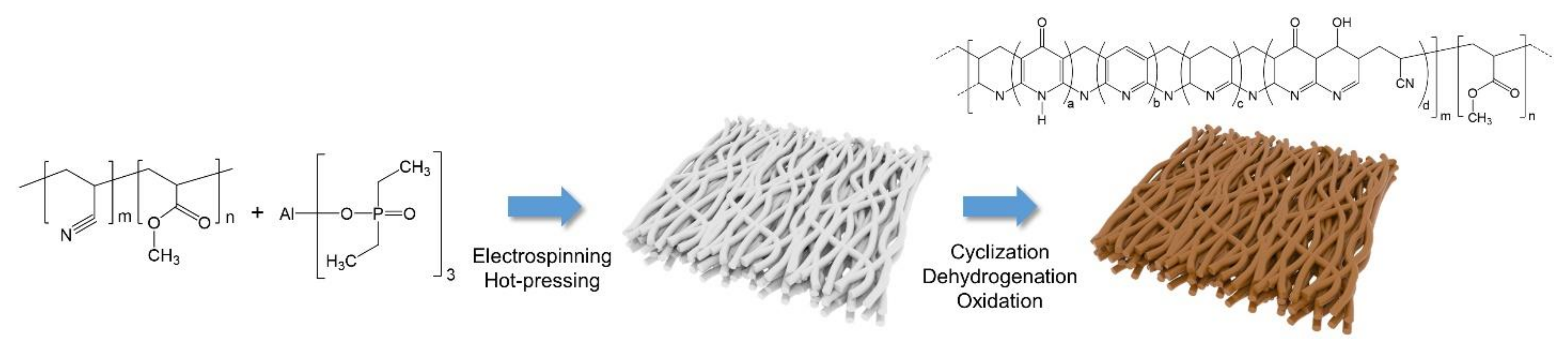
2.2. Preparation of the PAN Composite-Based Porous Membranes
2.3. Characterization of the Membranes
2.4. Electrochemical Evaluation of the Membranes
3. Results and Discussion
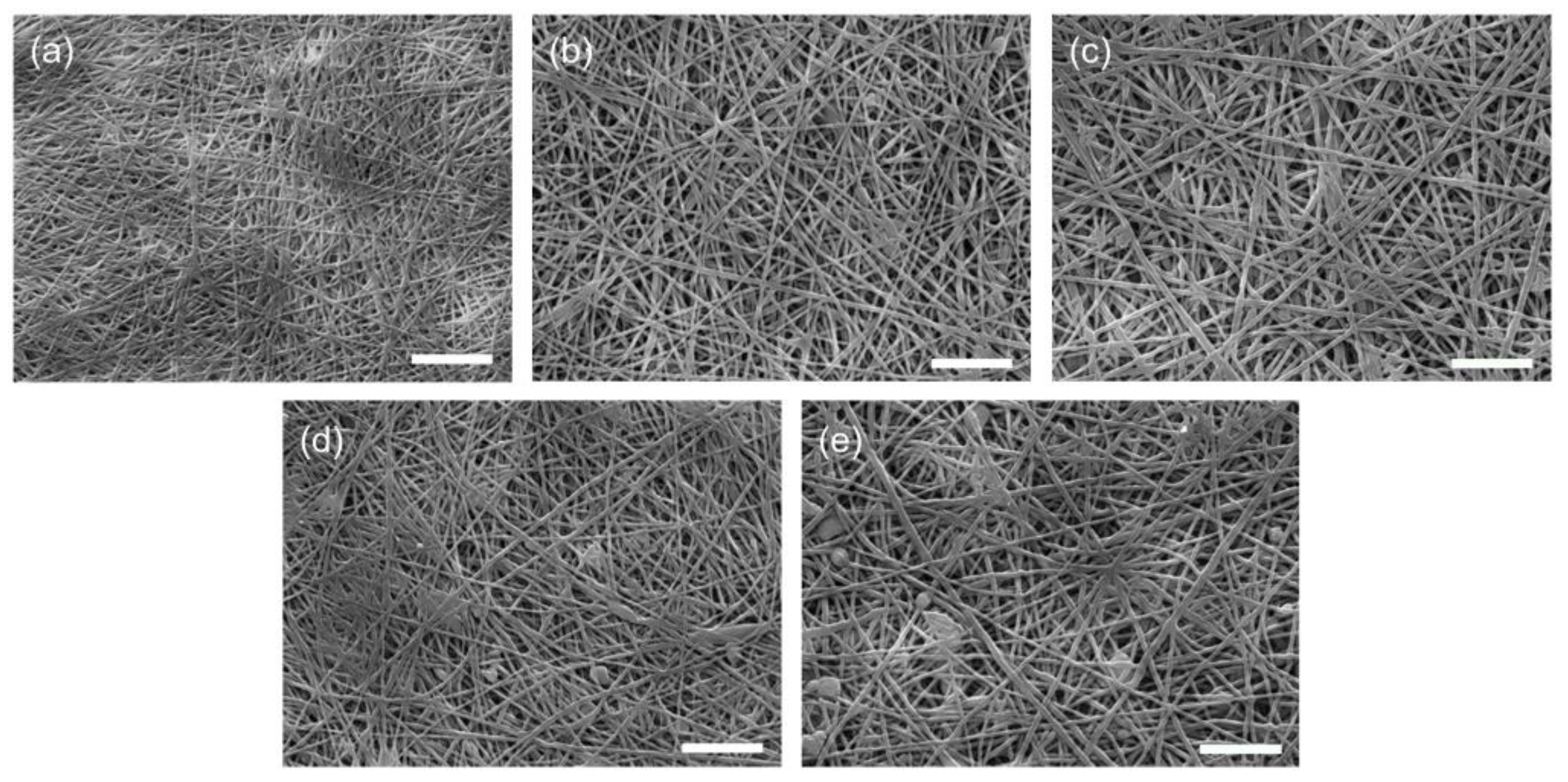
3.1. Characterization of the PAN/ADEP Composite Membranes
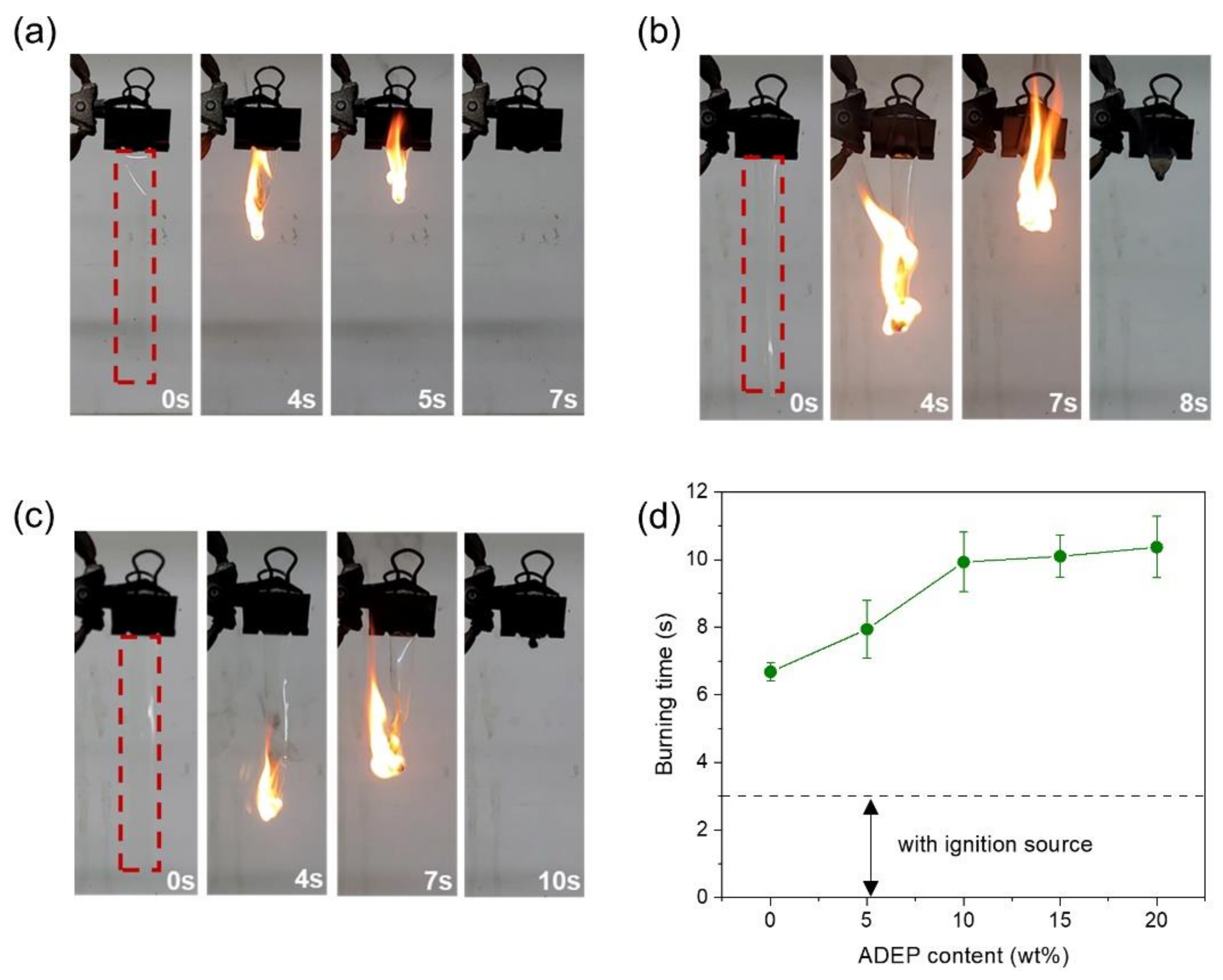
3.2. Flame Retardancy of the Membranes
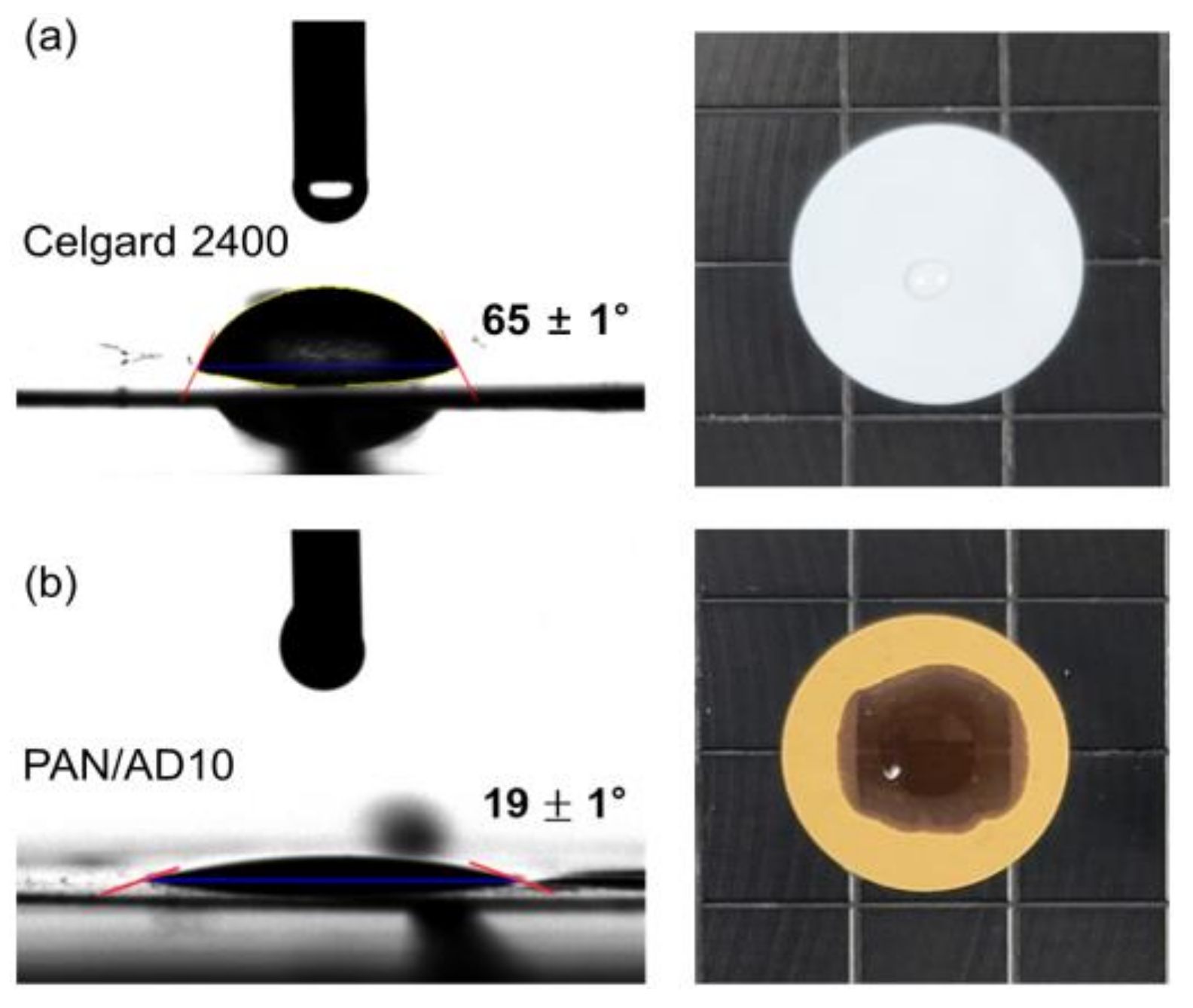
3.3. Electrolyte Wettability of the Membranes
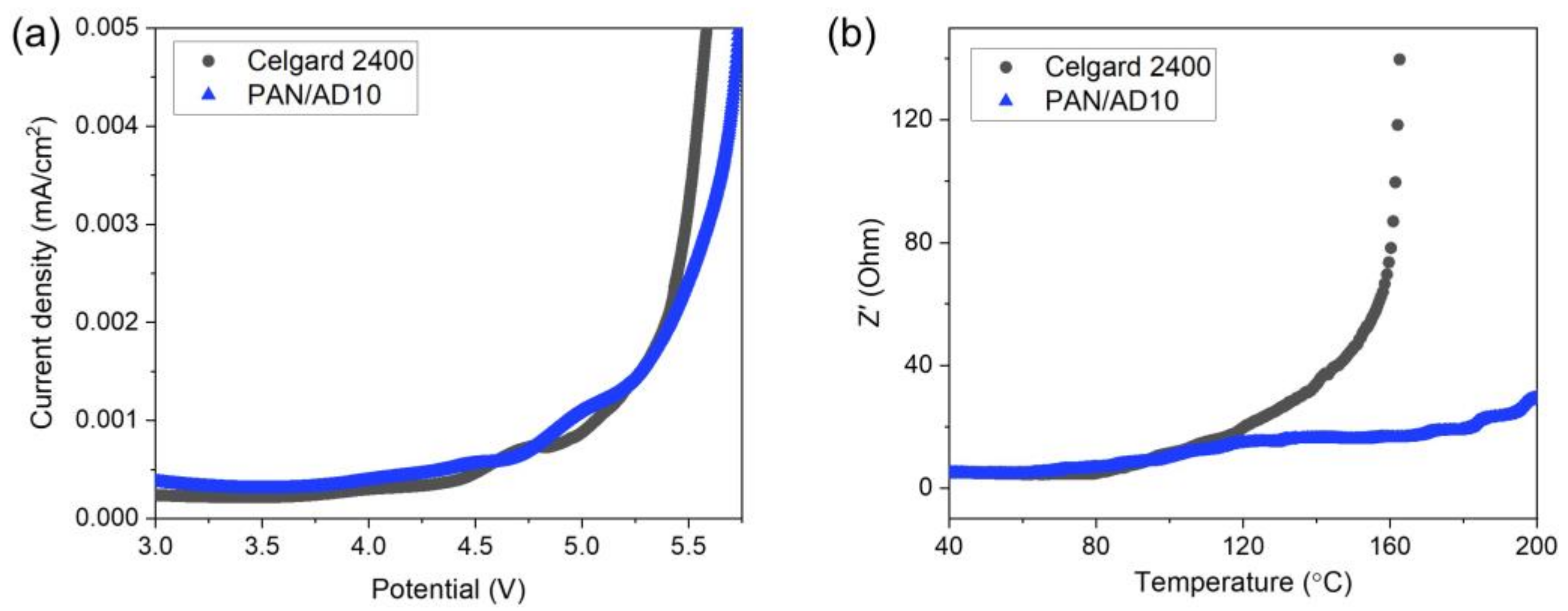
3.4. Electrochemical Stability and Heat Resistance of the Membranes
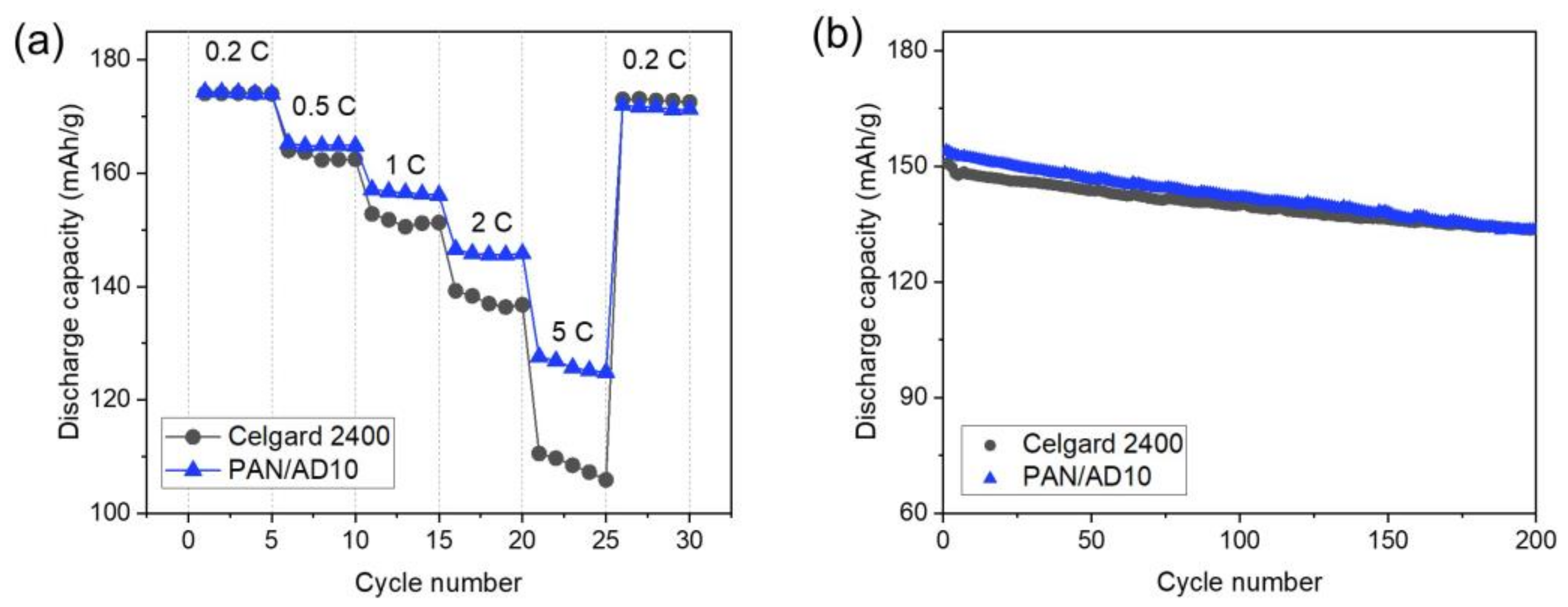
3.5. Electrochemical Performance of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A Critical Review of Thermal Issues in Lithium-Ion Batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on Lithium-Ion Batteries Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced Separators for Lithium-Ion and Lithium–Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef]
- Luiso, S.; Fedkiw, P. Lithium-ion battery separators: Recent developments and state of art. Curr. Opin. Electrochem. 2020, 20, 99–107. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Chung, T.C.M. Functional Polyolefins for Energy Applications. Macromolecules 2013, 46, 6671–6698. [Google Scholar] [CrossRef]
- Hao, J.; Lei, G.; Li, Z.; Wu, L.; Xiao, Q.; Wang, L. A novel polyethylene terephthalate nonwoven separator based on electrospinning technique for lithium ion battery. J. Membr. Sci. 2013, 428, 11–16. [Google Scholar] [CrossRef]
- Li, D.; Shi, D.; Xia, Y.; Qiao, L.; Li, X.; Zhang, H. Superior Thermally Stable and Nonflammable Porous Polybenzimidazole Membrane with High Wettability for High-Power Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 8742–8750. [Google Scholar] [CrossRef]
- Kim, M.; Hong, S.Y.; Bang, J.; Lee, S.-S. Highly sustainable polyphenylene sulfide membrane of tailored porous architecture for high-performance lithium-ion battery applications. Mater. Today Adv. 2021, 12, 100186. [Google Scholar] [CrossRef]
- Cho, T.H.; Sakai, T.; Tanase, S.; Kimura, K.; Kondo, Y.; Tarao, T.; Tanaka, M. Electrochemical Performances of Polyacrylonitrile Nanofiber-Based Nonwoven Separator for Lithium-Ion Battery. Electrochem. Solid-State Lett. 2007, 10, A159. [Google Scholar] [CrossRef]
- Ma, X.; Kolla, P.; Yang, R.; Wang, Z.; Zhao, Y.; Smirnova, A.L.; Fong, H. Electrospun polyacrylonitrile nanofibrous membranes with varied fiber diameters and different membrane porosities as lithium-ion battery separators. Electrochim. Acta 2017, 236, 417–423. [Google Scholar] [CrossRef]
- Lee, J.H.; Manuel, J.; Choi, H.; Park, W.H.; Ahn, J.-H. Partially oxidized polyacrylonitrile nanofibrous membrane as a thermally stable separator for lithium ion batteries. Polymer 2015, 68, 335–343. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, A.B.; Gilman, J.W. An overview of flame retardancy of polymeric materials: Application, technology, and future directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sun, Q.; Zhen, C.; Niu, Y.; Han, Y.; Zeng, G.; Chen, D.; Feng, C.; Chen, N.; Lv, W.; et al. Recent progress in flame-retardant separators for safe lithium-ion batteries. Energy Storage Mater. 2021, 37, 628–647. [Google Scholar] [CrossRef]
- Yusuf, A.; Avvaru, V.S.; Dirican, M.; Changchun, S.; Wang, D.-Y. Low heat yielding electrospun phosphenanthrene oxide loaded polyacrylonitrile composite separators for safer high energy density lithium-ion batteries. Appl. Mater. Today 2020, 20, 100675. [Google Scholar] [CrossRef]
- Li, J.-L.; Gao, C.-T.; Sun, X.; Peng, S.-G.; Wang, Y.-W.; Qin, S.-H. Synergistic Flame-Retardant Effect of Aluminum Diethyl Phosphinate in PP/IFR System and the Flame-Retardant Mechanism. Int. Polym. Proc. 2021, 36, 519–528. [Google Scholar] [CrossRef]
- Ding, Y.; Stoliarov, S.I.; Kraemer, R.H. Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate. Polymers 2018, 10, 1137. [Google Scholar]
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [Green Version]
- Brehme, S.; Schartel, B.; Goebbels, J.; Fischer, O.; Pospiech, D.; Bykov, Y.; Döring, M. Phosphorous polyester versus aluminium phosphinate in poly(butylene terephthalate) (PBT): Flame retardancy performance and mechanisms. Polym. Degrad. Stab. 2011, 96, 875–884. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-H.; Hon, J.-M.; Wang, M.-W.; Juang, T.-Y. First halogen and phosphorus-free, flame-retardant benzoxazine thermosets derived from main-chain type bishydroxydeoxybenzoin-based benzoxazine polymers. Polymer 2018, 154, 35–41. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Dirican, M.; Zhang, X. Evaluation of electrospun SiO2/nylon 6,6 nanofiber membranes as a thermally-stable separator for lithium-ion batteries. Electrochim. Acta 2014, 133, 501–508. [Google Scholar] [CrossRef]
- Watt, W.; Johnson, W. Mechanism of oxidisation of polyacrylonitrile fibres. Nature 1975, 257, 210–212. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Li, Z.; Cao, T.; Zhang, Y.; Han, Y.; Xu, S.; Xu, Z. Novel lithium ion battery separator based on hydroxymethyl functionalized poly(ether ether ketone). J. Membr. Sci. 2017, 540, 422–429. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Choi, E.-S.; Kim, J.H.; Lee, S.-Y. Nanoporous polymer scaffold-embedded nonwoven composite separator membranes for high-rate lithium-ion batteries. RSC Adv. 2014, 4, 54312–54321. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, H.-S.; Doh, C.-H.; Kim, S.H.; Lee, S.-M. Technological potential and issues of polyacrylonitrile based nanofiber non-woven separator for Li-ion rechargeable batteries. J. Power Sources 2013, 244, 196–206. [Google Scholar] [CrossRef]


| Sample | ADEP Content (%) | Fiber Size (μm) | Average Pore Size (μm) | Porosity (%) | Tensile Strength (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|---|---|
| PAN | 0 | 0.31 ± 0.05 | 0.22 | 46 ± 2 | 47.2 ± 1.4 | 1.62 ± 0.05 |
| PAN/AD5 | 5 | 0.48 ± 0.08 | 0.37 | 51 ± 2 | 28.9 ± 0.5 | 1.14 ± 0.02 |
| PAN/AD10 | 10 | 0.49 ± 0.08 | 0.38 | 46 ± 2 | 27.7 ± 0.7 | 1.14 ± 0.03 |
| PAN/AD15 | 15 | 0.49 ± 0.09 | 0.37 | 41 ± 2 | 23.4 ± 0.4 | 0.91 ± 0.02 |
| PAN/AD20 | 20 | 0.50 ± 0.10 | 0.38 | 38 ± 2 | 19.4 ± 0.5 | 0.81 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.H.; Jeong, H.Y.; Kim, T.H.; Lee, J.Y.; Hong, S.K.; Hong, Y.T.; Choi, J.; So, S.; Yoon, S.J.; Yu, D.M. Aluminum Diethylphosphinate-Incorporated Flame-Retardant Polyacrylonitrile Separators for Safety of Lithium-Ion Batteries. Polymers 2022, 14, 1649. https://doi.org/10.3390/polym14091649
Kang SH, Jeong HY, Kim TH, Lee JY, Hong SK, Hong YT, Choi J, So S, Yoon SJ, Yu DM. Aluminum Diethylphosphinate-Incorporated Flame-Retardant Polyacrylonitrile Separators for Safety of Lithium-Ion Batteries. Polymers. 2022; 14(9):1649. https://doi.org/10.3390/polym14091649
Chicago/Turabian StyleKang, Seok Hyeon, Hwan Yeop Jeong, Tae Ho Kim, Jang Yong Lee, Sung Kwon Hong, Young Taik Hong, Jaewon Choi, Soonyong So, Sang Jun Yoon, and Duk Man Yu. 2022. "Aluminum Diethylphosphinate-Incorporated Flame-Retardant Polyacrylonitrile Separators for Safety of Lithium-Ion Batteries" Polymers 14, no. 9: 1649. https://doi.org/10.3390/polym14091649
APA StyleKang, S. H., Jeong, H. Y., Kim, T. H., Lee, J. Y., Hong, S. K., Hong, Y. T., Choi, J., So, S., Yoon, S. J., & Yu, D. M. (2022). Aluminum Diethylphosphinate-Incorporated Flame-Retardant Polyacrylonitrile Separators for Safety of Lithium-Ion Batteries. Polymers, 14(9), 1649. https://doi.org/10.3390/polym14091649








