Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Coating of Alginate Microcapsules
2.3. Characterisation of Coated and Uncoated Microcapsules
2.4. Cell Viability Test
2.5. Thermotolerance Test under Dry and Wet Heat Treatment
2.6. Dissolution Test in Simulated Gastrointestinal Fluids
2.7. Cell Release Profiles and Live/Dead Cell Determination from Coated and Uncoated Microcapsules (Number of Cells by Flow Cytometry)
2.8. Synthesis of FITC-Labeled Chitosan
2.9. Determination of Dissolved Chitosan Coat
2.10. Statistical Analysis
3. Results
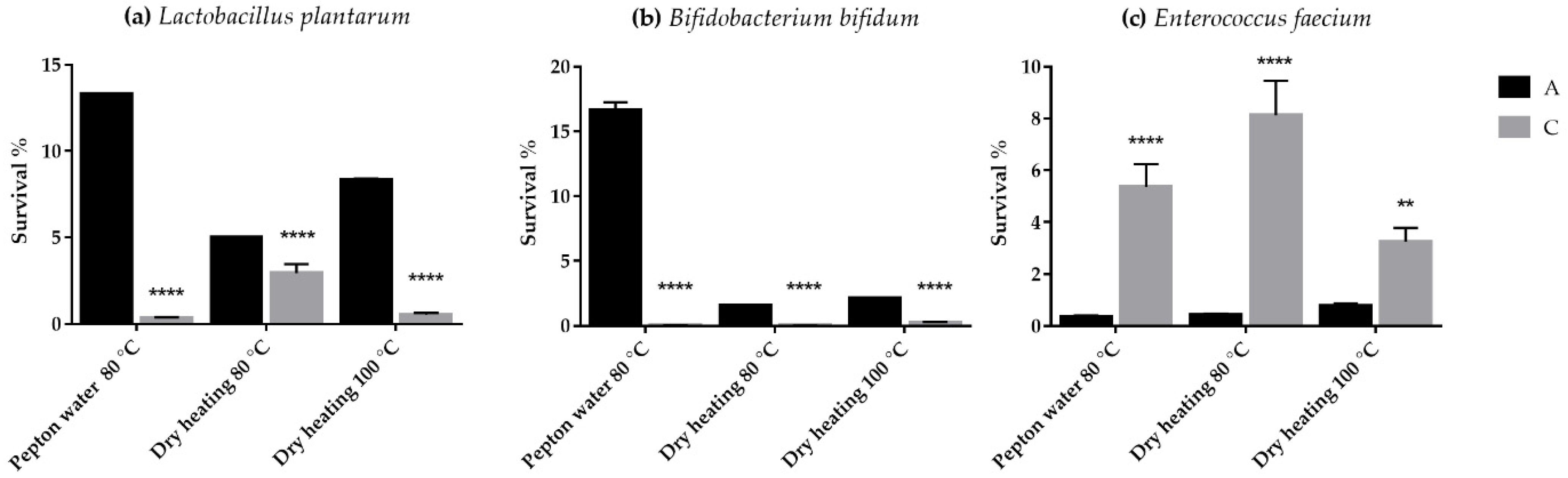
3.1. Thermotolerance Test under Dry and Wet Heat Treatment
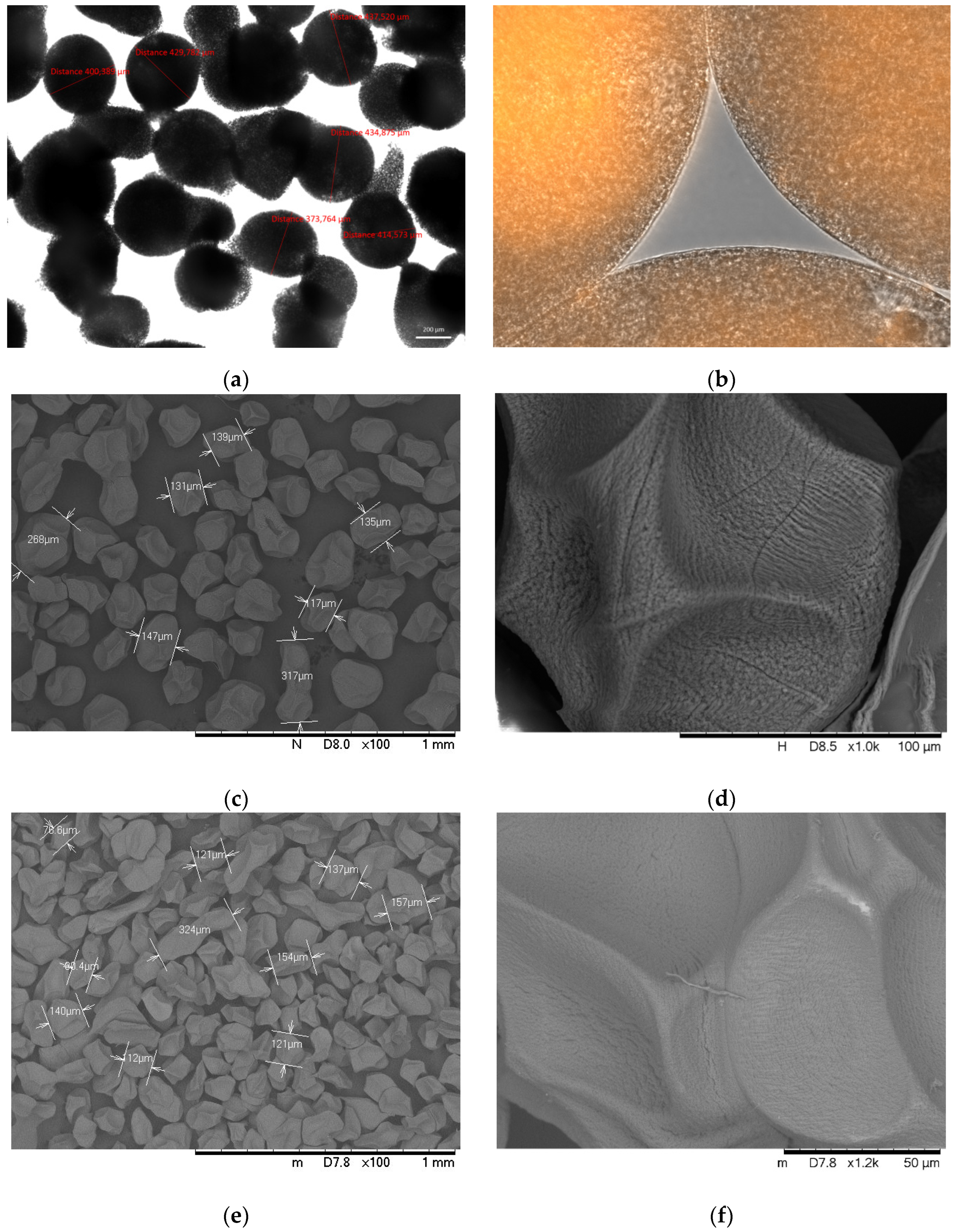
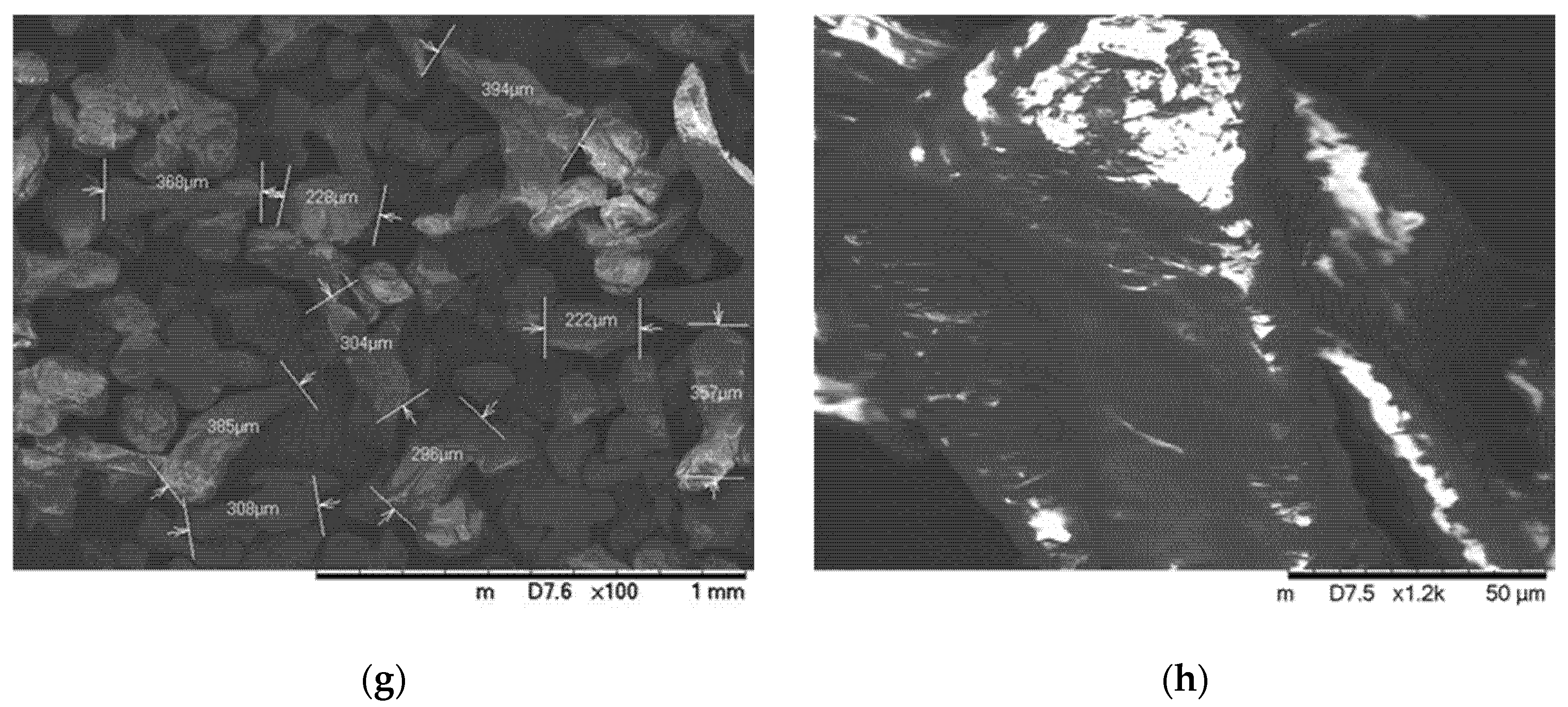
3.2. Characterization of Coated and Uncoated Microcapsules
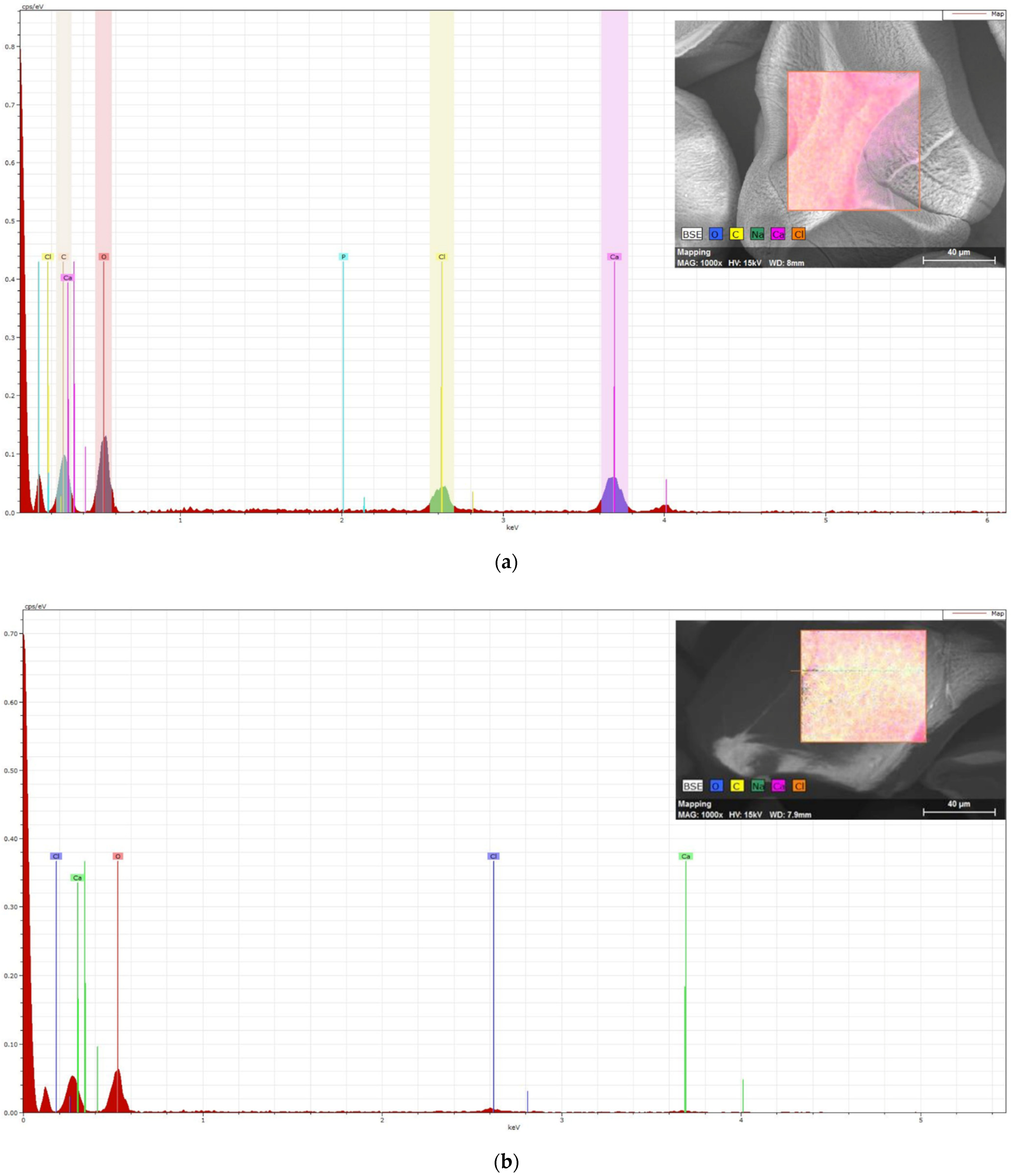
3.3. Elemental Spectroscopy
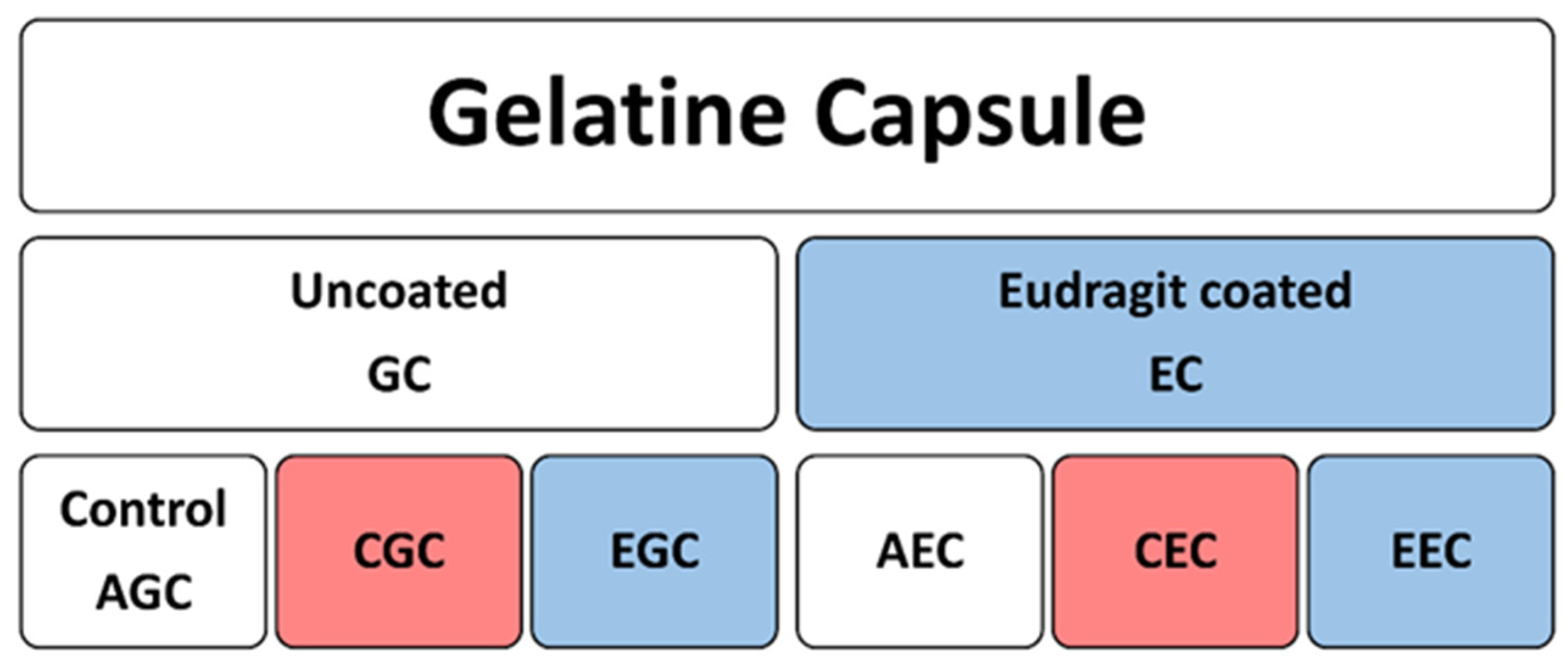
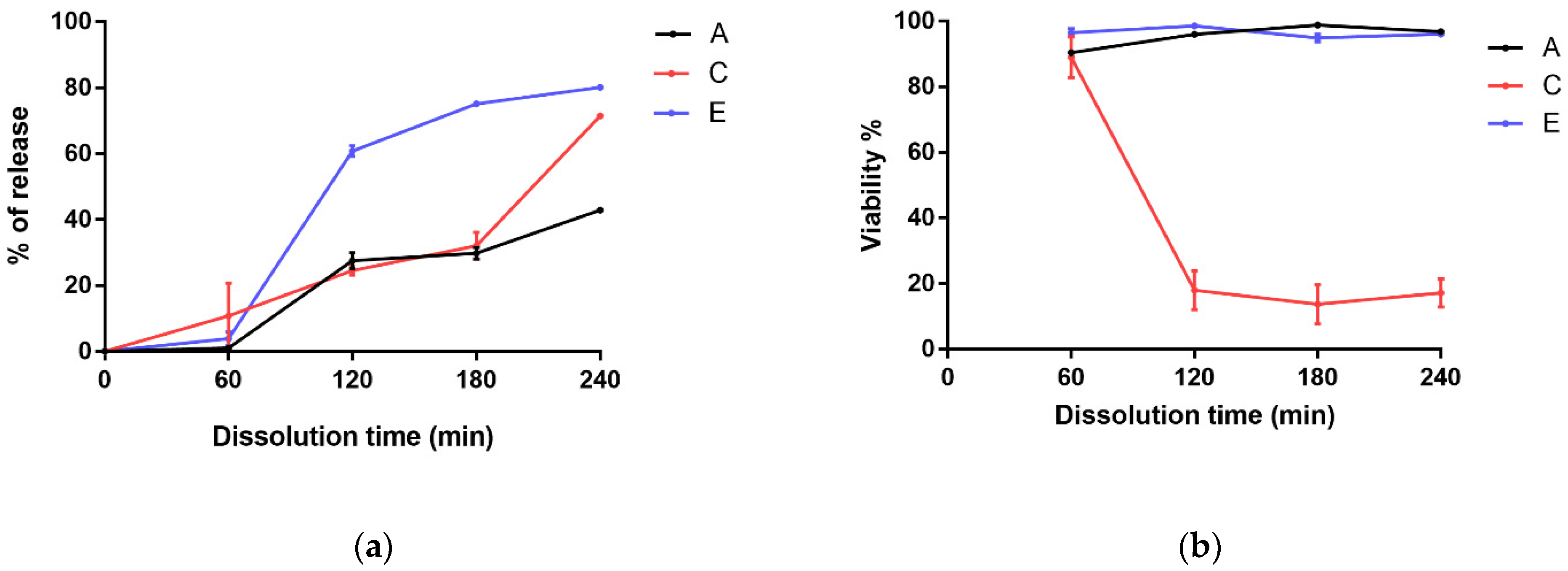
3.4. Dissolution Test of Microcapsule-Filled Gelatin Capsules
3.5. Cell Release Profiles and Live/Dead Cell Determination from Coated and Uncoated Microcapsules (Number of Cells by Flow Cytometry)
3.6. Determination of Dissolved Chitosan Coat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zamojska, D.; Nowak, A.; Nowak, I.; Macierzyńska-Piotrowska, E. Probiotics and Postbiotics as Substitutes of Antibiotics in Farm Animals: A Review. Animals 2021, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, G.H.; Jun, J.; Son, M.; Kang, M.J. Multiple-unit tablet of probiotic bacteria for improved storage stability, acid tolerability, and in vivo intestinal protective effect. Drug Des. Devel. Ther. 2016, 10, 1355–1364. [Google Scholar] [PubMed] [Green Version]
- Stadler, M.; Viernstein, H. Optimization of a formulation containing viable lactic acid bacteria. Int. J. Pharm. 2003, 256, 117–122. [Google Scholar] [CrossRef]
- Arnal, M.E.; Denis, S.; Uriot, O.; Lambert, C.; Holowacz, S.; Paul, F.; Kuylle, S.; Pereira, B.; Alric, M.; Blanquet-Diot, S. Impact of oral galenic formulations of Lactobacillus salivarius on probiotic survival and interactions with microbiota in human in vitro gut models. Benef. Microbes 2021, 12, 75–90. [Google Scholar] [CrossRef]
- Venema, K.; Verhoeven, J.; Beckman, C.; Keller, D. Survival of a probiotic-containing product using capsule-within-capsule technology in an in vitro model of the stomach and small intestine (TIM-1). Benef. Microbes 2020, 11, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Surono, I.; Verhoeven, J.; Verbruggen, S.; Venema, K. Microencapsulation increases survival of the probiotic Lactobacillus plantarum IS-10506, but not Enterococcus faecium IS-27526 in a dynamic, computer-controlled in vitro model of the upper gastrointestinal tract. J. Appl. Microbiol. 2018, 124, 1604–1609. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Dalbhagat, C.G.; Mishra, H.N. Emerging Technologies and Coating Materials for Improved Probiotication in Food Products: A Review. Food Bioprocess Technol. 2022, 1–42. [Google Scholar] [CrossRef]
- Liao, K.; Cai, J.; Shi, Z.; Tian, G.; Yan, D.; Chen, D. Effects of raw material extrusion and steam conditioning on feed pellet quality and nutrient digestibility of growing meat rabbits. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2017, 3, 151–155. [Google Scholar] [CrossRef]
- Itani, K.; Svihus, B. Feed processing and structural components affect starch digestion dynamics in broiler chickens. Br. Poult. Sci. 2019, 60, 246–255. [Google Scholar] [CrossRef]
- Rodrigues, B.M.; Olivo, P.M.; Osmari, M.P.; Vasconcellos, R.S.; Ribeiro, L.B.; Bankuti, F.I.; Pozza, M.S.S. Microencapsulation of Probiotic Strains by Lyophilization Is Efficient in Maintaining the Viability of Microorganisms and Modulation of Fecal Microbiota in Cats. Int. J. Microbiol. 2020, 2020, 1293481. [Google Scholar] [CrossRef] [PubMed]
- Simon, O.; Jadamus, A.; Vahjen, W. Probiotic feed additives-effectiveness and expected modes of action. J. Anim. Feed Sci. 2001, 10, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The Probiotic Properties of Lactic Acid Bacteria and Their Applications in Animal Husbandry. Curr. Microbiol. 2021, 79, 22. [Google Scholar] [CrossRef] [PubMed]
- Rodklongtan, A.; La-ongkham, O.; Nitisinprasert, S.; Chitprasert, P. Enhancement of Lactobacillus reuteri KUB-AC5 survival in broiler gastrointestinal tract by microencapsulation with alginate-chitosan semi-interpenetrating polymer networks. J. Appl. Microbiol. 2014, 117, 227–238. [Google Scholar] [CrossRef]
- Ross, G.R.; Gusils, C.; Gonzalez, S.N. Microencapsulation of probiotic strains for swine feeding. Biol. Pharm. Bull. 2008, 31, 2121–2125. [Google Scholar] [CrossRef] [Green Version]
- Iyer, C.; Phillips, M.; Kailasapathy, K. Release studies of Lactobacillus casei strain Shirota from chitosan-coated alginate-starch microcapsules in ex vivo porcine gastrointestinal contents. Lett. Appl. Microbiol. 2005, 41, 493–497. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Yun, T.T.; Li, A.K.; Qi, W.T.; Liang, X.X.; Wang, Y.W.; Liu, S. Evaluation of pilot-scale microencapsulation of probiotics and product effect on broilers1. J. Anim. Sci. 2015, 93, 4796–4807. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. Acid, bile, and heat tolerance of free and microencapsulated probiotic bacteria. J. Food Sci. 2007, 72, M446–M450. [Google Scholar] [CrossRef]
- Jiménez-Pranteda, M.L.; Poncelet, D.; Náder-Macías, M.E.; Arcos, A.; Aguilera, M.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A. Stability of lactobacilli encapsulated in various microbial polymers. J. Biosci. Bioeng. 2012, 113, 179–184. [Google Scholar] [CrossRef]
- Huq, T.; Fraschini, C.; Khan, A.; Riedl, B.; Bouchard, J.; Lacroix, M. Alginate based nanocomposite for microencapsulation of probiotic: Effect of cellulose nanocrystal (CNC) and lecithin. Carbohydr. Polym. 2017, 168, 61–69. [Google Scholar] [CrossRef]
- Huang, X.; Gänzle, M.; Zhang, H.; Zhao, M.; Fang, Y.; Nishinari, K. Microencapsulation of probiotic lactobacilli with shellac as moisture barrier and to allow controlled release. J. Sci. Food Agric. 2021, 101, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Thangrongthong, S.; Puttarat, N.; Ladda, B.; Itthisoponkul, T.; Pinket, W.; Kasemwong, K.; Taweechotipatr, M. Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci. Biotechnol. 2020, 29, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Shaharuddin, S.; Muhamad, I.I. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: Enhancement of survivability and thermotolerance. Carbohydr. Polym. 2015, 119, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Controlled release of Lactobacillus rhamnosus biofilm probiotics from alginate-locust bean gum microcapsules. Carbohydr. Polym. 2014, 103, 587–595. [Google Scholar] [CrossRef]
- Cheow, W.S.; Hadinoto, K. Biofilm-like Lactobacillus rhamnosus probiotics encapsulated in alginate and carrageenan microcapsules exhibiting enhanced thermotolerance and freeze-drying resistance. Biomacromolecules 2013, 14, 3214–3222. [Google Scholar] [CrossRef]
- Jiang, T.; Kim, Y.-K.; Singh, B.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Effect of microencapsulation of Lactobacillus plantarum 25 into alginate/chitosan/alginate microcapsules on viability and cytokine induction. J. Nanosci. Nanotechnol. 2013, 13, 5291–5295. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Production and evaluation of dry alginate-chitosan microcapsules as an enteric delivery vehicle for probiotic bacteria. Biomacromolecules 2011, 12, 2834–2840. [Google Scholar] [CrossRef]
- Tan, E.W.; Tan, K.Y.; Phang, L.V.; Kumar, P.V.; In, L.L.A. Enhanced gastrointestinal survivability of recombinant Lactococcus lactis using a double coated mucoadhesive film approach. PLoS ONE 2019, 14, e0219912. [Google Scholar] [CrossRef]
- Zawari, M.; Poller, B.; Walker, G.; Pearson, A.; Hampton, M.; Carr, A.C. Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants 2019, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Gartziandia, O.; Lasa, A.; Pedraz, J.L.; Miranda, J.; Portillo, M.P.; Igartua, M.; Hernández, R.M. Preparation and Characterization of Resveratrol Loaded Pectin/Alginate Blend Gastro-Resistant Microparticles. Molecules 2018, 23, 1886. [Google Scholar] [CrossRef] [Green Version]
- Bunthof, C.J.; Bloemen, K.; Breeuwer, P.; Rombouts, F.M.; Abee, T. Flow cytometric assessment of viability of lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 2326–2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, M.; Obermajer, N.; Bogovic Matijasić, B.; Rogelj, I.; Kmetec, V. Quantification of live and dead probiotic bacteria in lyophilised product by real-time PCR and by flow cytometry. Appl. Microbiol. Biotechnol. 2009, 84, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Matulyte, I.; Kasparaviciene, G.; Bernatoniene, J. Development of New Formula Microcapsules from Nutmeg Essential Oil Using Sucrose Esters and Magnesium Aluminometasilicate. Pharmaceutics 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Poot, M.; Yue, S.T.; Millard, P.J. Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl. Environ. Microbiol. 1997, 63, 2421–2431. [Google Scholar] [CrossRef] [Green Version]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef]
- Mawad, A.; Helmy, Y.A.; Shalkami, A.-G.; Kathayat, D.; Rajashekara, G. E. coli Nissle microencapsulation in alginate-chitosan nanoparticles and its effect on Campylobacter jejuni in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 10675–10690. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of probiotics by efficient vibration technology. J. Microencapsul. 2017, 34, 667–674. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdélyi, L.; Fenyvesi, F.; Gál, B.; Haimhoffer, Á.; Vasvári, G.; Budai, I.; Remenyik, J.; Bereczki, I.; Fehér, P.; Ujhelyi, Z.; et al. Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules. Polymers 2022, 14, 1664. https://doi.org/10.3390/polym14091664
Erdélyi L, Fenyvesi F, Gál B, Haimhoffer Á, Vasvári G, Budai I, Remenyik J, Bereczki I, Fehér P, Ujhelyi Z, et al. Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules. Polymers. 2022; 14(9):1664. https://doi.org/10.3390/polym14091664
Chicago/Turabian StyleErdélyi, Lóránd, Ferenc Fenyvesi, Bernadett Gál, Ádám Haimhoffer, Gábor Vasvári, István Budai, Judit Remenyik, Ilona Bereczki, Pálma Fehér, Zoltán Ujhelyi, and et al. 2022. "Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules" Polymers 14, no. 9: 1664. https://doi.org/10.3390/polym14091664
APA StyleErdélyi, L., Fenyvesi, F., Gál, B., Haimhoffer, Á., Vasvári, G., Budai, I., Remenyik, J., Bereczki, I., Fehér, P., Ujhelyi, Z., Bácskay, I., Vecsernyés, M., Kovács, R., & Váradi, J. (2022). Investigation of the Role and Effectiveness of Chitosan Coating on Probiotic Microcapsules. Polymers, 14(9), 1664. https://doi.org/10.3390/polym14091664
















