PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery
Abstract
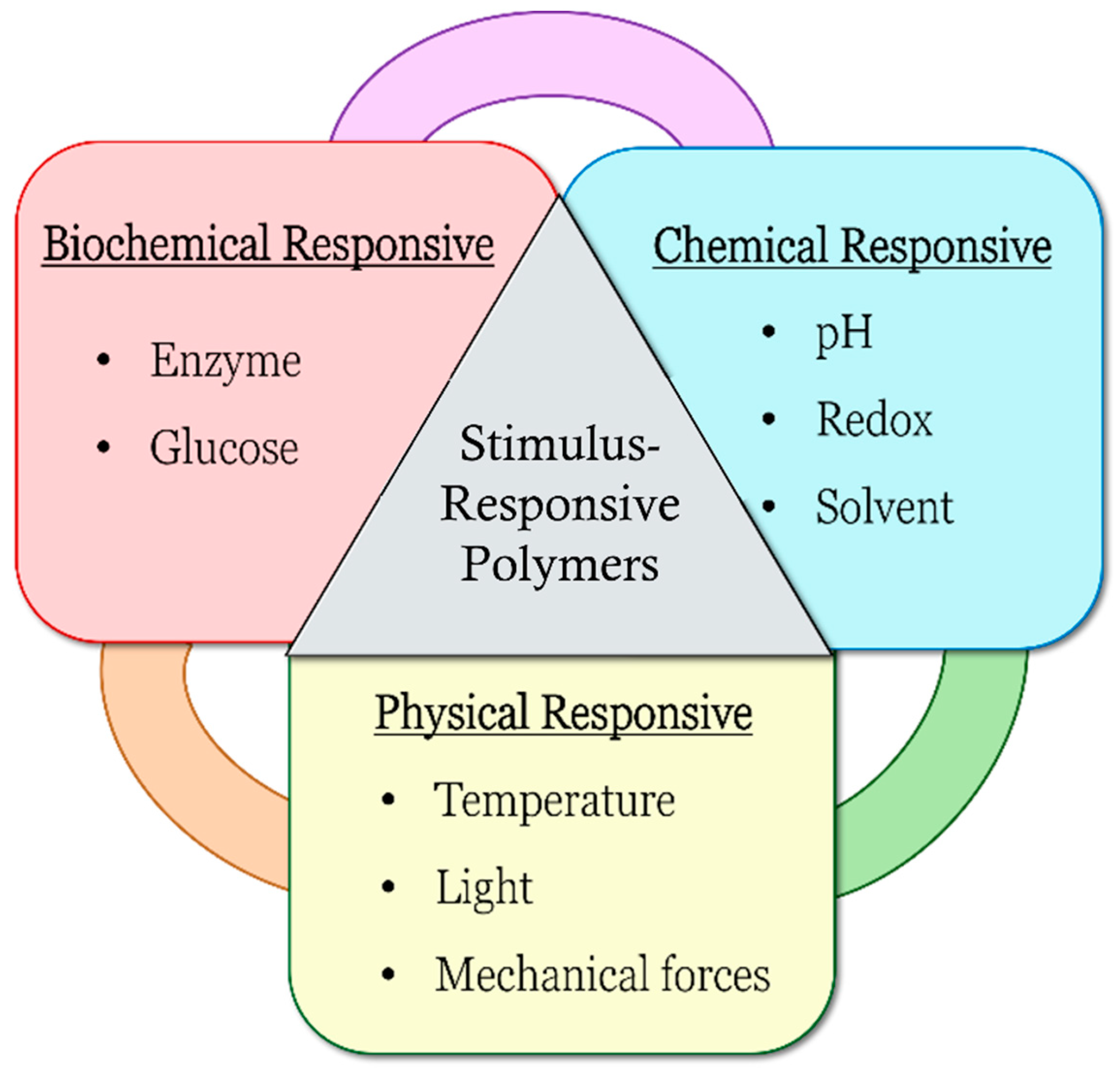
:1. Introduction
pH-Responsive Polymers
| Mechanism | Pendant Groups | Ionizable Group/Linkage | Responsive pH | Polymer Type | Type of Response | Application | Ref |
|---|---|---|---|---|---|---|---|
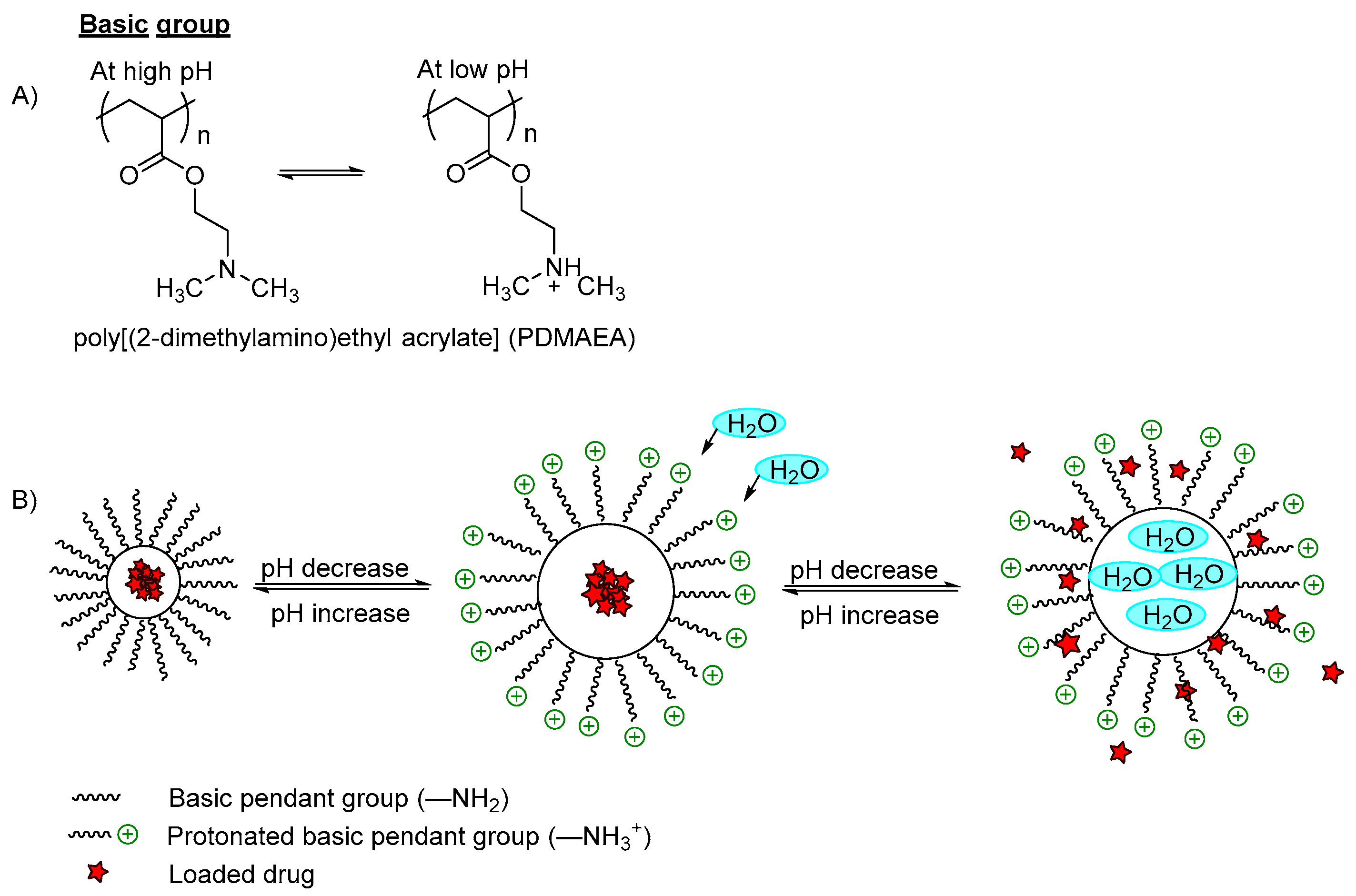
| (A) Ionizable Moieties | Basic pendant groups, such as amine and its derivatives | 2-aminoethyl methacrylate | 6.5 and 6.8 | Polyplex Nanoparticles (NPs) | Improve cellular uptake and transfection efficiency | Gene Delivery | [7] |
| 2-methoxy-4-aminomethyl-1,3-dioxolan | 5.5 | Poly(vinyl alcohol) PVA | Rapid drug release | Drug carrier for tumor therapy | [24] | ||
| Histidine | 5.0 | Poly(ethylene glycol)-polycaprolactone PEG-PCL | Increase drug release | Chemotherapy | [25] | ||
| 2-dimethylamino ethyl methacrylate | 4.0 and 7.0 | Poly(lactic acid) PLA | Swells and speeds drug release | Targeted drug delivery vehicles | [26] | ||
| 2-dimethylamino ethyl methacrylate | 5.0 | PCL | Swells and enhances drug release | Drug delivery | [27] | ||
| Chitosan | 5.5 | Poly(lactic-co-glycolic acid) PLGA | Efficient release | Tacrolimus delivery | [28] | ||
| Chitosan | 5.5 | Poly(N-isopropylacrylamide)-co-itaconic acid NIPAM | Fast release | Local breast cancer therapy | [29] | ||
| 2-(diisopropylamino) ethyl methacrylate (DPA) | 6.5 | Hydroxyethyl methacrylate-co-DPA copolymers | Increased drug release | Ocular drug delivery | [30] | ||
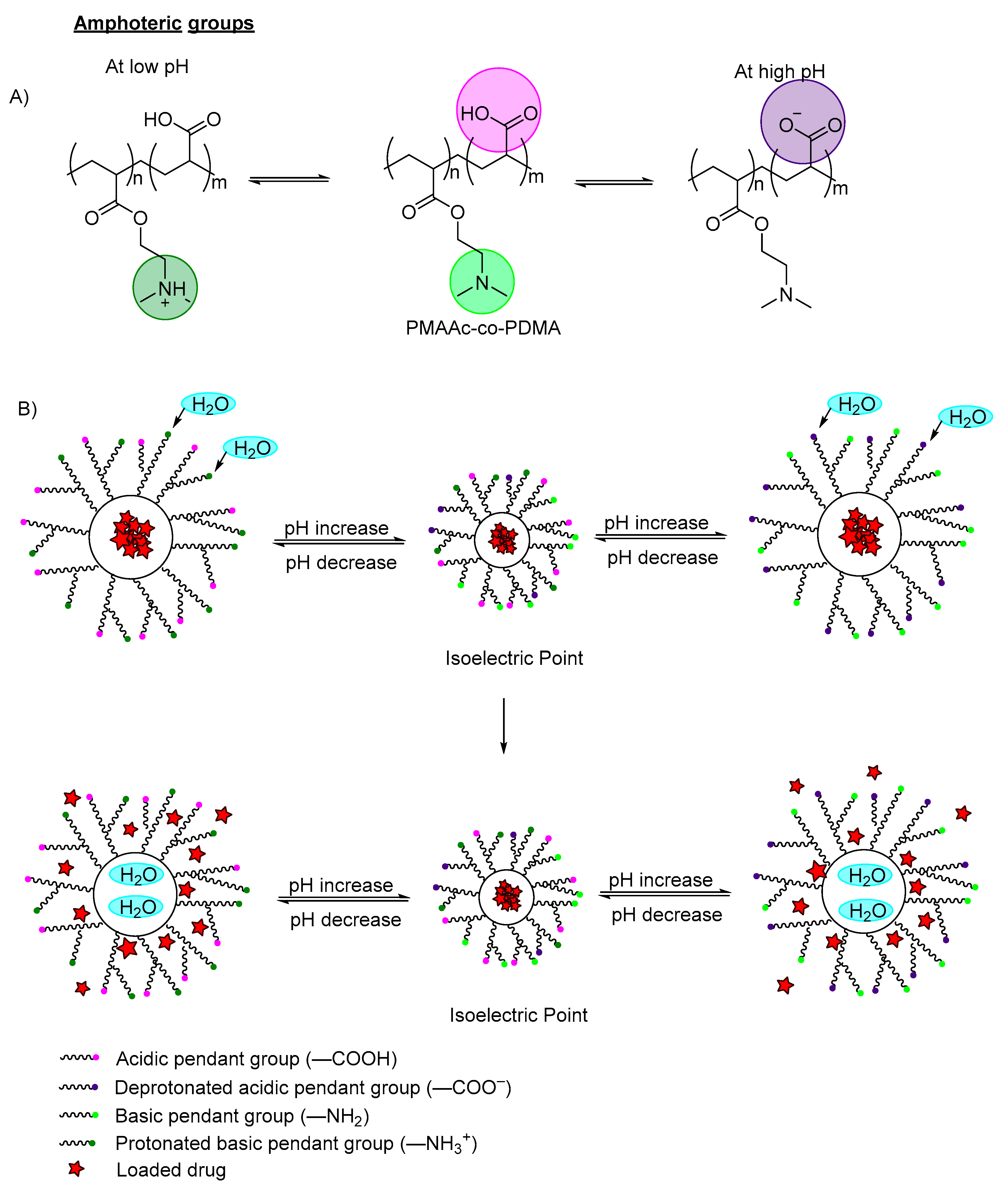
| Carboxylic acid pendant groups | Methacrylic acid (MAA) | 7.4 | Amine-modified bimodal mesopores silica | Swells and speeds drug release | Drug delivery carrier | [31] | |
| Acrylic acid | 7.4 | Poly(ethylene glycol) PEG | Increased drug release | Targeted drug delivery | [32] | ||
| Mechanism | Acid-Labile Groups | Responsive pH | Polymer Type | Type of Response | Application | Ref | |
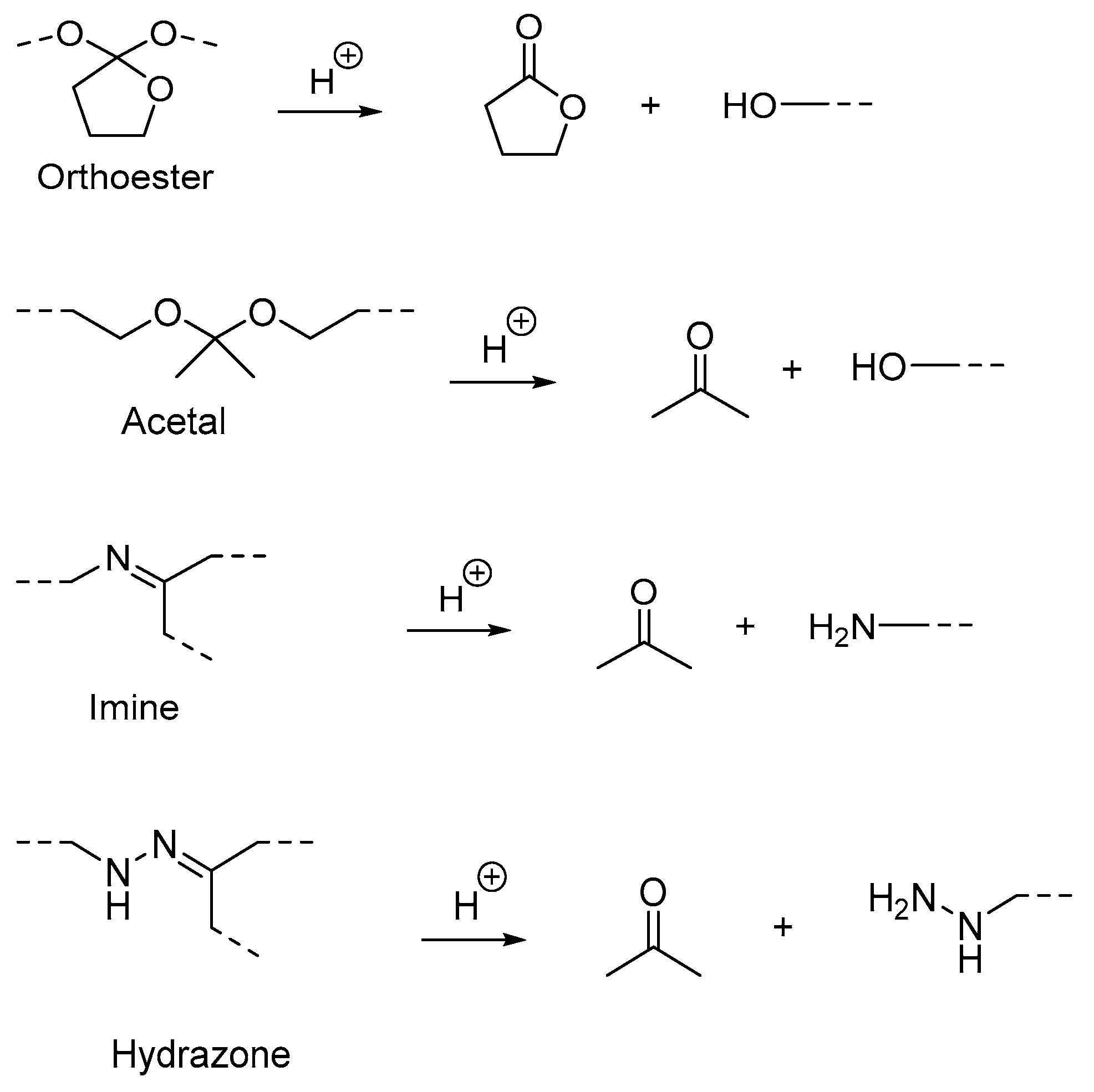
| (B) Acid-labile linkages | β-Thiopropionate | 5.0 | PEG | Increased drug release | Targeted cancer cell treatment | [33] | |
| β-Thiopropionate | 5.0 | Poly(beta-thioether ester)-PEG | Rapid drug release | Nanocarrier for drug delivery | [34] | ||
| Hydrazone linkage | 5.5 | Lipid Polymer Hybrid NPs | Fast drug release | Biomedical and chemotherapy | [17,35] | ||
| Hydrazone linkage | 5.4 | N-isopropylacrylamide-co-glycidyl methacrylate (NIPAM-co-GMA) | Increased drug release | Chemotherapy drug delivery | [36] | ||
| Hydrazone linkage | 5.6 | Poly(β-benzyl malate) | Rapid drug release | Antitumor drug carrier | [37] | ||
| Oxazoline | 6.0 | Poly(lactic acid)-poly(β-amino ester) | Increased drug release | Colon cancer adjuvant therapy | [38] | ||
| Boron-ester linkage | 6.8 | Polymer dots | Better fluorescent intensity | Bioimaging probe | [39] | ||
| Borate-ester linkage | 5.5 | PNIPAAm Poly(N-isopropylacrylamide) | Rapid drug release | Cancer therapy | [40] | ||
| Others | Metal ligand (Fe3+) | 5.0 | PEG-PLGA | Rapid drug release | MRI-guided therapy | [41] | |
2. Stimulus-Responsive PU
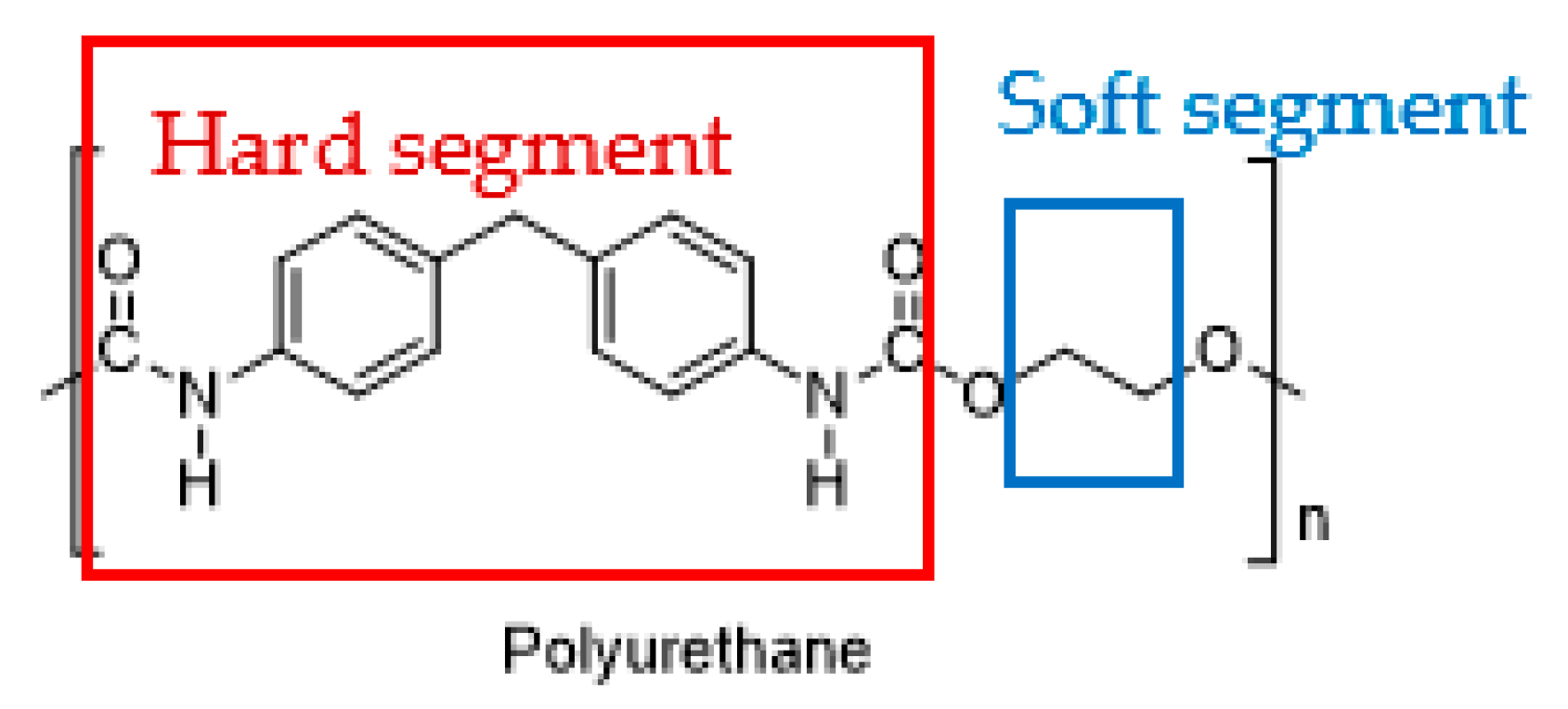
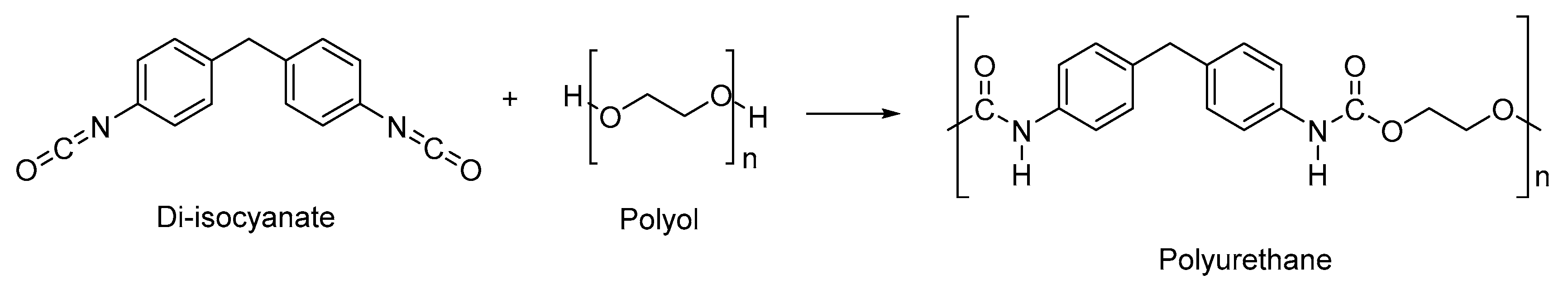
2.1. Polyurethane
2.2. Types of Stimuli/Stimulus-Responsive Polyurethanes Used in Biomedical and Drug Delivery Applications
| Type of Stimuli/Stimulus-Responsive PU | Type of Stimuli/Stimulus | * PU Type | PU Name | Reactants for Synthesis of PU | Biocompatibility Evaluation | Biodegradability Evaluation | Applications | Ref |
|---|---|---|---|---|---|---|---|---|
| Single | Thermo | A + C | Poly(ether urethane) (PEU) |
| Ex vivo in rodent model for hydrogel injectability and gelation | ** N/A | Controlled and triggered release drug | [64] |
| Redox | A + C | PU Nanoparticles |
|
| Degrade and reach 50% weight loss (polymers with increase of disulfide bonds) in 10 mM glutathione (GSH) after 14 days | Chemotherapy drug delivery | [56] | |
| Redox | A + C | PU micelles with mPEG block and PLA block with disulfide bonds mPEG-PUSS-mPEG |
|
| Decompose within 24 h in the presence of 10 mM dithiothreitol (DTT) | Anticancer drug delivery | [4] | |
| Redox | A + C | PU with disulfide bonds, pendant carboxyl groups, and primary amine group PU-SS-COOH-NH2 micelles |
|
| ** N/A | Drug delivery | [59] | |
| Light | B + C | Serinol-based PU nanoparticles |
| ** N/A | Nanoparticle count rate decrease ~>30% after 15 min of UV irradiation | Nanocarrier for controlled drug release | [65] | |
| Shape memory | N/A | Shape memory polyurethane (SMPU) | Commercial SMPU, MM3520 | ** N/A | ** N/A | Endovascular embolization | [66] | |
| Dual | Shape memory + water | N/A | Thermoplastic PU/hydroxyethyl cotton cellulose nanofibers (TPU/CNF-C/CNTs) | Commercial TPU, BT-70ARYU | ** N/A | ** N/A | Sensors, actuators | [67] |
| Thermo + light | A + C | PUA Nanoparticles |
|
| Weight loss of approximately >~10% in 28 days | 3D cell-laden bioprinting | [68] | |
| Thermo + shape memory | A + C | PCL-based PU (PCLAU/Fe3O4) |
|
| Weight loss of 67% after 13 weeks | Vascular stents | [69] | |
| Thermo + enzyme | B + C | Poly(ester urethane) Nanoparticles |
|
| ** N/A | Chemotherapy drug delivery | [60] | |
| Multi | Thermo + shape + water | A + C | PU/nanoporous cellulose gel (PU/NCG) |
| ** N/A | ** N/A | Biomaterials, sensors | [70] |
3. pH-Responsive Polyurethane
| Type of pH-Responsive PU | PU System | Reactants Used in Synthesis of PU | Ionizable Group/Linkage | Additional Stimulus Response | pH Responsiveness | * Applications | Reference | Reference Materials | Improvement to the Reference Materials |
|---|---|---|---|---|---|---|---|---|---|
| Single | PU micelles |
| Hydrazone linkage | N/A | pH ranging from 4.0–6.0, cleavage of hydrazone bond and degraded | A | [61,63] | N/A | N/A |
| PU micelles |
| Hydrazone linkage | N/A | At pH 4.4, particle size increase due to swelling, drug release to 98% | A | [71] | Same PU without hydrazone bond | N/A | |
| PU/DEA copolymer |
| 2-(diethylamino) ethyl methacrylate | N/A | At pH 4.0, dynamic swelling and drug release | A | [11,72] | Same PU without DEA monomers | N/A | |
| PU micelles |
| Diethanolamine | N/A | At pH 5.5, highest drug release | A | [73] | N/A | N/A | |
| PU copolymer |
| HEP | N/A | At pH 4.5, swelled twofold and close to zero drug release; however, sodium diclofenac incorporated release at elevation to pH 7.0 | A | [3,74] | PEG-HD-MDI-HD without HEP monomers | Reversible and sharp switch between “on” and “off” drug release, serves as window membrane in reservoir-type intravaginal rings | |
| PU copolymer NPs |
|
| N/A | PU containing higher HEP ratio, swelled and highest drug release at pH 5.0 | A | [75] | N/A | N/A | |
| PU copolymer hydrogel |
| DMPA | N/A | Drug release at pH 7.0 | A | [76] | N/A | N/A | |
| PU hydrogels- |
| Poly(azomethine-urethane) (PAMU) | N/A | Highest swelling degree at pH 3.0; increase of PAMU, swelling degree increase, release of drug increase | A | [77] | N/A | N/A | |
| PU nanomicelles |
| 2-[N,N-bis (2-hydroxy-ethyl)] aminoethanesulfonic acid sodium salt (BES-Na) | N/A | Drug release rate: pH 5.0 > pH 6.8 > pH 7.4 | A | [12] | N/A | N/A | |
| PU-sodium alginate (SA) blend |
| Sodium Alginate | N/A | Swelled at pH 7.4, sustained and prolonged release of incorporated protein or insulin | A | [78,79] | N/A | BHET derived from PET waste, biocompatible | |
| Cellulose crosslinked PU |
|
| N/A | All 3 PUs swelled and highest release of incorporated drugs at pH 7.4 | A | [80] | N/A | Control release rate by changing chain extender; Drug release rate LAPU > DAPU > GAPU | |
| PEG-HTPB (g-COOH)-PEG triblock copolymer |
| Mercaptoacetic acid | N/A | At pH 7.4, micelles swelled rapidly and released drug | A | [81] | N/A | N/A | |
| PU/cellulose acetate phthalate (CAP) fibers | Commercial PU | CAP | N/A | Rapid release of Rhodamine B at pH 7.4 within 1 min | A | [82] | - Pure CAP - Pure PU fibers | Improved tensile strength compared to previously reported CAP fibers | |
| PU films |
|
| N/A | PU-Arginine shows highest drug release at pH 4.4; All PU shows average drug release of 64% at pH 10.4 | A | [83] | N/A | N/A | |
| PEG-PU copolymers |
|
| N/A | Highest pH buffering capacity 7.02, specific responsiveness not mentioned | B | [84] | N/A | N/A | |
| Dual | PU |
|
| Thermo-responsive | PU-MDEA: Swells at pH 4.0–5.5 PU-DMPA: Swells at pH 8.5–10.0 | N/A | [85] | N/A | N/A |
| PU Micelles |
|
| Thermo-responsive | HDI-MDEA and HDI-BDEA, rapid drug release at pH 4.0 | A | [86] | N/A | N/A | |
| PU/DPA |
| DPA | Thermo-responsive | Increase of DPA, results in highest swelling degree at pH 4.0 | A | [87] | Same PU without addition of DPA/PPGDA/PEGMA mixture | N/A | |
| PEG-PCL based PU blend with cellulose nanocrystals (CNC) |
|
| Shape memory | CNC-COOH; At pH 4.0, folded strip of CNC-C6H4NO2 recovers to straight | C | [88] | N/A | N/A | |
| PU |
| Pyridine | Shape memory | Swells at pH 1.3, drug release and shape recovers | A, C | [10] | N/A | N/A | |
| Azo-cationic waterborne polyurethane (CWPU) |
|
| Photo-responsive | Shows different color in different pH medium | B | [89] | N/A | N/A | |
| PU micelles with disulfide linkage |
| MDEA | Reduction-responsive | Rapid drug release at pH 5.5 | A | [90] | N/A | N/A | |
| PU with disulfide bonds |
| Poly(2-ethyl-2-oxazoline)(PEOz) | Reduction-responsive | Drug release rate higher at pH 5.0 | A | [91] | - End-group-carboxylated PEOz-PLA - PEOz-hydrazone-DOX | Cumulative drug release increase with presence of 1, 4-dithio-D, L-threitol (DTT) | |
| MPEG/PU triblock copolymers with disulfide linkage |
| Bis-1,4-(hydroxy-ethyl) piperazine (HEP) | Reduction-responsive | pH 5.5 and 6.8, swells and faster drug release | A | [92,93] | N/A | N/A | |
| Multi | PU |
| DMPA | Thermo-responsive and shape memory | For PEG-30%-MDI-DMPA, fixes deformed shape at pH 2.0, recovers shape at pH 9.0 | N/A | [94] | N/A | N/A |
3.1. Applications of pH-Responsive Polyurethanes in Drug Delivery Systems
3.1.1. Oral Administration
3.1.2. Intravaginal Administration
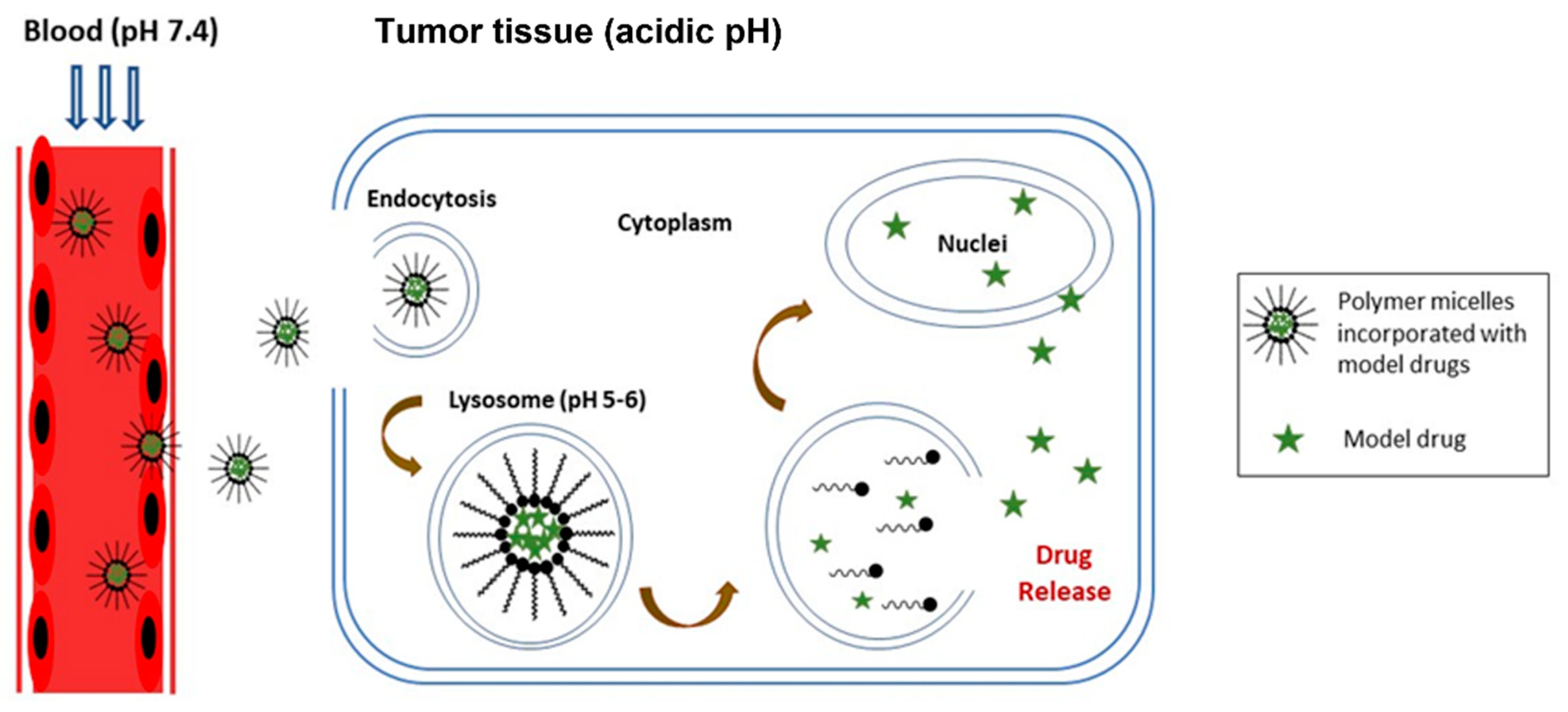
3.1.3. Intravenous Administration for Chemotherapeutic Drugs
3.1.4. Controlled Drug Delivery
3.2. Applications of pH-Responsive Polyurethanes as Biomaterials
3.3. Applications of pH-Responsive Polyurethanes in Optical Imaging
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Fernández, L.; Mora-Boza, A.; Reyes-Ortega, F. pH-Responsive Polymers: Properties, Synthesis, and Applications. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–86. [Google Scholar]
- Polo Fonseca, L.; Trinca, R.B.; Felisberti, M.I. Amphiphilic polyurethane hydrogels as smart carriers for acidic hydrophobic drugs. Int. J. Pharm. 2018, 546, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, Y.; Ho, E.A.; Liu, S. Reversibly pH-responsive polyurethane membranes for on-demand intravaginal drug delivery. Acta Biomater. 2017, 47, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Tan, X.; Xu, Z.; Zhu, G.; Teng, W.; Zhao, Q.; Liang, Z.; Wu, Z.; Xiong, D. Smart drug carrier based on polyurethane material for enhanced and controlled DOX release triggered by redox stimulus. React. Funct. Polym. 2020, 148, 104507. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, F.; Zhang, Z.; Cheng, Y.; Wang, Z.; Li, Y. Stimuli-responsive polydopamine-based smart materials. Chem. Soc. Rev. 2021, 50, 8319–8343. [Google Scholar] [CrossRef]
- Ooi, Y.J.; Wen, Y.; Zhu, J.; Song, X.; Li, J. Surface Charge Switchable Polymer/DNA Nanoparticles Responsive to Tumor Extracellular pH for Tumor-Triggered Enhanced Gene Delivery. Biomacromolecules 2020, 21, 1136–1148. [Google Scholar] [CrossRef]
- Chen, K.; Gou, W.; Wang, X.; Zeng, C.; Ge, F.; Dong, Z.; Wang, C. UV-Cured Fluoride-Free Polyurethane Functionalized Textile with pH-Induced Switchable Superhydrophobicity and Underwater Superoleophobicity for Controllable Oil/Water Separation. ACS Sustain. Chem. Eng. 2018, 6, 16616–16628. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Li, L.; Zhu, F.; Ren, X.; Huang, Q.; Cheng, Y.; Li, Y. Bioinspired Integration of Naturally Occurring Molecules towards Universal and Smart Antibacterial Coatings. Adv. Funct. Mater. 2022, 32, 2108749. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Liu, Y.; Gong, T.; Wang, L.; Zhou, S. Highly pH-sensitive polyurethane exhibiting shape memory and drug release. Polym. Chem. 2014, 5, 5168–5174. [Google Scholar] [CrossRef]
- Pardini, F.; Faccia, P.; Amalvy, J. Evaluation of pH-sensitive polyurethane/2-diethylaminoethyl methacrylate hybrids potentially useful for drug delivery developments. J. Drug Deliv. Sci. Technol. 2015, 30, 199–208. [Google Scholar] [CrossRef]
- Song, Y.; Chai, Y.; Xu, K.; Zhang, P. Functional polyurethane nanomicelle with pH-responsive drug delivery property. E-Polymers 2018, 18, 409–417. [Google Scholar] [CrossRef]
- Sgreccia, E.; Pasquini, L.; Ercolani, G.; Knauth, P.; Di Vona, M.L. Stimuli-responsive amphoteric ion exchange polymers bearing carboxylic and amine groups grafted to a cross-linkable silica network. Eur. Polym. J. 2019, 112, 255–262. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, H.; Wu, J.; Sun, J.; Wang, Y. Novel amphoteric pH-sensitive hydrogels derived from ethylenediaminetetraacetic dianhydride, butanediamine and amino-terminated poly(ethylene glycol): Design, synthesis and swelling behavior. Eur. Polym. J. 2011, 47, 40–47. [Google Scholar] [CrossRef]
- Abou Taleb, M.F. Radiation synthesis of multifunctional polymeric hydrogels for oral delivery of insulin. Int. J. Biol. Macromol. 2013, 62, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Binauld, S.; Stenzel, M.H. Acid-degradable polymers for drug delivery: A decade of innovation. Chem. Commun. 2013, 49, 2082–2102. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xing, H.; Sun, Y.; Feng, S.; Wang, S. Non-small cell lung cancer combination therapy: Hyaluronic acid modified, epidermal growth factor receptor targeted, pH sensitive lipid-polymer hybrid nanoparticles for the delivery of erlotinib plus bevacizumab. Biomed. Pharmacother. 2020, 125, 109861. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Sihao, Z.; Sun, H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017, 37, 1253–1260. [Google Scholar] [CrossRef]
- Kumar, A.; Montemagno, C.; Choi, H.J. Smart Microparticles with a pH-responsive Macropore for Targeted Oral Drug Delivery. Sci. Rep. 2017, 7, 3059. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, J.; Zhang, C.; Cao, Z.; Cheng, D.; Liu, J.; Shuai, X. Stimuli-Responsive Polymeric Nanocarriers for Efficient Gene Delivery. Top. Curr. Chem. 2017, 375, 167–215. [Google Scholar] [CrossRef]
- Yang, W.; Bai, T.; Carr, L.R.; Keefe, A.J.; Xu, J.; Xue, H.; Irvin, C.A.; Chen, S.; Wang, J.; Jiang, S. The effect of lightly crosslinked poly(carboxybetaine) hydrogel coating on the performance of sensors in whole blood. Biomaterials 2012, 33, 7945–7951. [Google Scholar] [CrossRef]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit®: A versatile family of polymers for hot melt extrusion and 3D printing processes in pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Akhil, G.; Anuj, M.; Kumar, G.A. Colon targeted drug delivery systems—A review. Asian J. Pharm. Res. 2011, 1, 25–33. [Google Scholar]
- Li, D.; Qin, J.; Sun, M.; Yan, G.; Tang, R. pH-sensitive, dynamic graft polymer micelles via simple synthesis for enhanced chemotherapeutic efficacy. J. Biomater. Appl. 2020, 34, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.; Liu, S.; Yang, R.; Pu, Y.; Zhang, W.; Wang, X.; Liu, X.; Ren, Y.; Chi, B. pH-responsive nanomicelles of poly(ethylene glycol)-poly(ε-caprolactone)-poly(L-histidine) for targeted drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 277–292. [Google Scholar] [CrossRef]
- Ifra; Saha, S. Fabrication of topologically anisotropic microparticles and their surface modification with pH responsive polymer brush. Mater. Sci. Eng. C 2019, 104, 109894. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran- graft-poly[(2-dimethylamino)ethyl methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Mansouri, A.; Abnous, K.; Alibolandi, M.; Taghdisi, S.M.; Ramezani, M. Targeted delivery of tacrolimus to T cells by pH-responsive aptamer-chitosan-poly(lactic-co-glycolic acid) nanocomplex. J. Cell. Physiol. 2019, 234, 18262–18271. [Google Scholar] [CrossRef]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef]
- Faccia, P.A.; Pardini, F.M.; Amalvy, J.I. Uptake and release of Dexamethasone using pH-responsive poly(2-hydroxyethyl methacrylate-co-2-(diisopropylamino)ethyl methacrylate) hydrogels for potential use in ocular drug delivery. J. Drug Deliv. Sci. Technol. 2019, 51, 45–54. [Google Scholar] [CrossRef]
- Ma, J.Y.; Han, J.; Sun, J.H.; Fan, L.; Bai, S.Y.; Jiao, Y.W. pH-sensitive controlled release in vitro and pharmacokinetics of ibuprofen from hybrid nanocomposite using amine-modified bimodal mesopores silica as core and poly(methylacrylic acid) as shell. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1023–1033. [Google Scholar] [CrossRef]
- Yue, Z.; Che, Y.J.; Jin, Z.; Wang, S.; Ma, Q.; Zhang, Q.; Tan, Y.; Meng, F. A facile method to fabricate thermo- and pH-sensitive hydrogels with good mechanical performance based on poly(ethylene glycol) methyl ether methacrylate and acrylic acid as a potential drug carriers. J. Biomater. Sci. Polym. Ed. 2019, 30, 1375–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, T.; Yu, Q.; Yang, Z.; Sun, Y.; Cai, Z.; Cang, H. pH-Sensitive betulinic acid polymer prodrug nanoparticles for efficient and targeted cancer cells treatment. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 659–668. [Google Scholar] [CrossRef]
- Wu, W.X.; Yang, X.L.; Liu, B.Y.; Deng, Q.F.; Xun, M.M.; Wang, N.; Yu, X.Q. Lipase-catalyzed synthesis of oxidation-responsive poly(ethylene glycol)-b-poly(β-thioether ester) amphiphilic block copolymers. RSC Adv. 2016, 6, 11870–11879. [Google Scholar] [CrossRef]
- Wang, J. Combination treatment of cervical cancer using folate-decorated, pH-sensitive, carboplatin and paclitaxel co-loaded lipid-polymer hybrid nanoparticles. Drug Des. Devel. Ther. 2020, 14, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C 2020, 108, 110418. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, C.; Liu, B.; Peng, Y.; Meng, H.; Yang, T.; Zhou, Q.; Guo, S.; Wu, H. Erratum: Enhanced endocytic and pH-sensitive poly(malic acid) micelles for antitumor drug delivery. J. Biomed. Nanotechnol. 2019, 15, 28–41. [Google Scholar] [CrossRef]
- Hao, D.L.; Xie, R.; De, G.J.; Yi, H.; Zang, C.; Yang, M.Y.; Liu, L.; Ma, H.; Cai, W.Y.; Zhao, Q.H.; et al. PH-responsive artesunate polymer prodrugs with enhanced ablation effect on rodent xenograft colon cancer. Int. J. Nanomed. 2020, 15, 1771. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Ryplida, B.; Phuong, P.T.M.; Won, H.J.; Lee, G.; Bhang, S.H.; Park, S.Y. Reduction-triggered paclitaxel release nano-hybrid system based on core-crosslinked polymer dots with a pH-responsive shell-cleavable colorimetric biosensor. Int. J. Mol. Sci. 2019, 20, 5368. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Wu, S.; Hegazy, M.; Li, H.; Xu, X.; Lu, H.; Huang, X. Engineered borate ester conjugated protein-polymer nanoconjugates for pH-responsive drug delivery. Mater. Sci. Eng. C 2019, 104, 109914. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Yang, C.; Gong, S.; Jiang, H.; Sun, M.; Qian, C. A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 23, 102071. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; ur Rahman, M.S.; Iqbal, S.; Arshad, M.I.; Tahir, M.A.; Mahmooda, T. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Technol. 2017, 39, 277–282. [Google Scholar] [CrossRef]
- Aksoy, E.A.; Taskor, G.; Gultekinoglu, M.; Kara, F.; Ulubayram, K. Synthesis of biodegradable polyurethanes chain-extended with (2S)-bis(2-hydroxypropyl) 2-aminopentane dioate. J. Appl. Polym. Sci. 2018, 135, 45764. [Google Scholar] [CrossRef]
- Yeoh, F.H.; Lee, C.S.; Kang, Y.B.; Wong, S.F.; Cheng, S.F.; Ng, W.S. Production of biodegradable palm oil-based polyurethane as potential biomaterial for biomedical applications. Polymers 2020, 12, 1842. [Google Scholar] [CrossRef]
- Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. In Elastomers; Çankaya, N., Ed.; IntechOpen: London, UK, 2017; pp. 137–157. [Google Scholar] [CrossRef] [Green Version]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jéroîme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.M.; et al. Non-Isocyanate Polyurethanes from Carbonated Soybean Oil Using Monomeric or Oligomeric Diamines to Achieve Thermosets or Thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Díaz, L.E.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Vilariño-Feltrer, G.; Serrano, M.A.; Valero, M.F. Candidate polyurethanes based on castor oil (ricinus communis), with polycaprolactone diol and chitosan additions, for use in biomedical applications. Molecules 2019, 24, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar] [CrossRef]
- Zhang, C.; Madbouly, S.A.; Kessler, M.R. Biobased polyurethanes prepared from different vegetable oils. ACS Appl. Mater. Interfaces 2015, 7, 1226–1233. [Google Scholar] [CrossRef]
- Barksby, N.; Dormish, J.F.; Haider, K.W. Encyclopedia of Polymeric Nanomaterials; Springer: Berlin, Germany, 2020; pp. 1–14. [Google Scholar] [CrossRef]
- Szycher, M. Structure–Property Relations in Polyurethanes. In Szycher’s Handbook of Polyurethanes; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9781439839584. [Google Scholar]
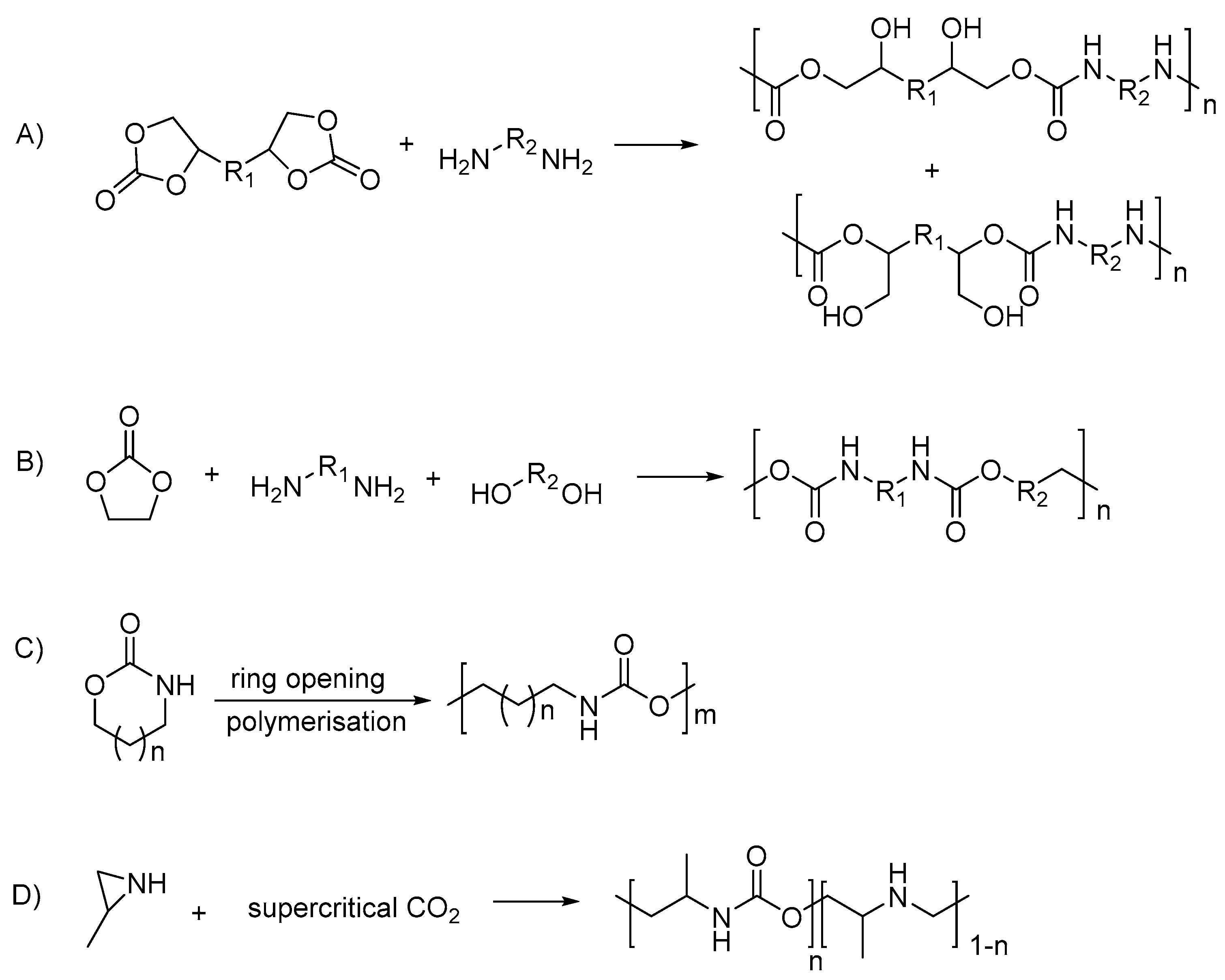
- Keul, H.; Mommer, S.; Möller, M. Poly(amide urethane)s with functional/reactive side groups based on a bis-cyclic bio-based monomer/coupling agent. Eur. Polym. J. 2013, 49, 853–864. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Neffgen, S.; Keul, H.; Höcker, H. Cationic Ring-Opening Polymerization of Trimethylene Urethane: A Mechanistic Study. Macromolecules 1997, 30, 1289–1297. [Google Scholar] [CrossRef]
- Ihata, O.; Kayaki, Y.; Ikariya, T. Aliphatic Poly(urethane−amine)s Synthesized by Copolymerization of Aziridines and Supercritical Carbon Dioxide. Macromolecules 2005, 38, 6429–6434. [Google Scholar] [CrossRef]
- Iyer, R.; Nguyen, T.; Padanilam, D.; Xu, C.; Saha, D.; Nguyen, K.T.; Hong, Y. Glutathione-responsive biodegradable polyurethane nanoparticles for lung cancer treatment. J. Control. Release 2020, 321, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, E.; Rios-Ramrez, A. Biodegradation of Medical Purpose Polymeric Materials and Their Impact on Biocompatibility. In Biodegradation—Life of Science; Chamy, R., Ed.; IntechOpen: London, UK, 2013; pp. 3–30. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, T.; Karak, N. Silicone-Containing Biodegradable Smart Elastomeric Thermoplastic Hyperbranched Polyurethane. ACS Omega 2018, 3, 6849–6859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, C.; Qiao, Z.; Yao, Y.; Luo, J. Reduction responsive and surface charge switchable polyurethane micelles with acid cleavable crosslinks for intracellular drug delivery. RSC Adv. 2018, 8, 17888–17897. [Google Scholar] [CrossRef] [Green Version]
- Aluri, R.; Saxena, S.; Joshi, D.C.; Jayakannan, M. Multistimuli-Responsive Amphiphilic Poly(ester-urethane) Nanoassemblies Based on l-Tyrosine for Intracellular Drug Delivery to Cancer Cells. Biomacromolecules 2018, 19, 2166–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q. Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules 2011, 44, 857–864. [Google Scholar] [CrossRef]
- Bera, A.; Singh Chandel, A.K.; Uday Kumar, C.; Jewrajka, S.K. Degradable/cytocompatible and pH responsive amphiphilic conetwork gels based on agarose-graft copolymers and polycaprolactone. J. Mater. Chem. B 2015, 3, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liang, D.; He, X.; Li, J.; Tan, H.; Li, J.; Fu, Q.; Gu, Q. The degradation and biocompatibility of pH-sensitive biodegradable polyurethanes for intracellular multifunctional antitumor drug delivery. Biomaterials 2012, 33, 2734–2745. [Google Scholar] [CrossRef]
- Boffito, M.; Torchio, A.; Tonda-Turo, C.; Laurano, R.; Gisbert-Garzarán, M.; Berkmann, J.C.; Cassino, C.; Manzano, M.; Duda, G.N.; Vallet-Regí, M.; et al. Hybrid Injectable Sol-Gel Systems Based on Thermo-Sensitive Polyurethane Hydrogels Carrying pH-Sensitive Mesoporous Silica Nanoparticles for the Controlled and Triggered Release of Therapeutic Agents. Front. Bioeng. Biotechnol. 2020, 8, 384. [Google Scholar] [CrossRef]
- Sun, J.; Rust, T.; Kuckling, D. Light-Responsive Serinol-Based Polyurethane Nanocarrier for Controlled Drug Release. Macromol. Rapid Commun. 2019, 40, 1900348. [Google Scholar] [CrossRef]
- Kashyap, D.; Kishore Kumar, P.; Kanagaraj, S. 4D printed porous radiopaque shape memory polyurethane for endovascular embolization. Addit. Manuf. 2018, 24, 687–695. [Google Scholar] [CrossRef]
- Wu, G.; Gu, Y.; Hou, X.; Li, R.; Ke, H.; Xiao, X. Hybrid nanocomposites of cellulose/carbon-nanotubes/polyurethane with rapidly water sensitive shape memory effect and strain sensing performance. Polymers 2019, 11, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, S.H.; Hsu, S.H. Synthesis and Characterization of Dual Stimuli-Sensitive Biodegradable Polyurethane Soft Hydrogels for 3D Cell-Laden Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Y.; Chang, K.; Jin, S.P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. J. Appl. Polym. Sci. 2018, 135, 45686. [Google Scholar] [CrossRef]
- Li, K.; Wei, P.; Huang, J.; Xu, D.; Zhong, Y.; Hu, L.; Zhang, L.; Cai, J. Mechanically Strong Shape-Memory and Solvent-Resistant Double-Network Polyurethane/Nanoporous Cellulose Gel Nanocomposites. ACS Sustain. Chem. Eng. 2019, 7, 15974–15982. [Google Scholar] [CrossRef]
- Song, N.; Zhou, L.; Li, J.; Pan, Z.; He, X.; Tan, H.; Wan, X.; Li, J.; Ran, R.; Fu, Q. Inspired by nonenveloped viruses escaping from endo-lysosomes: A pH-sensitive polyurethane micelle for effective intracellular trafficking. Nanoscale 2016, 8, 7711–7722. [Google Scholar] [CrossRef]
- Pardini, F.M.; Amalvy, J.I. Synthesis and swelling behavior of pH-responsive polyurethane/poly[2-(diethylamino)ethyl methacrylate] hybrid materials. J. Appl. Polym. Sci. 2014, 131, 39799. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, D.; Liu, C.; Guan, Y.; Zhang, J.; Su, Y.; Zhao, L.; Meng, F.; Luo, J. Biodegradable pH-sensitive polyurethane micelles with different polyethylene glycol (PEG) locations for anti-cancer drug carrier applications. RSC Adv. 2016, 6, 97684–97693. [Google Scholar] [CrossRef]
- Kim, S.; Traore, Y.L.; Ho, E.A.; Shafiq, M.; Kim, S.H.; Liu, S. Design and development of pH-responsive polyurethane membranes for intravaginal release of nanomedicines. Acta Biomater. 2018, 82, 12–23. [Google Scholar] [CrossRef]
- He, W.; Zheng, X.; Zhao, Q.; Duan, L.; Lv, Q.; Gao, G.H.; Yu, S. pH-Triggered Charge-Reversal Polyurethane Micelles for Controlled Release of Doxorubicin. Macromol. Biosci. 2016, 16, 925–935. [Google Scholar] [CrossRef]
- Kim, S.; Traore, Y.L.; Chen, Y.; Ho, E.A.; Liu, S. Switchable on-demand release of a nanocarrier from a segmented reservoir type intravaginal ring filled with a pH-responsive supramolecular polyurethane hydrogel. ACS Appl. Bio Mater. 2018, 1, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Duru Kamacı, U.; Kamacı, M. Preparation of polyvinyl alcohol, chitosan and polyurethane-based pH-sensitive and biodegradable hydrogels for controlled drug release applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 1167–1177. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukhopadhyay, P.; Kundu, P.P. Synthesis of a novel pH-sensitive polyurethane-alginate blend with poly(ethylene terephthalate) waste for the oral delivery of protein. J. Appl. Polym. Sci. 2014, 131, 40650. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukhopadhyay, P.; Pramanik, N.; Kundu, P.P. Effect of Polyethylene Glycol on Bis(2-hydroxyethyl) terephthalate-Based Polyurethane/Alginate pH-Sensitive Blend for Oral Protein Delivery. Adv. Polym. Technol. 2016, 35, 21525. [Google Scholar] [CrossRef]
- Solanki, A.; Thakore, S. Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers. Int. J. Biol. Macromol. 2015, 80, 683–691. [Google Scholar] [CrossRef]
- Nabid, M.R.; Omrani, I. Facile preparation of pH-responsive polyurethane nanocarrier for oral delivery. Mater. Sci. Eng. C 2016, 69, 532–537. [Google Scholar] [CrossRef]
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S.K.; De Smedt, S.C.; et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydr. Polym. 2016, 151, 1240–1244. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Bahadur, A.; Saeed, A.; ur Rahman, M.S.; Naseer, M.M. Biocompatible, pH-responsive, and biodegradable polyurethanes as smart anti-cancer drug delivery carriers. React. Funct. Polym. 2018, 127, 153–160. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, X.M.; Duan, L.J.; Zhang, M.Y.; Gao, G.H.; Zhang, H.X. Environmental pH-responsive fluorescent PEG-polyurethane for potential optical imaging. J. Appl. Polym. Sci. 2013, 129, 846–852. [Google Scholar] [CrossRef]
- Zhou, H.; Xun, R.; Liu, Q.; Fan, H.; Liu, Y. Preparation of thermal and pH dually sensitive polyurethane membranes and their properties. J. Macromol. Sci. Part B Phys. 2014, 53, 398–411. [Google Scholar] [CrossRef]
- Wang, A.; Gao, H.; Sun, Y.; Sun, Y.L.; Yang, Y.W.; Wu, G.; Wang, Y.; Fan, Y.; Ma, J. Temperature- and pH-responsive nanoparticles of biocompatible polyurethanes for doxorubicin delivery. Int. J. Pharm. 2013, 441, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Pardini, F.M.; Faccia, P.A.; Pardini, O.R.; Amalvy, J.I. Thermal and pH dual responsive polyurethane/2-(diisopropylamino)ethyl methacrylate hybrids: Synthesis, characterization, and swelling behavior. Int. J. Polym. Anal. Charact. 2018, 23, 207–225. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liu, D.; Wang, W.; Liu, Y.; Zhou, S. PH-Responsive Shape Memory Poly(ethylene glycol)-Poly(ε-caprolactone)-based Polyurethane/Cellulose Nanocrystals Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 12988–12999. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Gooch, J.; Sun, W.; Wang, H.; Wang, K. Photo- and pH-sensitive azo-containing cationic waterborne polyurethane. Polym. Bull. 2015, 72, 881–895. [Google Scholar] [CrossRef]
- Guan, Y.; Su, Y.; Zhao, L.; Meng, F.; Wang, Q.; Yao, Y.; Luo, J. Biodegradable polyurethane micelles with pH and reduction responsive properties for intracellular drug delivery. Mater. Sci. Eng. C 2017, 75, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhang, H.; Xu, K.; Du, B.; Zhu, C.; Li, Y. pH and reduction dual-responsive micelles based on novel polyurethanes with detachable poly(2-ethyl-2-oxazoline) shell for controlled release of doxorubicin. Drug Deliv. 2019, 26, 300–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; He, C.; Ding, J.; Cheng, Y.; Song, W.; Zhuang, X.; Chen, X. PH and reduction dual responsive polyurethane triblock copolymers for efficient intracellular drug delivery. Soft Matter 2013, 9, 2637–2645. [Google Scholar] [CrossRef]
- Yu, S.; He, C.; Lv, Q.; Sun, H.; Chen, X. PH and reduction dual responsive cross-linked polyurethane micelles as an intracellular drug delivery system. RSC Adv. 2014, 4, 63070–63078. [Google Scholar] [CrossRef]
- Song, Q.; Chen, H.; Zhou, S.; Zhao, K.; Wang, B.; Hu, P. Thermo- and pH-sensitive shape memory polyurethane containing carboxyl groups. Polym. Chem. 2016, 7, 1739–1746. [Google Scholar] [CrossRef]
- Santra, S.; Sk, M.A.; Mondal, A.; Molla, M.R. Self-Immolative Polyurethane-Based Nanoassemblies: Surface Charge Modulation at Tumor-Relevant pH and Redox-Responsive Guest Release. Langmuir 2020, 36, 8282–8289. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Zhai, Y.; Lian, H.; Luo, C.; Li, L.; Du, Y.; Zhang, D.; Ding, W.; Qiu, S.; et al. Enteric Polymer Based on pH-Responsive Aliphatic Polycarbonate Functionalized with Vitamin E to Facilitate Oral Delivery of Tacrolimus. Biomacromolecules 2015, 16, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Joshi, A.; Toor, A.; Verma, G. Drug delivery: Advancements and challenges. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 865–886. ISBN 9780323461436. [Google Scholar]
- Pearce, A.K.; Blok, A.J.; Yilmaz, G.; Singh, N.; Cavanagh, R.J.; Abelha, T.; Taresco, V.; Alexander, C. Functional polymers for drug delivery: Prospects and challenges. Chim. Oggi/Chem. Today 2018, 36, 38–42. [Google Scholar]
- Yang, W.-W.; Pierstorff, E. Reservoir-Based Polymer Drug Delivery Systems. J. Lab. Autom. 2012, 17, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Kumar, C.U.; Jewrajka, S.K. Effect of polyethylene glycol on properties and drug encapsulation-release performance of biodegradable/cytocompatible agarose-polyethylene glycol-polycaprolactone amphiphilic Co-network gels. ACS Appl. Mater. Interfaces 2016, 8, 3182–3192. [Google Scholar] [CrossRef]
- Huang, C.; Soenen, S.J.; van Gulck, E.; Vanham, G.; Rejman, J.; Van Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M.; et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 2012, 33, 962–969. [Google Scholar] [CrossRef]
- John, J.V.; Uthaman, S.; Augustine, R.; Chen, H.; Park, I.-K.; Kim, I. PH/redox dual stimuli-responsive sheddable nanodaisies for efficient intracellular tumour-triggered drug delivery. J. Mater. Chem. B 2017, 5, 5027–5036. [Google Scholar] [CrossRef]
- Long, Y.B.; Gu, W.X.; Pang, C.; Ma, J.; Gao, H. Construction of coumarin-based cross-linked micelles with pH responsive hydrazone bond and tumor targeting moiety. J. Mater. Chem. B 2016, 4, 1480–1488. [Google Scholar] [CrossRef]
- Liao, Z.S.; Huang, S.Y.; Huang, J.J.; Chen, J.K.; Lee, A.W.; Lai, J.Y.; Lee, D.J.; Cheng, C.C. Self-Assembled pH-Responsive Polymeric Micelles for Highly Efficient, Noncytotoxic Delivery of Doxorubicin Chemotherapy to Inhibit Macrophage Activation: In Vitro Investigation. Biomacromolecules 2018, 19, 2772–2781. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Heng, P.W.S. Controlled release drug delivery systems. Pharm. Dev. Technol. 2018, 23, 833. [Google Scholar] [CrossRef]
- Zhu, A.; Pan, Y.; Dai, S.; Li, F.; Shen, J. Preparation of N-Maleoylchitosan Nanocapsules for Loading and Sustained Release of Felodipine. Biomacromolecules 2009, 10, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
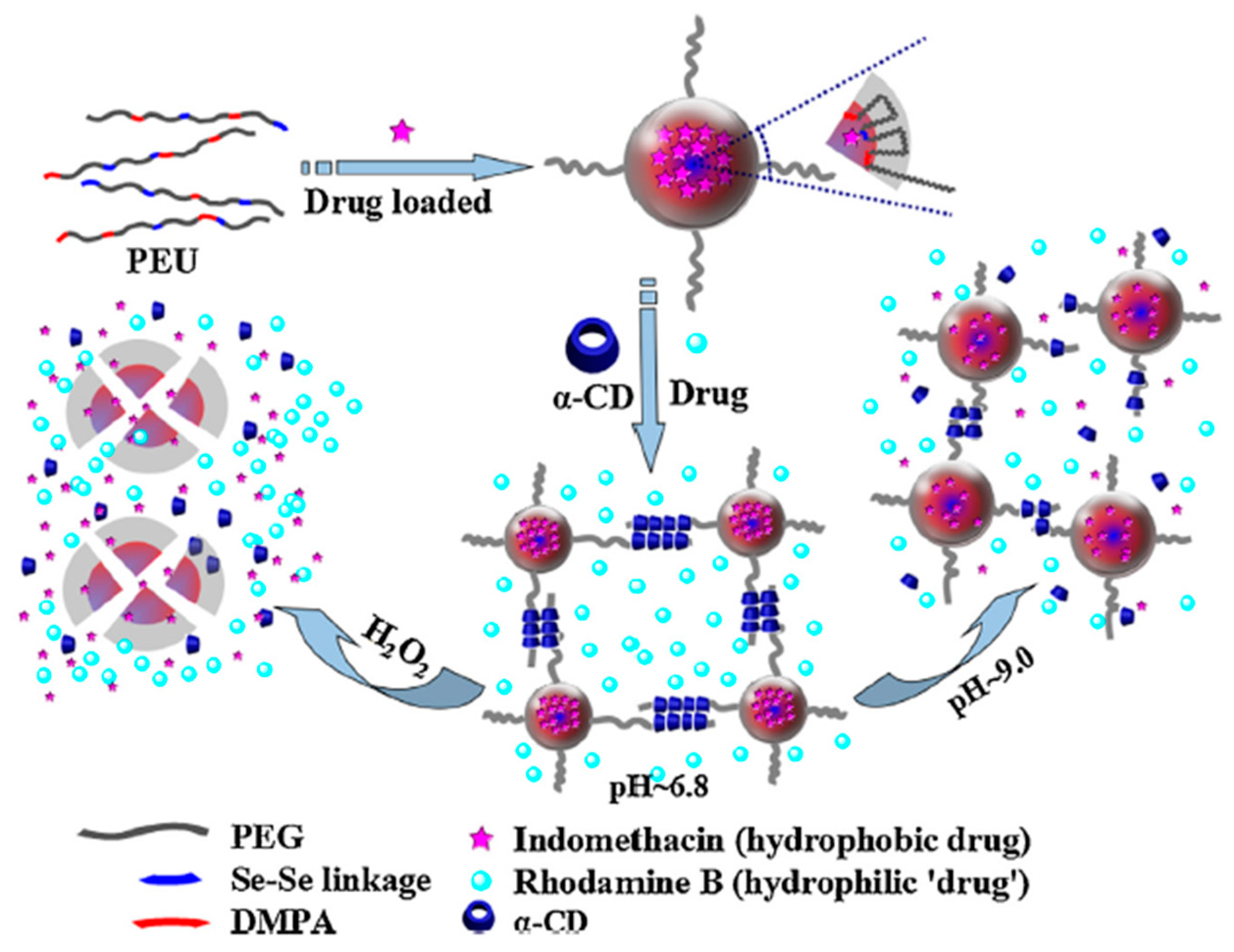
- Cheng, X.; Jin, Y.; Qi, R.; Fan, W.; Li, H.; Sun, X.; Lai, S. Dual pH and oxidation-responsive nanogels crosslinked by diselenide bonds for controlled drug delivery. Polymer 2016, 101, 370–378. [Google Scholar] [CrossRef]
- Cheng, X.; Jin, Y.; Sun, T.; Qi, R.; Li, H.; Fan, W. An injectable, dual pH and oxidation-responsive supramolecular hydrogel for controlled dual drug delivery. Colloids Surf. B Biointerfaces 2016, 141, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Su, Y.; Chen, B. Mechanically Adaptive and Shape-Memory Behaviour of Chitosan-Modified Cellulose Whisker/Elastomer Composites in Different pH Environments. ChemPhysChem 2014, 15, 2794–2800. [Google Scholar] [CrossRef] [PubMed]
- Jinhao, G.A.O.; Hongwei, G.U.; Bing, X.U. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Liao, B.; Wang, W.; Long, P.; He, B.; Li, F.; Liu, Q. Synthesis of fluorescent carbon nanoparticles grafted with polystyrene and their fluorescent fibers processed by electrospinning. RSC Adv. 2014, 4, 57683–57690. [Google Scholar] [CrossRef]
- Rutkaite, R.; Swanson, L.; Li, Y.; Armes, S.P. Fluorescence studies of pyrene-labelled, pH-responsive diblock copolymer micelles in aqueous solution. Polymer 2008, 49, 1800–1811. [Google Scholar] [CrossRef]
- Nagireddy, N.R.; Yallapu, M.M.; Kokkarachedu, V.; Sakey, R.; Kanikireddy, V.; Alias, J.P.; Konduru, M.R. Preparation and characterization of magnetic nanoparticles embedded in hydrogels for protein purification and metal extraction. J. Polym. Res. 2011, 18, 2285–2294. [Google Scholar] [CrossRef]
- Klingstedt, T.; Nilsson, K.P.R. Conjugated polymers for enhanced bioimaging. Biochim. Biophys. Acta-Gen. Subj. 2011, 1810, 286–296. [Google Scholar] [CrossRef]
- Gao, L.; Song, Q.; Huang, X.; Huang, J. A new surfactant-fluorescence probe for detecting shape transitions in self-assembled systems. J. Colloid Interface Sci. 2008, 323, 420–425. [Google Scholar] [CrossRef]
- Xi, M.; Jiang, Y. A pH-responsive self-fluorescent polymeric micelle as a potential optical imaging probe. Polym. Adv. Technol. 2018, 29, 2002–2009. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Duan, L.; Gao, G.H.; Zhang, X. A potential dual-modality optical imaging probe based on the pH-responsive micelle. J. Polym. Res. 2016, 23, 179. [Google Scholar] [CrossRef]



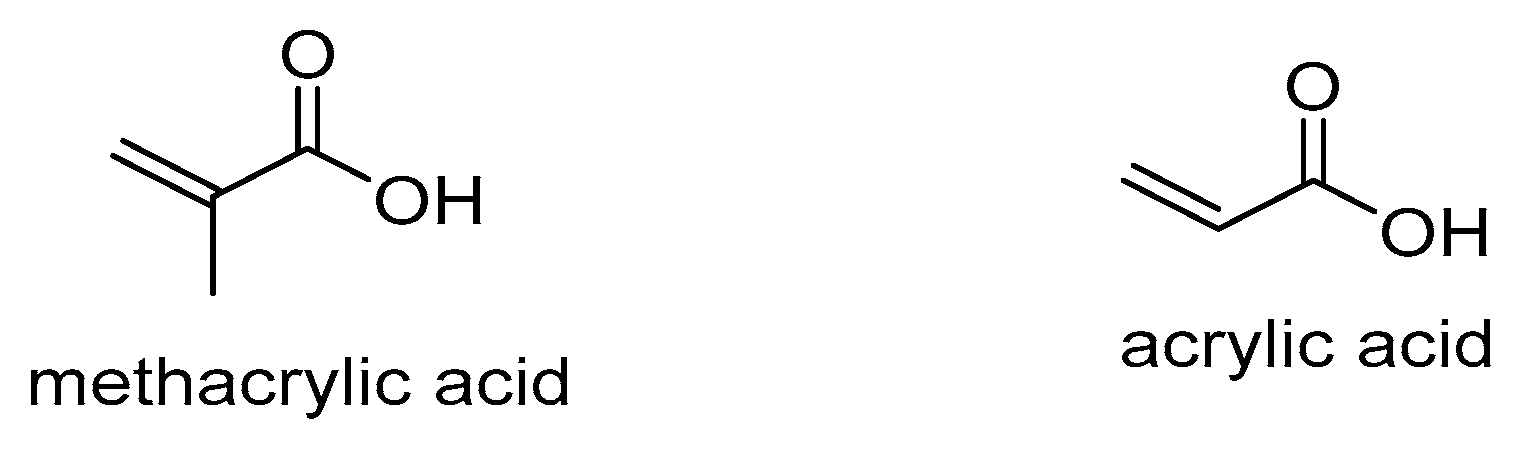

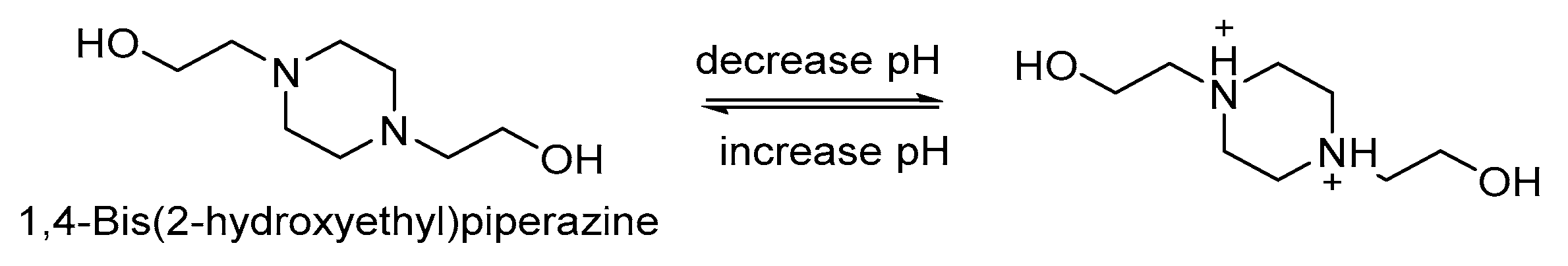

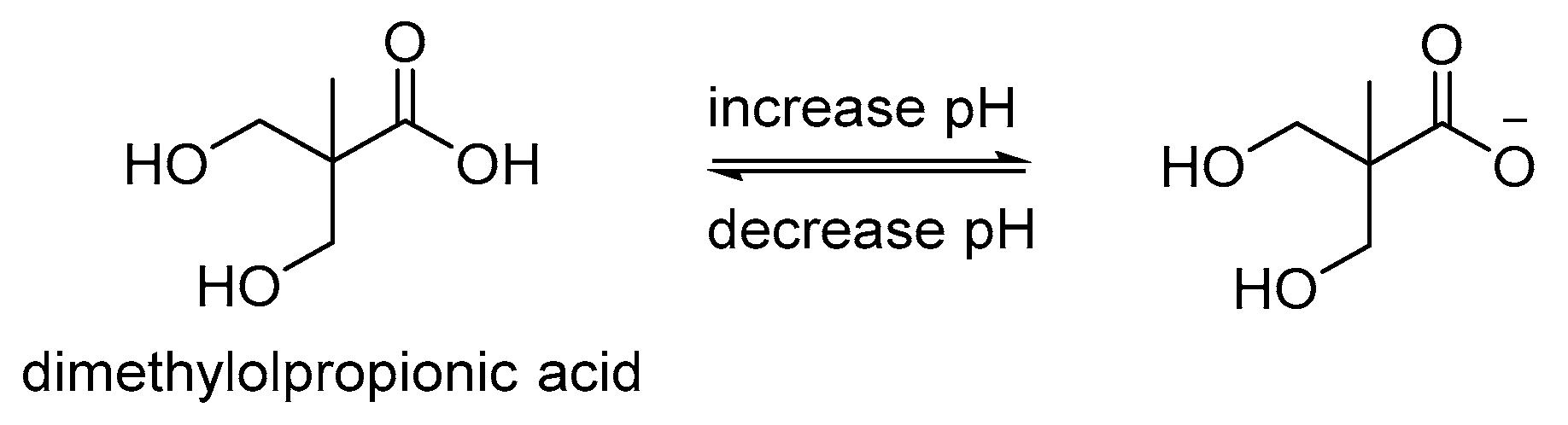



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.Y.H.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Lam, K.Y. PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers 2022, 14, 1672. https://doi.org/10.3390/polym14091672
Tan RYH, Lee CS, Pichika MR, Cheng SF, Lam KY. PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers. 2022; 14(9):1672. https://doi.org/10.3390/polym14091672
Chicago/Turabian StyleTan, Rachel Yie Hang, Choy Sin Lee, Mallikarjuna Rao Pichika, Sit Foon Cheng, and Ki Yan Lam. 2022. "PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery" Polymers 14, no. 9: 1672. https://doi.org/10.3390/polym14091672
APA StyleTan, R. Y. H., Lee, C. S., Pichika, M. R., Cheng, S. F., & Lam, K. Y. (2022). PH Responsive Polyurethane for the Advancement of Biomedical and Drug Delivery. Polymers, 14(9), 1672. https://doi.org/10.3390/polym14091672









