Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract
Abstract
:1. Introduction
2. Experimentation
2.1. Materials Preparation
2.2. Weight-Loss Measurements
2.3. Electrochemical Measurements
2.4. FTIR Spectroscopy
2.5. Hardness Studies
2.6. SEM Imaging
3. Results and Discussion
3.1. FTIR Characterization
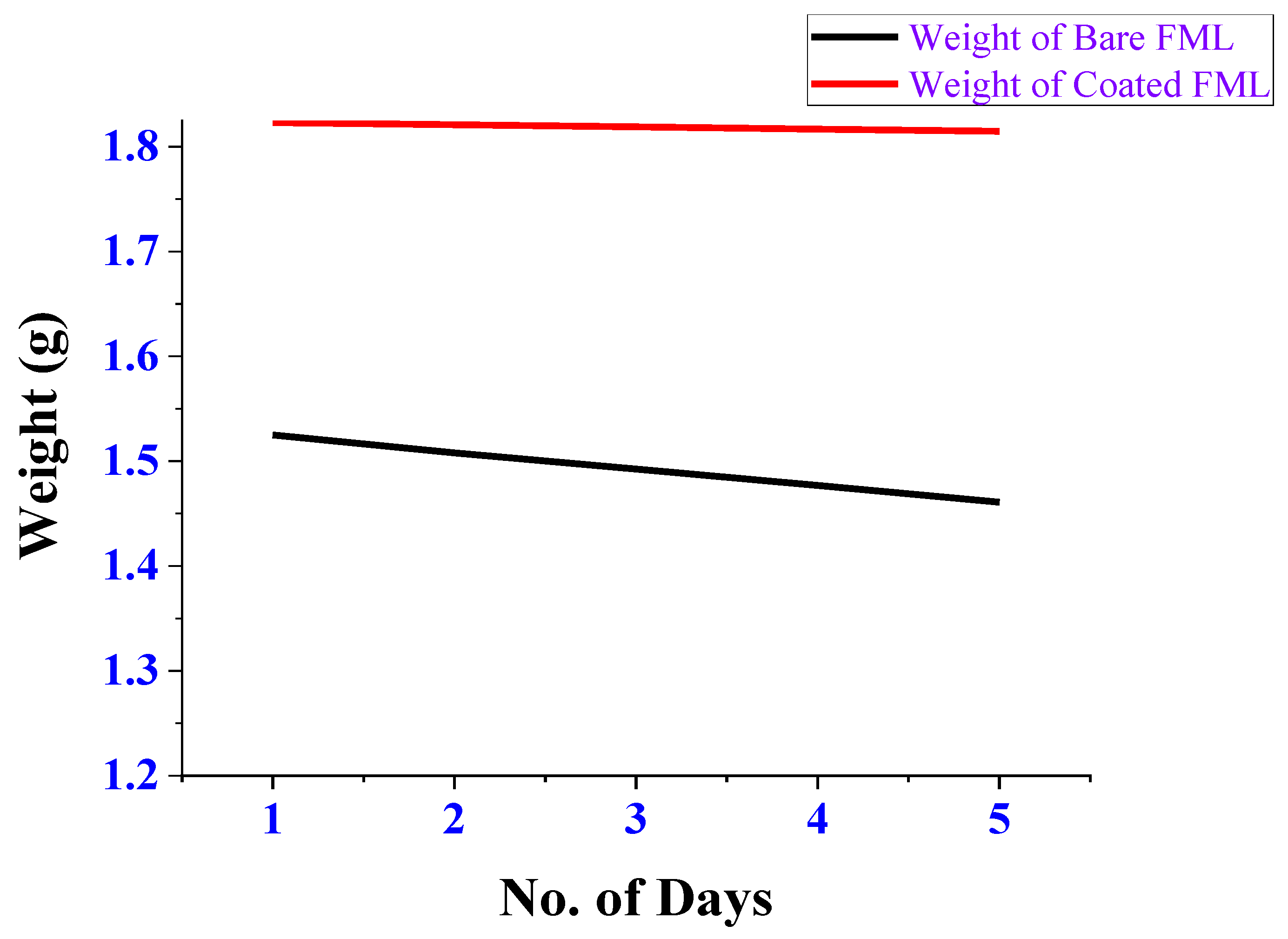
3.2. Weight-Loss Measurements
3.3. Electrochemical Study
3.3.1. Polarization Measurements
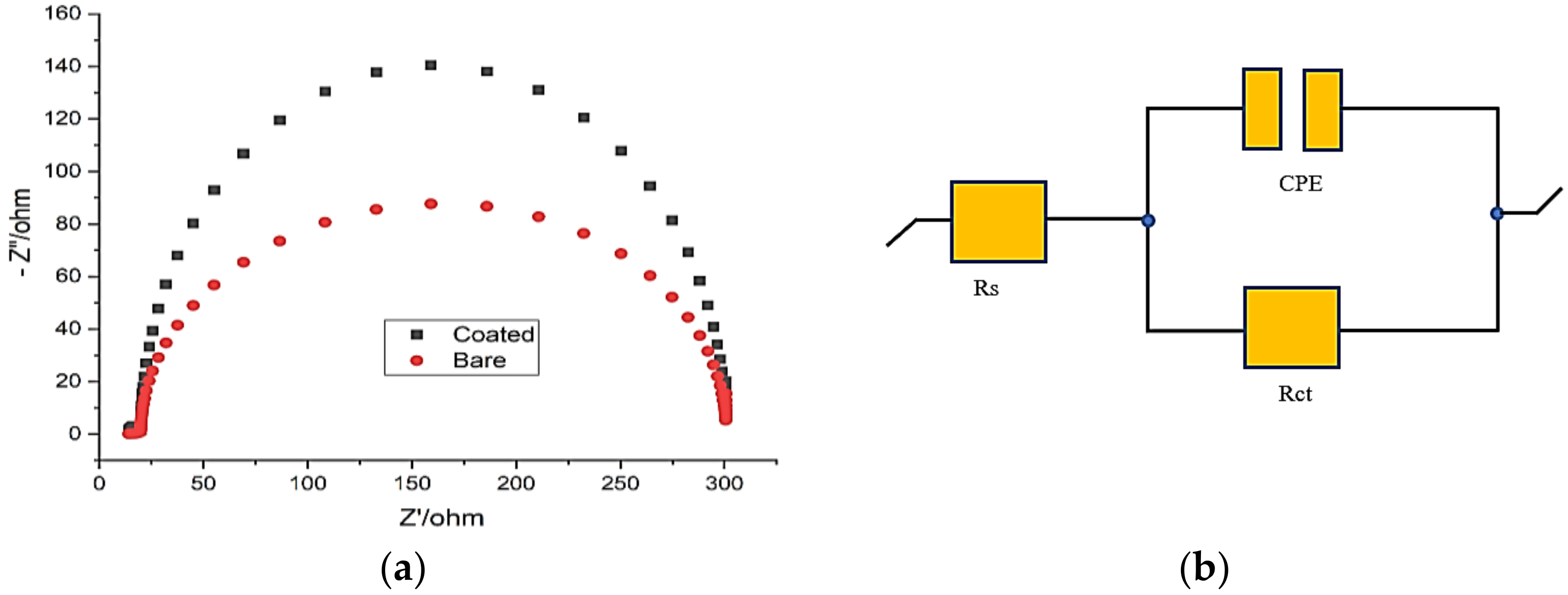
3.3.2. Electrochemical-Impedance Spectroscopy
3.4. Mechanical-Hardness Test
3.5. Surface Analysis
Scanning Electron Microscope
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinmazcelik, T.; Avcu, E.; Bora, M.O.; Çoban, O. A review: Fibre metal laminates, background, bonding types and applied test methods. Mater. Des. 2011, 32, 3671–3685. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Rajesh Jesudoss Hynes, N. Prospects of Joining Multi-Material Structures. AIP Conf. Proc. 2017, 1953, 130021–130025. [Google Scholar]
- Hynes, N.R.J.; Velu, P.S. Simulation of friction welding of alumina and steel with aluminum interlayer. Int. J. Adv. Manuf. Technol. 2017, 93, 121–127. [Google Scholar] [CrossRef]
- Velu, P.S.; Hynes, N.R.J. Numerical Analysis of Friction Welded Titanium Joints. J. Achiev. Mater. Manuf. 2016, 76, 26–29. [Google Scholar]
- Kumar, R.; Hynes, N.R.J. Finite-element simulation and validation of material flow in thermal drilling process. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 162–172. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Wu, S.; Suo, X.; Wei, X.; Li, H. Aluminum-polyethylene composite coatings with self-sealing induced anti-corrosion performances. J. Mater. Process. Technol. 2020, 282, 116642. [Google Scholar] [CrossRef]
- Jakubczak, P.; Bieniaś, J.; Dadej, K. Experimental and numerical investigation into the impact resistance of aluminium car-bon laminates. Compos. Struct. 2020, 244, 112319. [Google Scholar] [CrossRef]
- Li, F.; Zhao, M.; Zhan, Y.; Wu, C.; Zhang, Y.; Jiang, X.; Sun, Z. Facile fabrication of novel superhydrophobic Al2O3/polysiloxane hybrids coatings for aluminum alloy corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128444. [Google Scholar] [CrossRef]
- Tavakkolizadeh, M.; Saadatmanesh, H. Repair of Damaged Steel-Concrete Composite Girders Using Carbon fibre—Reinforced Polymer Sheets. Compos. Struct. 2003, 7, 311–322. [Google Scholar]
- Krishnegowda, P.; Venkatesha, V.; Krishnegowda, P.; Shivayogiraju, S. Acalypha torta leaf extract as green corrosion inhibitor for mild steel in hydrochloric acid solution. Ind. Eng. Chem. Res. 2013, 52, 722–728. [Google Scholar] [CrossRef]
- Hamill, L.; Hofmann, D.C.; Nutt, S. Galvanic Corrosion and Mechanical Behaviour of Fibre Metal Laminates of Metallic Glass and Carbon Fibre Composites. Adv. Eng. Mater. 2018, 2, 1700711. [Google Scholar] [CrossRef]
- Qu, D.; Qian, S.Y.; Baldock, B. Effects of galvanic coupling between carbon steel and stainless-steel reinforcements. NACE North. Area Conf. Proc. 2006, 45, 475–483. [Google Scholar]
- D’Urso, G.; Giardini, C.; Lorenzi, S.; Cabrini, M.; Pastore, T. The Effects of process parameters on mechanical properties and corrosion behavior in friction stir welding of aluminum alloys. Procedia Eng. 2017, 183, 270–276. [Google Scholar] [CrossRef]
- Gupta, N.K.; Joshi, P.G.; Srivastava, V.; Quraishi, M.A. A macromolecule as green corrosion inhibitor for mild steel in sulfamic acid useful for sugar industry. Int. J. Biol. Macromol. 2018, 106, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, E.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. J. Mol. Liq. 2018, 255, 185–198. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for aluminium in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; Aljuhani, A.; Aouad, M.R.; Hammouti, B.; Belghiti, M.E.; Chauhan, D.S.; Quraishi, M.A. Pyridazinium-based ionic liquids as novel and green corrosion inhibitors of carbon steel in acidmedium. J. Mol. Liq. 2018, 249, 997–1008. [Google Scholar] [CrossRef]
- Dohare, P.; Quraishi, M.A.; Lgaz, H.; Salghi, R. Ultrasound induced green synthesis of pyrazolo-pyridines as novel corrosion inhibitors useful for industrial pickling process: Experimental and theoretical approach. Results Phys. 2019, 13, 102344. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2015, 90, 107–117. [Google Scholar] [CrossRef]
- Rahim, A.; Rocca, E.; Steinmetz, J.; Kassim, M.; Adnan, R.; Ibrahim, M.S. Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros. Sci. 2007, 49, 402–417. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Jasminum nudiflorum Lindl leaves extract of the corrosion of aluminium in HCl solution. Corros. Sci. 2012, 64, 253–262. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, alpha-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Li, X.; Deng, S. Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros. Sci. 2012, 65, 299–308. [Google Scholar] [CrossRef]
- Torres, V. Inhibitory action of aqueous coffee ground extracts on the corrosion of carbon steel in HCl solution. Corros. Sci. 2011, 53, 2385–2392. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J.; Umoren, S.A. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Radojčić, I.; Berković, K.; Kovač, S.; Vorkapić-Furač, J. Natural honey and black radish juice as tin corrosion inhibitors. Corros. Sci. 2008, 50, 1498–1504. [Google Scholar] [CrossRef]
- Deng, S.; Li, X. Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros. Sci. 2012, 55, 407–415. [Google Scholar] [CrossRef]
- Goyal, M.; Pareek, A.; Nagori, B.P.; Sasmal, D. Aerva Lanata: A Review on Phytochemistry And Pharmacological Aspects. Pharm. Rev. 2011, 5, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Hynes, N.R.J.; Selvaraj, R.M.; Mohamed, T.; Mukesh, A.M.; Olfa, K.; Nikolova, P.M. Aerva lanata flowers extract as green corrosion inhibitor of low-carbon steel in HCl solution: An in vitro study. Chem. Pap. 2020, 75, 1165–1174. [Google Scholar] [CrossRef]
- Jafari, Y.; Nooshabadi, M.S.; Ghoreishi, S.M. Electropolymerized coatings of poly(oanisidine) and poly(o-anisidine)-TiO2 nanocomposite on aluminium alloy 3004 by using the galvanostatic method and their corrosion protection performance. Poly. Adv. Tech. 2014, 25, 279–287. [Google Scholar] [CrossRef]
- Egorkin, V.S.; Mashtalyar, D.V.; Gnedenkov, A.S.; Filonina, V.S.; Vyaliy, I.E.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Izotov, N.V.; Sinebryukhov, S.L.; et al. Icephobic Performance of Combined Fluorine-Containing Composite Layers on Al-Mg-Mn–Si Alloy Surface. Polymers 2021, 13, 3827. [Google Scholar] [CrossRef] [PubMed]






| Plant Name | Inhibition Efficiency | Material Used | Medium |
|---|---|---|---|
| Saraka Ashoka | 95.48 | carbon steel | 0.5 M H2SO4 |
| Chitosan | 92% | 300 mg/L on mild steel | 1M sulfamic acid |
| Glycyrrhiza glabra leaves | 88% | 800 ppm on mild steel | 1 M HCl |
| Sunflower-seed hull | 98% | 300 ppm on aluminium | 1 M HCl |
| Pyridazinium | 84% | 100 mg/L on mild steel | 1 M HCl |
| Pyrazolo-pyridines | 97% | 100 mg/L on carbon steel | 1 M HCl |
| Salvia officinalis | 96% | 2500 mg/L on stainless steel | HCl |
| Osmanthus fragran | 94% | 340 mg/L on carbon steel | HCl |
| Musa paradisica | 90% | 300 mg/L on carbon steel | HCl |
| Mangrove tannins | 89% | 6000 mg/L on metal | Acidic medium |
| Jasminumnudiflorum | 92% | 1000 mg/L on aluminium | HCl |
| Lawsonia inermis | 92% | 1200 mg/L on moderate steel | 1 m HCl |
| Dendrocalamus brandisii | 90% | 1000 mg/L on aluminium | HCl, H3PO4 |
| Aqueous coffee grounds | 83% | 400 mg/L on carbon steel | 1 M HCl |
| Phyllanthus amarus | 81% | 4000 mg/L on mild steel | Acidic media |
| Black radish | 92% | 1000 mg/L on carbon steel | - |
| Ginkgo | 80% | 100 mg/L on carbon steel | HCl and H2SO4 |
| Elemental Composition | % of Element |
|---|---|
| Manganese (Mn) | 0.0–0.15 |
| Iron (Fe) | 0.0–0.70 |
| Magnesium (Mg) | 0.80–1.20 |
| Silicon (Si) | 0.40–0.80 |
| Copper (Cu) | 0.15–0.40 |
| Zinc (Zn) | 0.0–0.25 |
| Titanium (Ti) | 0.0–0.15 |
| Chromium (Cr) | 0.04–0.35 |
| Other (Each) | 0.0–0.05 |
| Others (Total) | 0.0–0.15 |
| Aluminium (Al) | Balance |
| Days | Weight of the Bare FML (g) | Corrosion Rate (mmpy) | Weight of the Coated FML (g) | Corrosion Rate (mmpy) | Efficiency (%) |
|---|---|---|---|---|---|
| Initial | 1.5394 | - | 1.8249 | - | - |
| 1 | 1.5250 | 3.5358 | 1.8230 | 0.4665 | 86.8 |
| 2 | 1.5079 | 4.1982 | 1.8210 | 0.4912 | 88.29 |
| 3 | 1.4924 | 3.8065 | 1.8189 | 0.5056 | 86.71 |
| 4 | 1.4768 | 3.8312 | 1.8168 | 0.5147 | 86.56 |
| 5 | 1.4608 | 3.9287 | 1.8147 | 0.5184 | 86.8 |
| Average | 87.032 | ||||
| Concentration (ppm) | Before Coating (g) | After Coating (g) |
|---|---|---|
| Bare | 1.5394 | - |
| 600 | 1.5346 | 1.8890 |
| Specimen | Ecorr (V) | Icorr (A cm−2) | Efficiency (η %) |
|---|---|---|---|
| Brae FML | −0.79 | 0.001959 | - |
| Coated FML | −0.77 | 0.0002335 | 88 |
| Specimen | Rct (Ω cm2) | CPE (μF cm−2) | n | Efficiency (η %) |
|---|---|---|---|---|
| Brae FML (0) | 300.23 | 1.09 × 10−3 | 0.0715 | - |
| Coated FML (150) | 301.15 | 1.72 × 10−3 | 0.0785 | 85.9 |
| Specimen | Applied Force (P) in kg | Vickers Hardness Number (VHN) VHN = (1824 × P)/d2 | Average Vickers Hardness Number | Standard Deviation | ||
|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | ||||
| Coated FML after immersion | 30 | 293 | 307 | 297 | 299 | 5.89 |
| Bare FML after immersion | 30 | 211 | 215 | 201 | 209 | 5.89 |
| Untreated FML | 30 | 530 | 518 | 518 | 522 | 5.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hynes, N.R.J.; Vignesh, N.J.; Barile, C.; Velu, P.S.; Baskaran, T.; Jappes, J.T.W.; Al-Khashman, O.A.; Brykov, M.; Ene, A. Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract. Polymers 2022, 14, 1700. https://doi.org/10.3390/polym14091700
Hynes NRJ, Vignesh NJ, Barile C, Velu PS, Baskaran T, Jappes JTW, Al-Khashman OA, Brykov M, Ene A. Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract. Polymers. 2022; 14(9):1700. https://doi.org/10.3390/polym14091700
Chicago/Turabian StyleHynes, Navasingh Rajesh Jesudoss, Nagarajan Jawahar Vignesh, Claudia Barile, Pitchumani Shenbaga Velu, Thangagiri Baskaran, Jebas Thangiah Winowlin Jappes, Omar Ali Al-Khashman, Michail Brykov, and Antoaneta Ene. 2022. "Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract" Polymers 14, no. 9: 1700. https://doi.org/10.3390/polym14091700









